Abstract
Stress is the major predisposing and precipitating factor in the onset of depression which is the most significant mental health risk for women. Behavioral studies in animal models show that female sex though less affected by an acute stressor; exposure to repeated stressors induces coping deficits to impair adaptation in them. A decrease in the function of 5-hydroxytryptamine (5-HT; serotonin) in the hippocampus and an increased function of the 5-HT-1A receptor in the raphe nucleus coexist in depression. Pharmacological and neurochemical data are relevant that facilitation of serotonin neurotransmission via hippocampus due to desensitization of somatodendritic 5-HT1A receptors may lead to adaptation to stress. The present article reviews research on sex related differences of raphe-hippocampal serotonin neurotransmission to find a possible answer that may account for the sex differences of adaptation to stress reported in preclinical research and greater incidence of depression in women than men.
Keywords: Raphe, hippocampus, sex related differences, stress 5-HT-1A receptors, serotonin, depression.
1. INTRODUCTION
It is well accepted that males and females can behave differently but how much of that difference is attributable to genetic, environmental and neurobiological factors has often been a matter of debate [1-3]. Evidence suggests that the effects of sex hormones on brain organization occur so early in life that from the start the environment is acting on differently wired brains in the two sexes. Human studies on sex differences of brain function are often supported by preclinical research suggesting that the differences are attributable to biological differences between males and females because there are few environmental or socio-cultural factors to consider in preclinical research.
Women are at least twice as likely as men to suffer from depression and anxiety [4-6]. These sex differences are seen in different countries and cultures, suggesting a biological basis. Evidence from animal studies also suggests that behavioral and neurobiological effects of stress are sexually dimorphic. However, despite great interest in this area [7], mechanisms that may contribute to this striking sex difference have remained elusive.
A dysfunctional 5-hydroxytryptamine (5-HT; serotonin)-ergic system is a vulnerability factor for major depressive disorder and other forms of affective illnesses [2]. At least 14 different types and subtypes of serotonin receptors have been identified [8]. A number of these receptors such as 5-HT-2 [9, 10], 5-HT-3 [11] and 5-HT-1A [12] receptors play a role in the genesis of psychiatric illnesses. Studies on animal models show that raphe hippocampal serotonin neurotransmission and its regulation via 5-HT-1A receptors can explain vulnerability or resistance to stress stimuli. The 5-HT-1A receptor which is a key mediator of serotonergic signaling in the central nervous system is also implicated in the mechanism of action of selective serotonin reuptake inhibitors (SSRIs) [13-16], while some studies indicate that sex may moderate the response to antidepressants with women exhibiting a preferential response to SSRIs compared to tricyclic antidepressants (TCAs) [17].
Cell bodies of serotonin containing neurons are located in the raphe nuclei in the brain stem. The 5-HT-1A receptor is a G-protein-coupled receptor widely distributed in regions that receive serotonergic input from the raphe nuclei: the frontal cortex, septum, amygdale, hippocampus and hypothalamus [18, 19]. It also serves as somatodendritic autoreceptor of raphe nuclei reducing the firing rate of serotonergic neurons [8, 20-22]. The hippocampus has been extensively studied with regard to stress, depression and antidepressant action [23-25]. Sex related differences of raphe-hippocampal serotonin neurotransmission with a particular focus on 5-HT-1A receptors are accumulated in the present review as this may account for the gender differences of adaptation to stress and greater incidence of depression in women than men.
2. STRESS, DEPRESSION AND THE GENDER DIFFERENCE
The hypothesis that stress is the major precipitating factor in the onset of depression is consistently supported by clinical and preclinical studies [26-28] showing the relationship between previous traumatic stressful event (predisposing factor) and subsequent other stressor (precipitating factor). Both physical and psychological stressors have been shown to lead to the onset of a depressive episode. Sex in genetically predisoposed subjects may result in depression [29]. Studies in female twins show a clear interaction between genetic loading and exposure to a recent stressful life event in the precipitation of depressive episode [30].
In a study of 4,856 individuals (53% female) experiencing depression, it has been seen that different types of adverse life events are associated with different depressive symptoms profile [31]. In a follow-up study of over 7 years in 266 middle aged women, without a history of major depression at base line, 15.8% women met criteria for major depression [32]. These researchers reported that lifetime history of anxiety disorder and very stressful life events are important contributing factors in the onset of first episode of major depression.
Major depressive disorder is two times more prevalent in women than in men [4, 6, 33, 34]. The mean age of onset, the overall course of depression and the risk for chronic or recurrent depression does not differ between sexes [35], although some studies suggest that women have a higher rate of recurrent depression and slower recovery from a depressive episode [36].
Women are approximately three times more likely to develop depression in response to stress because they experience more stressful events [30, 37-39]. Women report more depressive symptoms than men, with an emphasis on worthlessness, decreased sexual interest, guilt feelings, insomnia, anxiety, and gastrointestinal symptoms [36, 40]. This prominent gender difference in depression begins in adolescence, prior to which the incidence of major depression is equal in girls and boys, suggesting the potential role of female sex hormones in female depression vulnerability [41]. Many depressed women also exhibit anxiety symptoms, and it has been suggested that women may be more likely to suffer from mixed anxiety-depressive disorder [42, 43]. Polymorphic variations in 5-HT transporter, MAO-A or 5-HT receptor may be involved in the sex related differences of adaptation to stress [29].
3. STRESS CONTROLLABILITY AND LEARNED HELPLESSNESS IN ANIMAL MODELS
Based upon the clinical evidence that links stressful life events with depressive episodes several animal models exhibiting stressor controllability and learned helplessness have been developed [44-47]. The most common animal model of 'stress and coping' is that of 'learned helplessness' [48, 49] in which animals are exposed to either controllable or uncontrollable stressful events and later, they are tested on a new task in which all animals are given the opportunity to control the stressor, usually by escape. In most reports, animals that are exposed to uncontrollable stressful events do not learn to escape during testing on the new task [50, 51]. This behavior has been equated with a sense of 'giving up', experienced by humans with major depression [52].
Animal models of depression should fulfill three major criteria [47]. The first criterion “face validity” assesses how well the symptoms observed in animals resemble those in human patients. The second criterion “predictive validity” addresses the question how well animals in the model respond favorably to the same drugs as human do under the same treatment conditions. The third criterion “construct validity” assesses to what extent the model is consistent with the theoretical rationale.
The learned helplessness paradigm was not developed to provide an animal model of depression or anxiety but it was shown in later studies that the model is sensitive to both antidepressants [53, 54] and anxiolytics [55-58]. Implications for the learned helplessness paradigm as an animal model of either depression or anxiety have been discussed [45,59]. The paradigm is widely used to understand neural mechanism and degree of behavioral adaptation to an uncontrollable stressor [60-63].
Animals exposed to other unpredictable and uncontrollable stressor e.g. restraint stress, elevated platform and forced swimming also show coping deficits for aversive but escapable situations [64, 65]. Chronic mild stress also causes behavioral changes in animals that parallels symptoms of depression [66, 67].
4. SEX RELATED DIFFERENCES IN ADAPTATION TO STRESS
Although learned helplessness is an established model for clinical depression and anxiety, and has been investigated for about 40 years, only few studies have used female animals in the learned helplessness paradigm. The female preponderance of depression is also, heretofore, not been consistently reflected in animal models (Table 1). Surprisingly, evidence from animal studies suggests that females are relatively resistant to the behavioral effects of an acute stress compared to males.
