Abstract
Heavy alcohol use in young adults has been prospectively associated with a host of psychosocial and alcohol-related problems. Recent studies have supported the interaction between serotonin transporter polymorphism and adverse environmental factors, as a predictor of alcohol use and the development of alcohol dependence. The current study examined the role of depressive symptoms in combination with the serotonin transporter polymorphism as a predictor of alcohol use and alcohol-related problems. Results revealed a significant genotype by depressive symptom interaction, such that heavier alcohol use was associated with depressive symptoms in L allele homozygotes but not among S allele carriers. These results remained significant after controlling for ethnicity and gender effects. These findings extend the emerging literature supporting 5-HTTLPR genotype as a risk factor for alcohol-related problems in the context of co-occurring symptoms of depression.
Keywords: 5-HTTLPR, alcohol, depression, serotonin, serotonin transporter polymorphism, SLC6A4
Introduction
Alcohol use disorders (AUDs) affect approximately 8.5% of the U.S. adult population (Grant et al., 2004) and account for $185 billion in costs related to the psychiatric and medical consequences of heavy drinking (Harwood, 2000). Given that the onset of alcoholism occurs largely before age 30, recent studies have focused on genetic and environmental factors that affect young people (Hingson and Zha, 2009).
Alcohol use in young adults is especially problematic as early age of first use is associated with a later increase in psychosocial and alcohol-related problems (Tapert et al., 1999; Rowe et al., 2004). Both genetic and environmental factors contribute to the onset of problem drinking in adolescence and early adulthood. Genetic factors account for an estimated 40–60% of the risk of developing AUDs (Kendler et al., 1994; Prescott et al., 1999; Schuckit, 2009). At the neurobiological level, the serotonin neurotransmitter system appears to be important to alcohol phenotypes via its relation to impulsivity, negative mood, craving, and response to alcohol (Heinz et al., 2004; Hinckers et al., 2006).
A polymorphism which has been directly associated with alcohol dependence in the 5′ promoter region (5-HTTLPR) of the serotonin transporter gene (locus ID SLC6A4) is of particular interest to neuropsychiatric genetics, including genetics of alcoholism. This 44-bp deletion/insertion polymorphism results in two common alleles, the 528-bp long allele (L) and the 448-bp short allele (S). The S allele has been shown to decrease transcription and decrease 5-HT reuptake in vitro (Heils et al., 1997) and in vivo (Heinz et al., 2000). The L allele has been associated with a predisposition to lowered level of response to alcohol, which is in turn associated with the onset of AUDs (Bleich et al., 2007). Additionally, the L allele has been associated with early onset of AUDs, earlier age of first drink (Buchmann et al., 2009), higher rates of AUD, a heavier drinking pattern, and higher alcohol craving (Hu et al., 2005; Olsson et al., 2005; Hinckers et al., 2006; Bleich et al., 2007).
In contrast to studies reporting an association between the L allele and alcohol problems, there is recent evidence suggesting that the S allele has a small, but direct relationship to the development of AUDs (Feinn et al., 2005; McHugh et al., 2010). Studies examining gene × environment interactions have produced mixed evidence regarding which allele contributes risk. A meta-analysis of 5-HTTLPR findings reported that the S allele was more often associated with the development of alcohol problems (Feinn et al., 2005). A separate study found that the S allele placed adolescents at risk for alcohol use (Brody et al., 2009). Interestingly, although Brody et al. (2009) found that the S allele represented a risk factor for alcohol use in youth, the 17 studies analyzed by Feinn et al. (2005) in their meta-analysis had a mean age range of 39–52 years of age. The relationship between the serotonin transporter polymorphism and AUDs may in fact differ by age, gender, and/or stage of addiction (Philibert et al., 2008; Merenakk et al., 2011). Contradictory findings regarding SLC6A4 may also be explained by the heterogeneity of alcohol use phenotypes, including variability in depression and anxiety symptoms (Schuckit and Smith, 2006), comorbidity, and age of onset (Johnson, 2004; Buchmann et al., 2009). The current study examines the interaction between depressive symptoms and 5-HTTLPR as a predictor of alcohol use and related problems in a sample of heavy-drinking young adults.