Table 1.
Sex Related Differences in Stress-Induced Behavioral Deficits Stressor
| Stressor | Behavior | Behavioral Deficits | References |
|---|---|---|---|
| Forced swimming | Latency and duration of immobility | Male > Female | [75-79] |
| Open space swimming | Latency and duration of immobility | Young Female > Young Male | [80] |
| Escapable shock Inescapable shock |
Escape latency Escape impairment |
Male> Female Male > Female Male = Female |
[73] [69-71, 73] [72] |
| Chronic mild stress | Disruption of sucrose intake & open field activity | Female > Male | [67, 84] |
| Chronic mild stress + Forced swimming | Latency and duration of immobility | Male > Female | [67, 84] |
| Single 2h restraint | Open field activity | Male > Female | [74, 82] |
| Repeated restraint | Open field activity | Female > Male | [74, 82] |
| Lipopolysaccharide challenge | Open field activity & Forced swim test |
Male > Female Male > Female |
[81] [81] |
Stressors to which female sex is more vulnerable are highlighted.
Exposure to inescapable foot shock disrupted shuttle box-escape performance of males, whereas, escape performance of females was unaffected [68]. Escape latencies increased in both males and females but the increases were greater in male rats [69]. In the elevated plus-maze also, exposure to inescapable foot shock resulted in suppression of “total number of arm entries” and “rearings” in males but not in females [70]. In addition “time on open arms” was reduced in both sexes, but this effect was stronger in males than in females. No sex based difference was found in learned helplessness behavior in another study [71] which reported that females to be more vulnerable than males to stress-induced elevations in homocysteine but not escape deficits.
Exposure to controllable stress (escapable foots hock) alleviated the expression of helplessness behavior in both females and males, but females learned to escape more rapidly than did males [72]. Moreover, modulation of controllability i.e exposure to inescapable foot shock produced helpless behavior in males but females were less likely to become helpless [72].
Male rats were more vulnerable to restraint stress than that of the female rats because open field behavior of female rats was less affected by a single 2h restraint stress than that of the male rats, though food intake was comparably decreased [73]. Male animals exhibited more immobility than females in the forced swimming test [3, 74-78] but young female rats were more vulnerable than males in an open space swim test [79].
Male rats were also more vulnerable to a mild lipopolysaccharide (LPS) challenge than that of female rats as assessed in both forced swim and open field test [80] because LPS challenge decreased open field activity in male but not female rats. LPS-treated female rats coped better with the stressful forced swimming procedure, as evidenced by an increase in swimming duration while in males swimming duration was not altered by LPS administration [80].
In striking contrast female but not male rats exhibited deficits of open field behavior after exposure to repeated restraint stresses [64, 73, 81, 82]. Female rats were more vulnerable to chronic mild stress as depicted by the disruption of sucrose intake and decreases of open field activity [66]. But in response to an additional forced swim test, females previously exposed to chronic mild stress, were found to cope better than males. The sex differences in helplessness behavior were not dependent on the presence of sex hormones in adulthood, because neither ovariectomy of females nor castration of males abolished those [83].
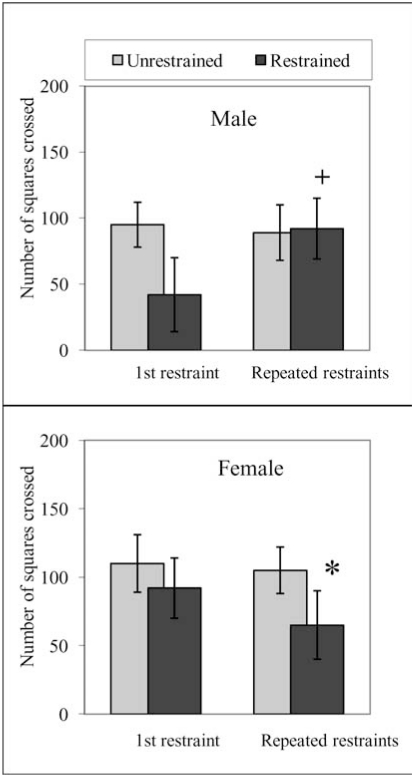
In general sex related studies on animal models show that female performance though better than males if exposed to an acute stressor but repeated or chronic exposure induces coping deficits to impair adaptation making female sex more vulnerable to depression. Data in Fig. (1) (Haleem unpublished data), similar to previously reported studies [64, 73, 81] show that male and female rats exposed to 2h restraint stress exhibited a decrease in open field activity monitored on the following day but the decreases were smaller and not significant in female rats. These differences were not attributable to the effects of entrained oestrus cycles in females as these were randomly distributed [73, 81]. Conversely, following repeated (2h/day for 5 days) exposure to restraint stress the deficits of open field exploration were present in female but not male rats (Fig. 1).
Fig. (1).
Activity of male and female rats in an open field 24 h after a single (2h) or repeated (2h/day for 5 days) restraints. Male (body weights 200-250 gm) and female (body weights 190-230) animals were restrained for 2h on wire grids and activity in an open field was monitored 24 h after the termination of the 1st or 5th restraint period as described by Haleem & Parveen (1994). Values are means ± S. D. (n=6). Significant differences by Newman Keuls test: *P<0.01 from respective unrestrained animals; +P<0.01 from 1st day (2h/day) restrained animals, following two way ANOVA. (Haleem unpublished data).
5. RAPHE-HIPPOCAMPAL SEROTONIN NEUROTRANSMISSION AND ADAPTATION TO STRESS
Early indications that central serotonin system may be involved in responses to stress came from studies on male rats demonstrating that acute stress procedures like immobilization, forced swimming and cold exposure increased brain 5-HT metabolism [84, 85]. Since then evidence has accumulated that 5-HT turnover is enhanced by following exposure to various stressors including exercise and foot shock although brain levels of 5-HT are not always altered [1, 4, 86-88]. It has been also shown that stress-induced increases of brain serotonin are caused by an increase in the availability of tryptophan [86, 88], the precursor of 5-HT, or an increase in the activity of tryptophan hydroxylase [89-92], the rate limiting enzyme of 5-HT biosynthesis. Microdialysis studies showed an increase in extracellular levels of serotonin [93-95] in different areas of the brain following exposure to different types of stressors.
A role of hippocampus in responses to stress, first reported from our laboratory [90], also emerged from studies on male rats. We found that acute exposure to an episode of 2h restraint stress increased 5-HT turnover in the hypothalamus, midbrain and cortex but the increases did not occur in the hippocampus [90]. Conversely, repeated daily exposure to 2h/day restraint, which produced behavioral adaptation, increased 5-HT turnover in the hippocampus only and not in other brain regions. It was suggested that an increase in serotonin neurotransmission via hippocampus is involved in adaptation to stress.
Later studies, performed on male animals, also consistently showed that hippocampus may mediate adaptation to severe inescapable stressor by the facilitation of serotonergic neurotransmission (Table 2). Acute exposure to an elevated platform enhanced 5-HT overflow in the prefrontal cortex but not dorsal hippocampus whereas repeated daily exposure to the same stressor increased extracellular 5-HT in the dorsal hippocampus but not the prefrontal cortex [65]. In another study rats received inescapable foot shock and were tested in a shuttle box 24 h later. Pre stressed animals exhibited impairment of escape responses. This effect was prevented by bilateral intra hippocampal injection of zimelidine, a serotonin reuptake blocker but not by desipramine, a noradrenaline reuptake blocker [96]. Neurogenesis in the dentate gyrus of the hippocampus was enhanced by the activation of serotonin receptors [97]. It was suppressed by stress and the suppression prevented by 5-HT-1A receptor agonists [98].