Previous work on the interaction effects of the serotonin transporter polymorphism has focused on stress and negative life events as moderators of the development of AUDs. Psychological stress has been found to moderate the effects of the 5-HTTLPR polymorphism on alcohol use both in non-human primates (Barr et al., 2004) and in human samples (Caspi et al., 2010). Alcohol use may represent a way to cope with depression and/or anxiety symptoms, suggesting that these symptoms may moderate the relationship between SLC6A4 and alcohol misuse (Armeli et al., 2008). Family history of depression and stressful life events have both been linked to the development of poor coping skills, which have been directly associated with alcohol dependence (Schuckit and Smith, 2006). More recently, two copies of the L allele at the SLC6A4 locus were associated with higher coping motivations to drink (Armeli et al., 2008). This relationship was strongest among women, underscoring the role of gender in determining SLC6A4 contribution to alcohol phenotypes. Genetic studies of family history reveal minimal shared genetic etiology between depression and alcoholism, despite high comorbidity. This is consistent with the hypothesis that symptoms of depression and anxiety may affect AUDs through an indirect path, rather than shared heritability (Swendsen and Merikangas, 2000; Kuo et al., 2006; Sihvola et al., 2008). These findings suggest that the high co-occurrence of depression and AUDs is the result of interactive effects, rather than direct genetic contribution.
Previous work examining the interactive effects of negative life events and stress with the serotonin transporter polymorphism has proved inconclusive. The S allele has been associated with more frequent and heavier drinking in college students who have experienced negative life events (Covault et al., 2007). However, the relationship between psychosocial adversity and hazardous drinking in 19-year-old men was such that L allele homozygotes reported more hazardous drinking after adversity than those with either the S allele or psychosocial adversity alone (Laucht et al., 2009).
Gender may play a role in explaining the contradictory findings, and has recently been found to affect 5HT mRNA transcription from the SLC6A4 (Philibert et al., 2008). In this study, both the L allele and male sex were associated with increased 5HT mRNA transcription, which in turn was associated with AUDs in the same sample. Gender differences in the incidence of AUDs and depression and also in transcriptional activity at the SLC6A4 may account for some of the mixed results regarding which allelic variation poses a risk for AUDs. The SS genotype has been associated with heightened risk for depression, a disorder that is more prevalent in women (Caspi, 2003; Hasin et al., 2007), while AUDs are three times more common in men than women. Additional work is needed to fully elucidate the relationship between the 5-HTTLPR polymorphism, affective symptoms, and alcohol use; including the relative contribution of age and gender.
To that end, the present study examined the independent and interactive effects of the serotonin transporter polymorphism and depressive symptoms as predictors of alcohol use and alcohol-related problems in a sample of heavy-drinking young adults. It is hypothesized that the LL genotype of the 5-HTTLPR polymorphism will be associated with higher levels of drinking and alcohol problems, based on previous findings that the L allele results in increased 5HT mRNA transcription and AUDs in a mixed sample of depression and alcohol use disordered patients (Philibert et al., 2008). Consistent with recent findings among youth, this relationship is predicted to be stronger at higher levels of depressive symptoms (Laucht et al., 2009).
Materials and Methods
Participants and procedures
Participants (n = 72) were heavy drinkers who met the following inclusion criteria: (1) age between 18 and 65; and (2) score of 8 or higher in the alcohol use disorders identification test (AUDIT; Allen et al., 1998), indicating a hazardous drinking pattern. Exclusion criteria were: (1) currently receiving treatment for alcohol problems, a history of treatment in the 30-days before enrollment, or currently seeking treatment; (2) a lifetime diagnosis of schizophrenia, bipolar disorder, or psychotic disorder; (3) current and regular (defined as once weekly) use of any psychoactive drug, other than marijuana, as determined by self-report. Sample characteristics are provided in Table 1.
Table 1.