Table 2.
Evidence that Facilitation of Serotonin Neurotransmission in the Hippocampus is Involved in Adaptation to Stress
| Challenge | Response | Hippocampal 5-HT | References |
|---|---|---|---|
| Restraint stress | Decrease in food intake & open field activity | 5-HT increased in many brain regions except the hippocampus | [91] |
| Repeated restraint | Normal food intake & open field activity | 5-HT increased only in the hippocampus | [91] |
| Acute exposure to elevated platform | Increase in plasma corticosterone | Extra cellular 5-HT increased in the frontal cortex but not the hippocampus | [66] |
| Repeated daily (10 days) exposure to elevated platform | Normal plasma corticosterone response | Extra cellular 5-HT increased in the hippocampus but not the frontal cortex | [66] |
| Inescapable foot shock | Escape impairment in shuttle box | The behavioral deficit normalized with bilateral intra hippocampal serotonin reuptake inhibitor | [97] |
| Subordination stress | Neurogenesis | Stress-induced suppression of neurogenesis in the hippocampus prevented by 5-HT-1A agonists | [98-99] |
| Forced swimming | Immobility | Decreased 5-HT-1A receptor binding in the hippocampus | [125] |
| Restraint stress | Feedback control over 5-HT | Exaggerated feedback control over hippocampal 5-HT | [107] |
| Restraint stress | Density of 5-HT-1A receptor | 5-HT-1A receptor binding decreased in the hippocampus | [99, 110, 119] |
| Unpredictable, mild to moderate stressors | 5-HT-1A mRNA | 5-HT-1A expression decreased in the hippocampus | [23] |
| Long term administration of SSRIs | Feedback control over 5-HT | Smaller feedback effects over hippocampal 5-HT | [13, 14, 104] |
The synthesis and release of 5-HT in all brain regions including the hippocampus [20-22, 99] is under the control of an effective feedback mechanism involving the stimulation of 5-HT-1A receptors located on the soma and dendrites [100] of the serotonergic neurons in the raphe nucleus. 5-HT1B receptors located at the terminal ends of the serotonergic neurons [101] also control the release of 5-HT via a feedback mechanism [102]. Studies on the mechanism of action of selective serotonin reuptake inhibitors (SSRIs) and other antidepressants showed that repeated administration of these drugs increased 5-HT neurotransmission by either decreasing the sensitivity of presynaptic receptor or increasing the sensitivity of postsynaptic 5-HT-1A receptor in the dorsal hippocampus [13, 14, 16, 103]. Consequently it was suggested that a decrease in the function of the 5-HT in the hippocampus and an increased function of the 5-HT-1A receptor in the raphe nucleus coexist in depression.
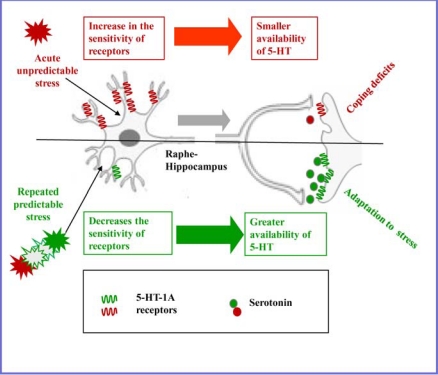
Studies using learned helplessness model of anxiety/depression and chronic stress model of depression also support the role of 5-HT-1A receptor in adaptation to stress. Thus, rats adapted to repeated restrain stress schedule of 2h/day for 5 days exhibited a decrease in the sensitivity of somatodendritic 5-HT-1A [60, 104] and terminal 5-HT-1B [105] receptors: an effect similar to antidepressant like effect. It was suggested that a decrease in the negative feedback control due to desensitization of auto receptors increases the availability of 5-HT in terminal regions to help cope the stress demand and produce adaptation to stress (Fig 2). Conversely, exposure to inescapable but not escapable stressors sensitized serotonergic neurons in the raphe region to subsequent input [85]. Acute exposure to 2h restraint stress [106] as well as long term starvation [107] increased the responsiveness of somatodendritic 5-HT-1A receptor to decrease serotonin neurotransmission particularly via raphe-hippcampal pathway (Fig. 2). A decrease in the density of 5-HT-1A receptor in the hippocampus also occurred in rats exposed to restraint stress [98, 108, 109]. Rats exposed to different mild to moderate stressors every day, therefore making the daily stress exposure unpredictable exhibited a significant decrease in 5-HT-1A mRNA and 5-HT-1A receptor binding in the hippocampus [23].
Fig. (2).
Attenuation (red) and enhancement (green) of raphe-hippocampal serotonin neurotransmission regulated by somatodendritic 5-HT-1A receptors.
It may be argued that a desensitization and super sensitization respectively of autoreceptors would be expected to increase and decrease the availability of 5-HT in all brain regions innervated by serotonergic neurons and not particularly in the hippocampus [90]. An explanation to this could be that postsynaptic 5-HT-1A receptors also control the synthesis and release of 5-HT via feedback mechanism. Hippocampus is enriched with 5-HT-1A receptor [110] and receives serotonergic innervations from median raphe [111]. Many innervated areas project back to raphe nuclei and these are interconnected [112]. It is therefore possible that postsynaptic 5-HT-1A receptors also alter the median raphe nucleus 5-HT neuronal firing [15]. It is also possible that the effects are mediated via stress-induced release of corticosteroid hormones. Hippocampus is enriched with high affinity mineralocorticoid receptors and lower affinity glucocorticoid receptors at which corticosteroids bind to alter 5-HT-1A receptor mediated responses, reviewed by Joel, [113].
6. SEX-DIFFERENCES IN RAPHE-HIPPOCAMPAL SEROTONIN NEUROTRANSMISSION
If facilitation of serotonin neurotransmission due to desensitization of somatodendritic 5-HT-1A receptor increasing the availability of 5-HT in the hippocampus mediates adaptation to stress, the sex differences of somatodendritic 5-HT-1A receptors become important. Sexual dimorphism in the serotonin was first reported in early 1960’s [114]. An increasing amount of later work supported the view that central serotonin metabolism synthesis and functional responses are greater in female than male rats [20, 115-117]. It was also observed that sex differences of 5-HT were particularly larger in the hippocampus [20].
Sex differences also occur in the regulation of serotonin neurotransmission via 5-HT-1A receptors [118]. Expression of serotonin-1A receptor messenger RNA was greater in males in the hypothalamus and amygdala, and less in males in the hippocampus [119]. The concentrations of 5-HT and 5-HIAA were greater in the hippocampus of female than male rats. The 5-HT-1A agonist 8-hydroxy-2 (di-n-propylamino) tetralin caused comparable decreases of 5-HT and 5-HIAA in both sexes in the hypothalamus, cortex and midbrain except the hippocampus where the decreases were twice as large in the females as in males [20]. It suggests that the sensitivity of 5-HT-1A receptors that control the availability of 5-HT in the hippocampus (Fig. 2) is greater in female sex.
A few studies have examined sex influence on the role of hippocampus in responses to stress. Female in proestrus exhibited greater density of dendritic spines in the area CA1 of the hippocampus than males [120]. In response to acute stressful event of intermittent shocks, spine density was enhanced in the male hippocampus but reduced in the female hippocampus. Effects of early experience on the dendritic structure of dentate gyrus were also sexually dimorphic [121]. Thus female rats raised in an enriched environment displayed increased dendritic bushiness relative to males raised in the same environment. Conversely, neonatal handling resulted in an increase in postsynaptic serotonin neurotransmission in the hippocampus of male rats but decreased it in females [122]. LPS treatment induced a female-specific enhancement of 5-HIAA levels in the hippocampus and some other regions [80].
In a study of sex influence and isolation housing on 5-HT-1A receptor binding female mice displayed lower postsynaptic 5-HT-1A receptor binding compared to males in the hippocampus. Subsequently, following 6 weeks isolation housing 5-HT-1A receptor binding was further increased in males but not in females [123]. Conversely, forced swimming was found to decrease 5-HT-1A receptor binding in the hippocampus of female but not male rats [124]. Serotonin-1A mRNA, protein and binding sites, were greater in the hippocampi of pre-pubertal female than male rats. These were decreased more by neonatal handling and the decreases were greater in female sex [122].
Sex related studies therefore show that male and female animals have different levels of serotonin neurotransmission via raphe- hippocampal pathway under unstressed condition, which can respond in opposite directions to the same stressor. Females have greater serotonin content but an exaggerated feedback control over raphe-hippocampal serotonin neurotransmission via 5-HT-1A receptors making this sex more vulnerable to depression.
It is worth considering that serotonin functions are modulated by corticosteroids [113, 125, 126] while stress-induced [81] as well as 5-HT-1A agonist-induced [82] increase of plasma corticosterone, the principal corticosteroid secreted by the rat adrenal gland, are greater in female than male rats suggesting an important role of circulating corticosteroids in the sex related differences of raphe hippocampal serotonin neurotransmission in adaptation to stress. Influence of MAO-A genotype on 5-HT-1A receptor availability or polymorphic variations of 5-HT transporters [108] may well be involved in these sex differences of 5-HT-1A expression.
5-HT-1A receptor dependent responses are also modulated by estrogen. Thus, acute estrogen treatment prevented 5-HT1A receptor-induced disruption of Prepulse inhibition in healthy women [127]. Although, estrogen treatment to ovariectomized rats had no effect on the number or affinity of 5-HT1A binding sites labeled with [3H]8-OH-DPAT but 5-HT1A-mediated inhibition of adenylate cyclase selectively increased in the hippocampus [128]. A role of glutamate receptors in the sex related differences of adaptation to stress is also possible because antidepressant like activity of chromium chloride in the forced swim test in mice was inhibited by antagonists of glutamate receptors as well as antagonists of 5-HT-1A receptors [129].
7. POSSIBLE CLINICAL RELEVANCE
There is wealth of clinical evidence supporting sex difference in 5-HT-1A receptor function. Investigations have also been made to show that variation in 5-HT-1A expression is genetically mediated [130, 131].
Age related sexual dimorphism of 5-HT-1A receptor binding potential was initially observed in various brain tissues obtained from autopsy subjects. In this study men exhibited a significant age dependent decrease in the dissociation constant (Kd) for 5-HT-1A receptor binding in the occipital cortex; in women maximum binding capacity (Bmax) decreased with aging in the parietal cortex and hippocampus [132].
Parsey et al. [133] did not find an age related decrease in 5-HT-1A binding potential in healthy men or women. However, they found higher 5-HT-1A binding potential in the dorsal raphe and many forebrain regions of women than men. Conversely, 5-HT-2 receptor binding capacity was higher in healthy men than healthy women [134]. Staley et al. [135] observed higher 5-HT transporter availability in healthy women than men, but lower 5-HT transporter availability in depressed women than depressed men [136]. Javanovic et al. [137] also observed that compared to healthy men healthy women had significantly higher 5-HT-1A receptor but lower 5-HT transporter binding potential in a wide array of cortical and subcortical brain regions but in the follicular phase, women did not differ from men in the 5-HT1A receptor binding [138].
Several strands of evidence have emerged that specifically implicate 5-HT-1A receptors in depression and therapeutic effects of antidepressant drugs. Men and women patients with major depression exhibited attenuation of 5-HT-1A receptor mediated neuroendocrine and hypothermic responses reflecting a decrease in the effectiveness of postsynaptic and somatodendritic 5-HT-1A receptors respectively [46, 139, 140]. A decrease in 5-HT-1A binding potential has been also observed in the multiple brain areas including raphe region of men and women patients with major depression and bipolar disorder [141-143]. In another study, patients with major depression who have never been exposed to medication were found to have higher 5-HT-1A receptor binding compared to the depressed patients with a history of medication and control [133] suggesting the 5-HT-1A binding potential to be affected by medication. Higher 5-HT1A binding potential in the raphe and hippocampus in bipolar depressed males but not in bipolar depressed females has been also reported [68].
Currently the most common class of effective antidepressants is SSRIs that acts by selectively blocking the high affinity reuptake of serotonin. Approximately 78% of the prescribed SSRIs are given to women [5], while some studies indicate that sex may moderate the response to antidepressants with women exhibiting a preferential response to SSRIs compared to TCAs. Investigations addressing gender differences in response to SSRI (sertraline) and imipramine treatment in male and female patients with chronic depression have reported that premenopausal women had a favorable response to sertraline than to imipramine [17, 144]. Postmenopausal women exhibited similar response to the two medications and men exhibited a more favorable response to impramine than to sertraline. Martenyi et al. [145] compared treatment efficacy of SSRI (fluoxetine) and SNRI (maprotiline) in men and women patients of unipolar depression. They found a significant difference between treatment groups in females but not in males. Amongst females the difference was significant in women aged <44 years but not >44 years suggesting that women in their reproductive period are more responsive to SSRIs than SNRIs. In a recent study, Young et al. [146] have reported that women have a better response to the SSRI citalopram than men, which may be due to sex-specific biological differences particularly in serotonergic systems.
CONCLUSION
The evidence accumulated in the present article suggests that raphe hippocampal serotonin neurotransmission and its regulation by 5-HT-1A receptors has an important role in the sex related differences of adaptation to stress. Greater 5-HT neurotransmission via postsynaptic 5-HT-1A receptors in the hippocampus makes female sex more resistant to an acute stressor. On the other hand, greater efficacy of feedback control over 5-HT synthesis and release mediated via 5-HT-1A receptors could impair adaptation making the female sex more vulnerable to repeated and/or chronic stressors. In the quest to understand the mechanism of sex related differences in adaptation to stress, the role of raphe-hippocampal serotonin neurotransmission and its regulation by 5-HT-1A receptors can only be a small part of a big picture. The mechanisms through which estrogen and glucocorticoids can modulate serotonin neurotransmission and functional polymorphisms in the 5-HT transporter gene are also worth considering for an understanding of sex related differences of adaptation to stress. Despite heightened complexity it implies that the issue of sex related differences of brain function is not less important because it may provide ways to understand novel mechanisms of brain function.
ACKNOWLEDGEMENT
The author would like to thank Higher education Commission, Pakistan Science Foundation and Karachi University for providing research grants.