Demographic characteristics by 5-HTTLPR genotype [M ± (SD)].
| SS (n = 22) | SL (n = 22) | LL (n = 28) | SS/SL1 (n = 44) vs. LL (n = 28) | |
|---|---|---|---|---|
| Age | 20.36 ± (1.95) | 21.45 ± (4.13) | 21.48 ± (3.49) | F(1, 70) = 1.284, p = 0.261 |
| Gender (male n) | 13 | 14 | 20 | χ2(2, 72) = 0.865, p = 0.649 |
| Ethnicity (n) | χ2(2, 68) = 35.75, p < 0.001* | |||
| African American | 0 | 1 | 0 | |
| Caucasian | 11 | 16 | 24 | |
| Asian | 9 | 3 | 2 | |
| Latino | 2 | 2 | 2 |
1Genotype was coded as: SS/SL = 1, LL = 0.
*Significant results are starred.
Participants were recruited through flyers and online advertisements for a laboratory study of alcohol craving (Ray, 2010). Data from this report were culled from the baseline assessment, before any experimental manipulation of alcohol craving took place. Interested individuals called the laboratory to complete telephone screening measures for study eligibility, including the AUDIT. A total of 161 interested individuals were screened over the telephone for eligibility and 72 participants were enrolled in the study. Eligible participants came to the laboratory and completed in-person assessments of depressive symptoms, alcohol use, and alcohol-related problems. All participants provided a saliva sample for DNA analyses. A breath alcohol concentration (BAC) of 0.000 was required upon each laboratory visit. This study was approved by the Human Research Committee at the University of California, Los Angeles. All participants provided written informed consent after receiving a full explanation of the study.
Assessments
The Beck depression inventory revised
The Beck is as 23-item measure of depressive symptoms occurring in the past 2 weeks. The Beck Depression Inventory is a widely used measure in psychological research and practice to identify clinical levels of depression symptoms. The Beck depression inventory revised (BDI-II) has high internal consistency (alpha-coefficients for clinical samples ≥ 0.88), and content validity compared with DSM-IV criteria for depression and observer ratings. In addition, the BDI-II is sensitive to symptom changes over time (Richter et al., 1998).
The Rutgers alcohol problem index
The Rutgers alcohol problem index (RAPI) is a 23-item measure used to assess alcohol-related problems in the past year. Possible total scores range from 0 to 69. RAPI items assess the impact of alcohol on social and health functioning (White and Labouvie, 1989), and has been found to be valid for use with college-aged participants (Martens et al., 2007).
30-day timeline follow back interview
The timeline follow back (TLFB) interview was used to assess drinking behavior in the past 30 days. The TLFB is a calendar-assisted interview that was used to obtain data on drinking quantity and frequency. The TLFB accurately estimates alcohol consumption for as long as a 12-month period (Vakili et al., 2008). For the purpose of this study, four measures of drinking were derived from the TLFB interview: (a) total number of drinks consumed in the past 30 days;(b) number of drinking days in the past 30 days; (c) number of binge-drinking days in the past 30 days. A binge-drinking episode is defined as consuming four or more drinks for a woman and five or more drinks for a man (Courtney and Polich, 2009); and (d) the average number of drinks per drinking day in the past 30 days.
Genotyping
Saliva samples were collected under researcher observation for DNA analyses using Oragene saliva collection kits. Genotyping was performed at the UCLA Genotyping and Sequencing (GenoSeq) Core. Polymerase chain reaction (PCR) primers were labeled with fluorescent dye (6-FAM, VIC, or NED), and PCR was performed on Applied Biosystems dual block PCR thermal cyclers. Microsatellite genotypes were run on an AB 3730 capillary DNA sequencer and analyzed using the AB GeneMapper software version 4.0. The 5-HTTLPR polymorphism at the SLC6A4 locus was assayed on an AB 7900HT Fast Real-Time PCR System and analyzed using the Sequence Detection Systems (SDS) software version 2.3. Each run included two positive control samples (individual 2 in CEPH family 1347; Coriell Institute). Genotypes were automatically scored by the allele calling software, and each genotype was verified by visual inspection. In process validation checks, the UCLA GenoSeq Core has average call, reproducibility, and concordance rates of 96, 99.7, and 99.8%, respectively. Quality values were computed for each genotype call in this sample, using a standard algorithm that combines various quality metrics. Genotype calls with a quality score of less than 95% were set to fail. The primer sequences resulted in alleles that were 484 bp (S allele) or 528 bp (L allele). Consistent with the existing literature, analyses compared L allele homozygotes to S allele carriers (LL vs. SL/SS).