REFERENCES
- 1.Andrews MH, Matthews SG. Programming of the Hypothalamo-Pituitary-Adrenal Axis: Serotonergic Involvement. Stress. 2004;7:5–27. doi: 10.1080/10253890310001650277. [DOI] [PubMed] [Google Scholar]
- 2.Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 3.Vigod SN, Stewart DE. Emergent research in the cause of mental illness in women across the life span. Curr. Opin. Psychiatry. 2009;22:396–400. doi: 10.1097/YCO.0b013e3283297127. [DOI] [PubMed] [Google Scholar]
- 4.Bebbington P, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H. The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Int. Rev. Psychiatry. 2003;15:74–83. doi: 10.1080/0954026021000045976. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R C. Epidemiology of women and depression. J. Affect. Disorder. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 6.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Ryan CE, Hess AL, Harrison W, Davis SM, Keller MB. Gender differences in chronic double and major depression. J. Affect. Disorder. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 7.De Vries GJ. Sex differences in adult and developing brain: Compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer D, Hannon JP, Martin GR. Molecular pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 9.Christiansen L, Tan Q, Iachina M, Bathum L, Kruse TA, McGue M, Christensen K. Candidate gene polymorphisms in the serotonergic pathway: influence on depression symptomlogy in an elderly population. Biol. Psychiatry. 2007;61:223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 10.McMahon FJ, Buervenich S, Charney D, Lipsky R, Rush AJ, Wilson AF, Sorant AJ, Papanicolaou GJ, Laje G, Fava M, Trivedi MH, Wisniewski SR, Manji H. Variation in the gene encoding the serotonin-2A receptor is associated with outcome of antidepressant treatment. Am. J. Hum. Genet. 2006;78:804–814. doi: 10.1086/503820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niesler B, Kapeller J, Hammer C, Rappold G. Serotonin type 3 receptor genes: HTR3A, B, C, D, E. Pharmacogenomics. 2008;9:501–504. doi: 10.2217/14622416.9.5.501. [DOI] [PubMed] [Google Scholar]
- 12. Savitz J, Lucki I, Drevets WC. 5-HT-1A receptor function in major depressive disorder. Prog. Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Artigas F, Bel N, Casanovas JM, Romero L. Adaptive changes of the serotonergic system after antidepressant treatments. Adv. Exp. Med. Biol. 1996;398:51–59. doi: 10.1007/978-1-4613-0381-7_6. [DOI] [PubMed] [Google Scholar]
- 14.Barton CL, Hutson PH. Inhibition of hippocampal 5-HT synthesis by fluoxetine and paroxetine: evidence for the involvement of both 5-HT-1A and 5-HT1B/D autoreceptors. Synapse. 1991;31:13–19. doi: 10.1002/(SICI)1098-2396(199901)31:1<13::AID-SYN3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 15.Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT-1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- 16.Gardier AM, Wurtman RJ. Persistent blockade of potassium-evoked serotonin release from rat frontocortical terminals after fluoxetine administration. Brain Res. 1991;540:325–330. doi: 10.1016/0006-8993(91)90530-9. [DOI] [PubMed] [Google Scholar]
- 17.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg A, Davis SD, Harrison WH, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am. J. Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 18.Lesch KP, Gutknecht L. Focus on the 5-HT-1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int. J. Neuropsychopharmacol. 2004;7:381–385. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- 19.Sharp T, Boothman L, Raley J, Queree P. Important measures in the post recent discoveries in 5-HT neuron feedback control. Trends Pharmacol. Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Haleem DJ, Kennett GA, Curzon G. Hippocampal 5-HT synhesis is greater in females than in males and is more decreased by 5-HT-1A agonist 8-OH-DPAT. J. Neural Transm. 1990;79:93–101. doi: 10.1007/BF01251004. [DOI] [PubMed] [Google Scholar]
- 21.Hutson PH, Dourish CT, Curzon Neurochemical and behavioral evidence for mediation of the hyperphagic action of 8-OH-DPAT by cell body autoreceptors. Eur. J. Pharmacol. 1986;129:347–352. doi: 10.1016/0014-2999(86)90445-0. [DOI] [PubMed] [Google Scholar]
- 22.Sharp T, Bramwell SR, Hjorth S, Grahame-Smith DJ. Pharmacological characterization of 8-OH-DPAT-induced inhibition of rat hippocampal 5-HT release in vivo as measured by microdialysis. Br. J. Pharmacol. 1989;98:989–997. doi: 10.1111/j.1476-5381.1989.tb14630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JF, Chalmers D, Little KY, Watson SJ. Regulation of 5-HT-1A receptor, glucocorticoid mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Stress and hippocampal plasticity. Ann. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 26.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implication for major depressive disorder. Rev. Psychiatr. Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 27.Bale TL. Stress sensitivity and the development of affective disorders. Horm. Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert G, Gilbert J, Irons C. Life events, entrapments and arrested anger in depression. J. Affect. Disorder. 2004;79:149–160. doi: 10.1016/S0165-0327(02)00405-6. [DOI] [PubMed] [Google Scholar]
- 29.Jabbi M, Korf J, Ormel J, Kema IP, Den Boer JA. Investigating the molecular basis of major depressive disorder etiology, A functional convergent genetic approach. Stress, neurotransmitter and hormones. Ann. NY. Acad. Sci. 2008;1148:42–56. doi: 10.1196/annals.1410.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendler KS, Kuhn J, Prescott CA. The relationship of neuroticism, sex and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 31.Keller MC, Neale MC, Kendler KS. Association of deifferent adverse life events with distinct pattern of depressive symptoms. Am. J. Psychiatry. 2007;164:1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- 32.Bromberger JT, Kravitz HM, Matthews K, Youk A, Brown C, Feng W. Predictors of first life time episodes of major depression in midlife women. Psychol. Med. 2009;39:55–64. doi: 10.1017/S0033291708003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler RC. The effects of life events on depression. Ann. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 34.Oquendo M, Ellis S, Greenwald S, Malone K, Weissman M, Mann J. Ethnic and sex differences in suicide rates relative to major depression in the United States. Am. J. Psychiatry. 2001;158:1652–1658. doi: 10.1176/appi.ajp.158.10.1652. [DOI] [PubMed] [Google Scholar]
- 35.Kessler R, McGonagle K, Swartz M, Blazer D, Nelson C. Sex and depression in the National Comorbidity Survey, I: Life-time prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 36.Ernst C, Angst J. The Zurich Study XII: Sex differences in depression: Evidence from longitudinal epidemiological data. Eur. Arch. Psychiatry Clin. Neurosci. 1992;241:222–230. doi: 10.1007/BF02190257. [DOI] [PubMed] [Google Scholar]
- 37.Kendler KS, Thrornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am. J. Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 38.Maciejewski P, Prigerson H, Mazure C. Sex differences in event-related risk for major depression. Psychol. Med. 2001;31:593–604. doi: 10.1017/s0033291701003877. [DOI] [PubMed] [Google Scholar]
- 39.Mazure C, Maciejewski P. The interplay of stress, gender and cognitive style. Arch. Women Ment. Health. 2003;6:5–8. doi: 10.1007/s00737-002-0161-3. [DOI] [PubMed] [Google Scholar]