Data analysis
Prior to data analysis, study variables were screened for missing data, outliers, and distribution abnormalities. Analyses of variance (ANOVAs) were conducted using the general linear model (Proc GLM) in SAS statistical package. The primary dependent measures in this study were: alcohol-related problems (RAPI score), total number of drinks in the past 30 days, frequency of drinking in the past 30 days, number of days of binge drinking in the past 30 days, and the average number of drinks per drinking day in the past 30 days (all derived from the TLFB interview).
Model parameters consisted of the main effects of depressive symptoms (BDI-II score), 5-HTTLPR genotype, and depressive symptoms × genotype interaction as predictors of alcohol use and alcohol problems in this sample. In order to maximize statistical power, depressive symptoms were treated as a continuous variable in all analyses. Depressive symptom scores were dichotomized into “high” and “low,” using a median split at a score of 8. This dichotomy was used solely for graphically representing the results. In order to quantify effect sizes, partial η2s were computed as recommended for ANOVA-based models. Partial η2s are defined as the proportion of variance associated with or accounted for by each of the main effects or interactions in the ANOVA model. A dichotomous variable was used for the 5-HTTLPR genotype (LL = 0, SL and SS = 1). Descriptive statistics and exploratory analyses were conducted using a three-level genotype variable (SS = 0, SL = 1, and LL = 2).
Results
Genotype comparisons
Allele frequency did not differ by sex [χ2(2, 68) = 0.68, p > 0.05], but varied significantly by ethnicity. The SS genotype was more common among African American, Asian, and Latino participants compared with Caucasian participants [χ2(2, 68) = 35.75, p < 0.001; see Table 1]. Follow-up analyses were conducted restricting the sample to Caucasian individuals in order to ensure that the findings were not confounded by population stratification. Scores on measures of depressive symptoms, alcohol use, and alcohol problems across genotype are presented in Table 2 and reveal that L allele homozygotes scored significantly higher on alcohol problems, and total reported a higher total number of drinks (p < 0.05) than S allele carriers. Follow-up analyses adding age to the models found no significant main effect of age and there were no significant genotype × age interactions on alcohol use or alcohol problems.
Table 2.
Alcohol use, problems, and depressive symptoms by 5-HTTLPR genotype1 [M ± (SD)].
| SS/SL (n = 44) | LL (n = 28) | SS/LL vs. LL | |
|---|---|---|---|
| RAPI total score (possible range: 0–69) |
17.2 (12.96) | 28.22 (17.73) |
t(70) = −3.04; p = 0.010* |
| BDI-II total score (possible range: 0–63) |
7.25 (7.03) | 8.82 (7.69) |
t(70) = −0.892; p = 0.376 |
| Drinks per drinking day in the past 30 days | 5.75 (2.57) | 7.12 (3.64) |
t(70) = 1.386; p = 0.173 |
| Drinking days in the past 30 days | 9.20 (5.55) | 11.67 (5.64) |
t(70) = 1.90; p = 0.062a |
| Binge-drinking days in the past 30 days | 5.76 (4.54) | 8.26 (5.70) |
t(70) = 1.954; p = 0.055a |
| Total drinks in the past 30 days | 55.86 (43.88) | 90.78 (72.05) |
t(70) = 2.23; p = 0.031* |
1Genotype was coded as: SS/SL = 1, LL = 0.
*Significant results are starred.
aTrend-level finding.
Analyses were repeated with ethnicity as a covariate. Genotype remained a significant predictor of RAPI score (p = 0.017). The addition of ethnicity to the model reduced the significance of genotype as a predictor of total drinks to p = 0.074.
Depressive symptoms as moderators of the relationship between 5-HTTLPR genotype and drinking outcomes
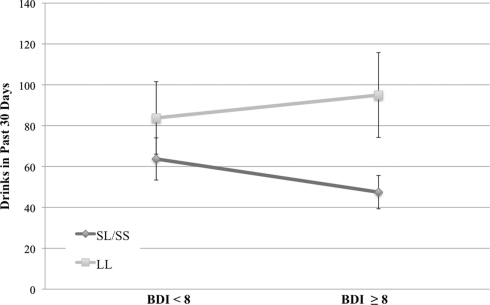
As shown in Table 3, results revealed a main effect of depressive symptoms on alcohol-related problems in the past year (p < 0.001) and on the total number of drinks consumed in a 30-day period (p < 0.01), such that alcohol-related problems and consumption increased as depressive symptoms increased. No main effects were found for genotype in predicting drinking frequency of drinking or total number of drinks in the past 30 days. There was a significant genotype × depressive symptom interaction on total number of drinks, such that at higher levels of depressive symptoms, participants who were homozygous for the L allele reported significantly higher total number of drinks (mean drinks for individuals above the median BDI-II score = 94.99) compared to S allele carriers (mean drinks for individuals above the median BDI-II score = 48.03; see Figure 1). The interaction between BDI-II and 5-HTTLPR genotype in predicting alcohol problems (RAPI scores) was only marginally significant (p = 0.071). Mean RAPI score for L allele homozygotes above the median BDI-II score was 31.57, and 22.94 for S allele carriers. 5-HTTLPR genotype, BDI-II score, and their interaction were not significant predictors of binge-drinking frequency, the number of drinking days in the past 30 days or the average number of drinks per drinking day; see Table 3.
Table 3.
Main analyses of the effect of depressive symptoms, 5-HTTLPR genotype1, and their interaction on alcohol use and alcohol problems.
| Dependent variable | Model | β | SE | t | p | η2 |
|---|---|---|---|---|---|---|
| Total drinks in past 30 days | 5-HTTLPR genotype1 | 6.988 | 19.506 | 0.36 | 0.721 | 0.002 |
| BDI-II | 3.805 | 1.344 | 2.830 | 0.006* | 0.105 | |
| Genotype*BDI-II | −4.751 | 1.779 | −2.670 | 0.009* | 0.095 | |
| Drinking days in past 30 days | 5-HTTLPR genotype | −0.124 | 2.572 | −0.050 | 0.962 | 0.000 |
| BDI-II | 0.261 | 0.177 | 1.470 | 0.146 | 0.031 | |
| Genotype*BDI-II | −0.377 | 0.235 | −1.610 | 0.112 | 0.037 | |
| Drinks per drinking day in past 30 days | 5-HTTLPR genotype | 0.147 | 1.086 | 0.135 | 0.893 | 0.002 |
| BDI-II | 0.088 | 0.074 | 1.182 | 0.241 | 0.054 | |
| Genotype*BDI-II | −0.147 | 0.098 | −1.505 | 0.137 | 0.028 | |
| Binge-drinking days in past 30 days | 5-HTTLPR genotype | −0.414 | 1.822 | −0.227 | 0.821 | 0.001 |
| BDI-II | 0.155 | 0.126 | 1.231 | 0.223 | 0.083 | |
| Genotype*BDI-II | −0.236 | 0.166 | −1.422 | 0.160 | 0.051 | |
| Rutgers alcohol problem index score (possible range: 0–69) | 5-HTTLPR genotype | −1.641 | 5.002 | −0.330 | 0.744 | 0.002 |
| BDI-II | 1.290 | 0.345 | 3.740 | <0.001* | 0.171 | |
| Genotype*BDI-II | −0.837 | 0.456 | −1.840 | 0.071a | 0.047 |
1Genotype was coded as: SS/SL = 1, LL = 0.
*Significant results are starred.
aTrend-level finding.
Figure 1.
Total drinks in the past 30 days (M ± SE) across serotonin transporter genotype (LL vs. SS/SL) and depressive groups (scores on the BDI, split at the median of 8). Analyses revealed a significant genotype × BDI interaction such that L allele homozygotes reporting high levels of depressive symptoms reported significantly more drinks in the past 30 days as compared to S allele carriers [F(3, 68) = 7.13, p = 0.009, η2 = 0.095].