- 40.Silverstein B. Gender differences in the prevalence of clinical depression: The role played by depression associated with somatic symptoms. Am. J. Psychiatry. 1999;156:480–482. doi: 10.1176/ajp.156.3.480. [DOI] [PubMed] [Google Scholar]
- 41.Payne JL. The role of estrogen in mood disorder in women. Intern. Rev. Psychiatry. 2003;15:280–290. doi: 10.1080/0954026031000136893. [DOI] [PubMed] [Google Scholar]
- 42.Lewinsohn PM, Rohde P, Seeley JR, Baldwin CL. Gender differences in suicide attempts from adolescence to young adulthood. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:427–434. doi: 10.1097/00004583-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 43. Piggot A. Gender differences in the epidemiology and treatment of anxiety disorders. J. Clin. Psychiatry. 1999;60(Suppl 18 ):4–15. [PubMed] [Google Scholar]
- 44.Chourbaji S, Zacher C, Sanchis-Sequra C, Dormann C, Vollmayr B, Gass P. Learned helplessness: validity and reliability of depressive like states in mice. Brain Res. Brain Res. Protoc. 2005;16:70–78. doi: 10.1016/j.brainresprot.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Maier SF, Watkin LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotrophin releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Shapira B, Newman M E, Gelfin Y, Lerer B. Blunted temperature and cortisol response to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology. 2000;25:421–438. doi: 10.1016/s0306-4530(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 47.Willner P. The validity of animal model of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 48.Maier SF. Learned helplessness and animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1984;8:435–446. [PubMed] [Google Scholar]
- 49. Seligman M E, Beagley G. Learned helplessness in the rat. J. Comp. Physiol. Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- 50.Overmier JB, Seligman M E. Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp. Physiol. Psychol. 1967;63: 28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 51. Seligman M E, Maier S F. Failure to escape traumatic shock. J. Exp. Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- 52.Miller WR, Seligman ME. Depression and learned helplessness in man. J. Abnorm. Psychol. 1975;84:228–238. doi: 10.1037/h0076720. [DOI] [PubMed] [Google Scholar]
- 53.Gambarana C, Scheggi S, Tagliamonte A, Pierluigi T, De Montis MG. Animal models for the study of antidepressant activity. Brain Res. Brain Res. Protoc. 2001;7:11–20. doi: 10.1016/s1385-299x(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 54.Petty F, Davis LL, Dabel D, Kramer GL. Serotonin dysfunction disorders: a behavioral neurochemistry perspective. J. Clin. Psychiat. 1996;57:11–16. [PubMed] [Google Scholar]
- 55.Drugan RC, Ryan SM, Minor TR, Maier SF. Librium prevents the analgesic and shuttlebox escape deficits typically observed following inescapable shock. Pharmacol. Biochem. Behav. 1984;21:749–754. doi: 10.1016/s0091-3057(84)80014-3. [DOI] [PubMed] [Google Scholar]
- 56.Maier SF, Kalman BA, Sutton LC, Wiertelak EP, Watkin LR. The role of the amygdale and dorsal raphe nucleus in mediating behavioral consequences of inescapable shock. Behav. Neurosci. 1993;107:377–388. doi: 10.1037//0735-7044.107.2.377. [DOI] [PubMed] [Google Scholar]
- 57.Maier SF, Kalman B A, Grahn R E. Chlordiazepoxide microinjected in the region of the dorsal raphe nucleus eliminates the interference with escape responding produced by inescacapable shock whether administered before inescapable shock or escape testing. Behav. Neurosci. 1997;108:121–130. doi: 10.1037//0735-7044.108.1.121. [DOI] [PubMed] [Google Scholar]
- 58.Short KR, Maier SF. Stressor controllability, social interaction and benzodiazepine systems. Pharmacol. Biochem. Behav. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- 59.Vollmayr B, Henn F A. Learned helplessness in the rat: improvements in the validity and reliability. Brain Res. Brain Res. Protoc. 2001;8:1–7. doi: 10.1016/s1385-299x(01)00067-8. [DOI] [PubMed] [Google Scholar]
- 60.Haleem DJ. Serotonergic mechanism of antidepressant action and adaptation to stress. J. Coll. Physician Surg. Pak. 1999;9:139–146. [Google Scholar]
- 61.McArthur R, Borsini F. Animal models of depression in drug discovery: a historical perspective. Pharmacol. Biochem. Behav. 2006;84:436–452. doi: 10.1016/j.pbb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Rupniak NM. Animal models of depression challenges for a drug development perspective. Behav. Pharmacol. 2003;14:385–390. doi: 10.1097/01.fbp.0000087738.21047.91. [DOI] [PubMed] [Google Scholar]
- 63.Willner P, Mitchell PJ. The validity of animal model of predisposition to depression Behav. Pharmacol. 2002;13:169–188. doi: 10.1097/00008877-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Kennett GA, Dickinson SL, Curzon G. Enhancement of some 5-HT dependent behavioral responses following repeated immobilization in rats. Brain Res. 1985;330:252–263. doi: 10.1016/0006-8993(85)90684-5. [DOI] [PubMed] [Google Scholar]
- 65.Storey JD, Robertson DA, Beattie JE, Reid IC, Mitchell SN, Balfour DJ. Behavioral and neurochemical responses evoked by repeated exposure to an elevated open platform. Behav. Brain Res. 2006;166:220–229. doi: 10.1016/j.bbr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z. Chronic mild stress: are females more vulnerable? Neuroscience. 2005;135:703–714. doi: 10.1016/j.neuroscience.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 67.Mineur YS, Beizung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression like behavior in mice. Behav. Brain Res. 2006;75:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 68.Steenbergen HL, Heinssbroek RPW, Van Harren F, Van de Poll N E. Sex dependent effects of inescapable shock administration on behavior and subsequent escape performance in rats. Physiol. Behav. 1989;45:781–787. doi: 10.1016/0031-9384(89)90295-3. [DOI] [PubMed] [Google Scholar]
- 69.Heinsbroek RP, Van Haaren F, Van de Poll NE, Steenbergen HL. Sex differences in the behavioral consequences of inescapable foot shocks depend on time since shock. Physiol. Behav. 1999;149: 1257–1263. doi: 10.1016/0031-9384(91)90360-z. [DOI] [PubMed] [Google Scholar]
- 70.Steenbergen H L, Heinsbroek R P, Van Hest A, Van de Poll N E. Sex-dependent effects of inescapable shock administration on shuttle box-escape performance and elevated plus-maze behavior. Physiol. Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- 71. Setnik B, de Souza F G, d’Almeida A, Nobrega JM. Increased homocysteine levels associated with sex and stress in the learned helplessness model of depression. Pharamacol. Biochem. Behav. 2004;77:155–161. doi: 10.1016/j.pbb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 72. Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol. Psychiatry. 2007;62:487–95. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 74.Alonso SJ, Castellano MA, Rodriguez M. Sex differences in behavioral despair: relationship between behavioral despair and open field activity. Physiol. Behav. 1991;49:69–72. doi: 10.1016/0031-9384(91)90232-d. [DOI] [PubMed] [Google Scholar]
- 75.Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced swimming test: gender differences. Neurosci. Biobehav. Rev.;1998, 23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 76.Contreras CM, Lara-Morales H, Molina-Hernandez M, Saavedra M, Arrelin-Rosas G. An early lesion of the lateral septal nuclei produces changes in the forced swim test depending on gender. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19:1277–1284. doi: 10.1016/0278-5846(95)00266-9. [DOI] [PubMed] [Google Scholar]