Follow-up analyses of genetic effects
Analyses of three levels of genotype
The effects of the 5-HTTLPR genotype were examined as a three-level variable: SS, SL, and LL. ANOVAs, using Proc GLM, revealed that the interaction of 5-HTTLPR genotype and depressive symptoms produced a marginally significant increase in total drinks consumed for each additional L allele. In short, analyses of the 5-HTTLPR as a three-level variable supported the findings achieved with the SS/SL and LL categories and suggest that L allele homozygotes are most distinct from S allele carriers. Consistent with other reports, SS and SL individuals were combined into a single group in order to enhance statistical power.
Analyses of gender and ethnicity effects
In order to account for the possibility that population stratification confounds the results reported above, we repeated the analyses described above using only self-identified Caucasian individuals (n = 51) and by controlling for ethnicity and gender in the full sample. Controlling for ethnicity and gender did not change the results of the main analyses, and revealed a main effect of ethnicity on the total number of drinks consumed in a 30-day period (p = 0.002), such that Caucasians had more drinks than any other group (M = 83.96, SD = 62.13) followed by Asians (M = 37.61, SD = 17.90) and Latinos (M = 16.83, SD = 16.50). Only one participant was African American. See Table 4 for full results. Repeating the analyses only in Caucasians significantly decreased statistical power to detect genetic effects. We therefore compared the results of the full sample with the Caucasian sample using partial η2s. Effect size estimates are ideal for comparisons with low power, as they are not biased by sample size. Results of analyses among Caucasian individuals revealed that the effect size estimates for the BDI-II × 5-HTTLPR were reduced slightly. The BDI-II × 5-HTTLPR genotype interaction on total number of drinks had an estimated partial η2 = 0.095 in the full sample (p = 0.009), as compared to partial η2 = 0.073 (p = 0.060) in the Caucasian-only sample. These results suggest that the observed genetic effects are not best explained by ethnicity and provide further support for the findings reported above.
Table 4.
Comparison of ANOVA models controlling for ethnicity and gender with main analyses.
| Dependent variable | Model 1 | β | SE | t | p | η2 |
|---|---|---|---|---|---|---|
| No of drinks (total) in past 30 days | 5-HTTLPR genotype1 | 6.988 | 19.506 | 0.360 | 0.721 | 0.002 |
| BDI-II | 3.805 | 1.344 | 2.830 | 0.006* | 0.105 | |
| Genotype*BDI-II | −4.751 | 1.779 | −2.670 | 0.009* | 0.095 | |
| Model 2 | ||||||
| Ethnicity | −18.430 | 5.827 | −3.163 | 0.002* | 0.058 | |
| 5-HTTLPR genotype | 10.179 | 18.358 | 0.554 | 0.581 | 0.002 | |
| BDI-II | 3.375 | 1.270 | 2.656 | 0.010* | 0.260 | |
| Genotype*BDI-II | −4.055 | 1.686 | −2.405 | 0.019* | 0.085 | |
| Rutgers alcohol problem index score (possible range: 0–69) | Model 1 | |||||
| 5-HTTLPR genotype | −1.641 | 5.002 | −0.330 | 0.744 | 0.002 | |
| BDI-II | 1.290 | 0.345 | 3.740 | <0.001* | 0.171 | |
| Genotype*BDI-II | −0.837 | 0.456 | −1.840 | 0.071a | 0.047 | |
| Model 2 | ||||||
| Ethnicity | −0.599 | 1.600 | −0.374 | 0.709 | 0.003 | |
| 5-HTTLPR genotype | −1.538 | 5.042 | −0.305 | 0.761 | 0.012 | |
| BDI-II | 1.276 | 0.349 | 3.658 | 0.001* | 0.332 | |
| Genotype*BDI-II | −0.815 | 0.436 | −1.760 | 0.083a | 0.055 | |
1Genotype was coded as: SS/SL = 1, LL = 0.
*Significant results are starred.
aTrend-level result.