- 77.Marvan ML, Chavez-Chavez L, Santana S. Clomipramine modifies fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch. Med. Res. 1996;27:83–86. [PubMed] [Google Scholar]
- 78.Marvan ML, Santana S, Chevez LC, Bertran M. Inescapable shocks attenuate fluctuations of forced swimming immobility in different phases of the rat estrous cycle. Arch. Med. Res. 1997;28:369–372. [PubMed] [Google Scholar]
- 79.Sun M-K, Alkon DL. Differential gender related vulnerability to depression induction and converging antidepressant responses in rats. J. Pharmacol. Exp. Ther. 2006;316:926–932. doi: 10.1124/jpet.105.093948. [DOI] [PubMed] [Google Scholar]
- 80.Pitychoutis PM, Nakamura K, Tsonis PA, PapadopoulouDaifoti Z. Neurochemical and behavioral alterations in an inflammatory model of depression: Sex differences exposed. Neuroscience. 2009;159:1216–1232. doi: 10.1016/j.neuroscience.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 81.Haleem DJ, Kennett G A, Curzon G. Adaptation of female rats to stress: shift to male pattern by inhibition of corticosterone synthesis. Brain Res. 1988;458:339–347. doi: 10.1016/0006-8993(88)90476-3. [DOI] [PubMed] [Google Scholar]
- 82.Haleem DJ, Kennett GA, Whitton PS, Curzon G. 8-OH-DPAT increases corticosterone but not other 5-HT-1A dependent responses more in females. Eur. J. Pharmacol. 1989;164:435–443. doi: 10.1016/0014-2999(89)90251-3. [DOI] [PubMed] [Google Scholar]
- 83.Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- 84.Bliss EL, Ailion J, Zwangziger J. Metabolism of norepinephrine, serotonin and dopamine in rat brain with stress. J. Pharmacol. Exp. Ther. 1968;164:122–134. [PubMed] [Google Scholar]
- 85.Curzon G, Green AR. Effects of immobilization on rat brain tryptophan pyrrolase and brain 5-hydroxytryptamine metabolism. Br. J. Pharmacol. 1969;37:689–697. doi: 10.1111/j.1476-5381.1969.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 87.Curzon G, Joseph MH, Knott PJ. Effects of immobilization and food deprivation on rat brain tryptophan metabolism. J. Neurochem. 1972;19:1967–1974. doi: 10.1111/j.1471-4159.1972.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 88. Kennett GA, Joseph MH. The functional importance of increased brain tryptophan in the serotonergic responses to restraint stress. Neuropharmacology. 1981;20:39–43. doi: 10.1016/0028-3908(81)90039-3. [DOI] [PubMed] [Google Scholar]
- 89.Dunn AJ. Foot shock -induced changes in brain catecholamines or indoleamines are not mediated by CRF or ACTH. Neurochem. Int. 2000;37:61–69. doi: 10.1016/s0197-0186(99)00163-1. [DOI] [PubMed] [Google Scholar]
- 90.Haleem DJ, Parveen T. Effects of restraint on rat brain regional 5-HT synthesis rate following adaptation to repeated restraint. NeuroReport. 1994;5:1785–1788. doi: 10.1097/00001756-199409080-00025. [DOI] [PubMed] [Google Scholar]
- 91.Shimizu , Oomura Y, Kai Y. Stress-induced anorexia in rats mediated via serotonergic mechanism in the hypothalamus. Physiol. Behav. 1989;46:835–841. doi: 10.1016/0031-9384(89)90045-0. [DOI] [PubMed] [Google Scholar]
- 92.Singh VB, Onaivi ES, Phan TH, Boadle-Biber MC. The increase in rat cortical and midbrain tryptophan hydroxylase activity in response to acute and repeated sound stress are blocked by bilateral lesions of the central nucleus of the amygdale. Brain Res. 1990;530:49–50. doi: 10.1016/0006-8993(90)90656-v. [DOI] [PubMed] [Google Scholar]
- 93.Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 94.Fujino K, Yoshitake T, Inoue O, Ibii N, Kehr J, Ishida J, Nohata H, Yamaguchi M. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci. Lett. 2002;320:91–95. doi: 10.1016/s0304-3940(02)00029-0. [DOI] [PubMed] [Google Scholar]
- 95.Shimizu N, Take S, Hori T, Oomura Y. In vivo measurement of hypothalamic serotonin release by intracerebral microdialysis: Significant enhancement by immobilization stress in rats. Brain Res. Bull. 1992;28:727–734. doi: 10.1016/0361-9230(92)90252-s. [DOI] [PubMed] [Google Scholar]
- 96.Joca SR, Zanelati T, Guimaraes FS. Post stress facilitation of serotonergic, but not noradrenergic neurotransmission in the dorsal hippocampus prevented learned helplessness development in rats. Brain Res. 2006;1087:67–74. doi: 10.1016/j.brainres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 97.Jason JR, Jacobs BL. 5-HT-1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 98.Gould E. Serotonin and hippocampal neurogenesis. Neuropharmacology. 1991;21(2 Suppl ):46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 99.Hjorth S, Auerbach SB. Further evidence for the importance of 5-HT-1A autoreceptors in the action of selective serotonin reuptake inhibitors. Eur. J. Pharmacol. 1994;260:251–255. doi: 10.1016/0014-2999(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 100.Verge D, Daval G, Patey A, Gozlan H, Mestikawy E, Hamon M. Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT-1A subtype. Eur. J. Pharmacol. 1985;113:463–464. doi: 10.1016/0014-2999(85)90099-8. [DOI] [PubMed] [Google Scholar]
- 101.Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine autoreceptors in the rat brain cortex with 5-HT-1B binding sites. Naunyn-Schmiedeberg s Arch. Pharmacol. 1986;332:1–7. doi: 10.1007/BF00633189. [DOI] [PubMed] [Google Scholar]
- 102. Adell A, Celada P, Artigas F. The role of 5-HT-1B receptors in the regulation of serotonin cell firing and release in the rat brain. J. Neuro chem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- 103.Blier P, Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorder and pain. J. Psychiatry Neurosci. 2001;26:37–43. [PMC free article] [PubMed] [Google Scholar]
- 104.Haleem DJ. Attenuation of 8-OH-DPAT-induced decreases in 5-HT synthesis in brain regions of rats adapted to a repeated stress schedule. Stress. 1999;3:123–129. doi: 10.3109/10253899909001117. [DOI] [PubMed] [Google Scholar]
- 105.Haleem DJ, Saify ZS, Siddiqui S, Batool F, Haleem M A. Pre and post synaptic responses to 1-(1-naphthylpiperazine) following adaptation to stress in rats. Prog. Neuro-psychopharmacol. Biol. Psychiatry. 2002;26:149–156. doi: 10.1016/s0278-5846(01)00240-8. [DOI] [PubMed] [Google Scholar]
- 106.Haleem D J, Samad N, Perveen T, Haider S, Haleem M A. Role of serotonin-1A receptors in restraint -induced behavioral deficits and adaptation to repeated restraint stress in rats. Int. J. Neurosci. 2007;117:243–257. doi: 10.1080/00207450500534084. [DOI] [PubMed] [Google Scholar]
- 107.Haleem DJ. Exaggerated feedback control over 5-HT and hyperactivity in a rat model of anorexia nervosa. Appetite. 2009;52:44–50. doi: 10.1016/j.appet.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 108.Mickey BJ, Ducci F, Hodgkinson CA, Langenecker S A, Goldman D, Zubieta J-K. Monoamine oxidase a genotype predicts human serotonin-1a receptor availability in vivo. J. Neurosci. 2008;28:11354 –11359. doi: 10.1523/JNEUROSCI.2391-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 110.Azmitia EC, Gannon PJ, Kheck NM, Azmitia PM. Cellular localization of 5-HT-1A receptors inn primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 111. Patel TB, Azmitia EC, Zhou FC. Increased 5-HT-1A receptor immunoreactivity in the rat hippocampus following 5, 7 dihydroxytryptamine lesions in the cingulum bundle and fimbria fornix. Behav. Brain Res. 1996;73:319–323. doi: 10.1016/0166-4328(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 112. Jacobs B L, Azmitia E C. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 113. Joels M. Functional actions of corticosteroids in the hippocampus. Eur. J. Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 114. Kato R. Serotonin content of rat brain in relation to sex and age. J. Neurochem. 1960;5:202P. doi: 10.1111/j.1471-4159.1960.tb13355.x. [DOI] [PubMed] [Google Scholar]
- 115.Carlsson M, Svensson K, Erriksson E, Carlsson A. Rat brain serotonin: Biochemical and functional evidence for a sex difference. J. Neural. Transm. 1985;63:297–313. doi: 10.1007/BF01252033. [DOI] [PubMed] [Google Scholar]
- 116.Rosecrans JA. Differences in brain area 5-hydroxyrtyptamine turnover and rearing behaviour in rats and mice of both sexes. Eur. J. Pharmacol. 1970;9:379–382. doi: 10.1016/0014-2999(70)90239-6. [DOI] [PubMed] [Google Scholar]
- 117.Simon A, Volicer L. Neonatal asphyxia in the rat: greater vulnerability of males and persistent effects on brain monoamine synthesis. J. Neurochem. 1976;26:893–900. doi: 10.1111/j.1471-4159.1976.tb06470.x. [DOI] [PubMed] [Google Scholar]
- 118.Mendelson SD, McEwen BS. Autoradiogrphic analysis of the effects of restraint-induced stress on 5-HT-1A, 5-HT-1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology. 1991;54:454–461. doi: 10.1159/000125951. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 120.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Juraska JM. Sex differences in ‘cognitive regions’ of the rat brain. Psychoneuroendocrinology. 1991;16:105–109. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- 122.Stamatakis A, Mantelas A, Papaioannou A, Pondiki S, Fameli M, Stylianopoulou F. Effects of neonatal handling on serotonin-1A subtype receptors in the rat hippocampus. Neuroscience. 2006;140:1–11. doi: 10.1016/j.neuroscience.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 123. Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre and postsynaptic 5-HT-1A receptors. Brain Res. 2006;1103:76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 124.Bellido I, Gomez-Luque A, Garcia-Carrera P, Rius F, de la Cuesta FS. Female rats show an increased sensibility to the forced swim test depressive-like stimulus in the hippocampus and frontal cortex 5-HT1A receptors. Neurosci. Lett. 2003;350:145–148. doi: 10.1016/s0304-3940(03)00882-6. [DOI] [PubMed] [Google Scholar]
- 125.Goel N, Bale TL. Examining the intersection of sex and stress in modeling neuropsychiatric disorders. J. Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haleem DJ. Repeated corticosterone treatment attenuates behavioral and neuroendocrine responses to 8-hydroxy-2-di-n-propylamino tetralin in rats. Life Sci. 1992;51:225–230. doi: 10.1016/0024-3205(92)90055-t. [DOI] [PubMed] [Google Scholar]
- 127.Gogos A, Nathan PJ, Guille V, Croft RJ, van den Buuse M. Estrogen prevents 5-HT1A receptor-induced disruptions of prepulse inhibition in healthy women. Neuropsychopharmacology. 2006;31:885–889. doi: 10.1038/sj.npp.1300933. [DOI] [PubMed] [Google Scholar]
- 128. Clarke WP, Saul MS. Estrogen effects on 5-HT1A receptors in hippocampal membranes from ovariectomized rats: functional and binding studies. Brain Res. 1990;518:287–291. doi: 10.1016/0006-8993(90)90983-i. [DOI] [PubMed] [Google Scholar]
- 129. Piotrowska A, Mtynie K, Siwek A, Dybata M, Opoka W, Poleszak E, Nowak G. Antidepressant like effects of chromium chloride in the mouse forced swim test: involvement of glutamatergic and serotonergic receptors. Pharmacol. Rep. 2008;60:991–995. [PubMed] [Google Scholar]
- 130.David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J. Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parsey RV, Oquenda MA, Ogden RT, Olvet DM, Simpson N, Huang YY, Van Heertum R, Arango V, Mann JJ. Altered serotonin-1A binding in major depression: a [carbonyl-C-11] WAY100635 positron emission tomography study. Biol. Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 132.Palego L, Marazzi D, Rossi A, Giannaccini G, Naccarato AG, Lucacchini A, Cassano GB. Apparent absence of aging and gender effects on serotonin-1A receptors in human neocortex and hippocampus. Brain Res. 1997;758:26–32. doi: 10.1016/s0006-8993(96)01415-1. [DOI] [PubMed] [Google Scholar]
- 133.Parsey R V, Oquenda M A, Simpson N R, Ogden R T, Van Heertum R, Arango V, Mann J J. Effects of sex age and aggressive traits in man on brain 5-HT-1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;945:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 134.Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5-HT-2 receptor in the living human brain. Neurosci. Lett. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- 135.Staley JK, Krishnan-Sarin S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB. Sex differences in beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and non smokers. Synapse. 2001;41:275–284. doi: 10.1002/syn.1084. [DOI] [PubMed] [Google Scholar]
- 136.Staley J K, Sanacora G, Tamagnan G, Maciejewski P K, Malison R T, Berman R M, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, Charney D, Innis R B. Sex differences in diencephalon serotonin transporter availability in major depression. Biol. Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 137. Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom A L. Sex differences in the serotonin-1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 138.Stein P, Savli MW, Mitterhauser M, Fink M, Spindelegger C, Mien L-K, Moser U, Dudczak R, Kletter K, Kasper S, Lanzenberger R. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C] WAY-100635. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:2159–2168. doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- 139.Cowen PJ, Power AC, Anderson IM. 5-HT-1A receptor sensitivity in major depression: A neuroendocrine study with buspirone. Br. J. Psychiatry. 1994;164:372–379. doi: 10.1192/bjp.164.3.372. [DOI] [PubMed] [Google Scholar]
- 140.Lesch KP. 5-HT-1A receptor responsitivity in anxiety disorder and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1991;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 141.Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nucl. Med. Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 142.Sargent PA, Kjaer KH, Bench C J, Rabiner AE, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen P J. Brain serotonin-1A receptor binding measured by positron emission tomography with WAY-100635: effects of depression and antidepressant treatment. Arch. Gen. Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 143.Sullivan GM, Ogden RT, Oquendo MA, Kumar JS, Simpson N, Huang YY, Mann JJ, Parsey RV. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol. Psychiatry. 2009;66:223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: Sex-Age Interactions. J. Women's Health. 2005;14:609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- 145.Martenyi F, Dossenbach M, Mraz K, Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double blind trial of antidepressants with serotonergic and norepinephrinergic reuptake inhibition profile. Eur. Neuropsychopharmacol. 2001;11:227–232. doi: 10.1016/s0924-977x(01)00089-x. [DOI] [PubMed] [Google Scholar]
- 146.Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, Rush AJ. Sex differences in response to citalopram: A STAR*D report. J. Psychiat. Res. 2009;43:503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]