Discussion
The current study extends the literature on the serotonin transporter polymorphism and its role in AUDs. Results indicated a main effect of 5-HTTLPR genotype such that L allele homozygotes had significantly more alcohol problems measured by the RAPI, and more drinks consumed over a 30-day period than S allele carriers. A main effect of depressive symptoms was found, wherein individuals reporting higher levels of depressive symptoms had more alcohol use and alcohol-related problems than those with fewer depressive symptoms. A significant 5-HTTLPR × BDI-II interaction was found; higher scores on the BDI-II were associated with more drinks consumed and more drinking days in the past 30 days among L allele homozygotes. Additionally, the interaction of 5-HTTLPR genotype and BDI-II scores approached significance in predicting more alcohol-related problems in the past year. Follow-up analyses using all three levels of genotype (SS, SL, and LL) revealed that each additional L allele was associated with an increase in drinking problems for participants reporting higher levels of depressive symptoms. These results indicate that the combination of LL genotype and depressive symptomatology is associated with alcohol use and alcohol-related problems. These findings support previous work examining serotonin transporter polymorphism in a mixed clinical sample of depression and AUDs, as well as previous studies examining gene × environment interactions with the 5-HTTLPR (Olsson et al., 2005; Philibert et al., 2008; Laucht et al., 2009). These results stand in contrast with work on the direct genetic contribution of the serotonin transporter polymorphism to AUDs, as we found no main effect of genotype in the present study (Feinn et al., 2005; McHugh et al., 2010).
A recent meta-analysis of serotonin transporter polymorphism and AUDs found a main effect of S allele across studies in predicting AUDs, but suggested that comorbid alcohol and depression results in a more complicated picture regarding the effects of the 5-HTTLPR polymorphism (McHugh et al., 2010). The current study offers support for the L allele as a risk for alcohol use problems when depressive symptoms are present. Given that this is a small sample and that few studies have examined the interaction of 5-HTTLPR and mood symptomatology as a predictor of alcohol problems, these results are preliminary.
Previous work examining interactions between stress and negative life events with the serotonin transporter polymorphism has been mixed, but evidence for the L allele as risk factor has come from both psychosocial (e.g., Olsson et al., 2005; Laucht et al., 2009) and biological studies (e.g., Philbert) of the serotonin transporter polymorphism. Olsson et al. (2005) reported a G × E interaction between 5-HTTLPR genotype and risky attachment style in predicting both anxiety symptoms and binge drinking. In risky settings, each additional copy of the S allele lowered the odds of anxiety (about 30% per allele), and binge drinking (about 35% per allele; Olsson et al., 2005). Laucht et al. (2009) found that high early life adversity and current stressful events interacted with the 5-HTTLPR genotype to predict the number of binge-drinking days in an adolescent sample. Independent risks for developing an AUD have also been associated with the L allele, including mechanisms of alcohol craving and low level of response to alcohol (Hinckers et al., 2006; Bleich et al., 2007). The L allele has also been associated with higher rates of AUD and drinking, and a lower age of onset of AUDs (Hu et al., 2005; Kweon et al., 2005).
The current findings stand in contrast to studies finding a direct relationship between the S allele of the serotonin transporter polymorphism and AUDs. Covault et al. (2007) found that S allele homozygotes with multiple negative life events in the prior year reported more frequent drinking and heavy drinking, stronger intentions to drink, and greater non-prescribed drug use. Among homozygotes for the L allele, drinking and drug use were unaffected by past-year negative life events. More recently, Brody et al. (2009) found that presence of one or two copies of the S allele was associated with increases in substance use over time. However, this association was greatly attenuated by supportive parenting. Johnson (2000) hypothesized that LL genotype is more common among early onset AUDs and that the LL genotype results in reduced levels of intra-synaptic 5-HT. The present results align with and extend previous work implicating the L allele as a risk for alcohol-related problems in young adults with depression symptomatology (Olsson et al., 2005; Laucht et al., 2009).
While studies examining the relationship between the L allele and alcohol use problems have been conducted in primarily male samples (Laucht et al., 2009), the present sample is fairly well balanced on gender across genotype categories. Furthermore, the relationship between the LL genotype and problematic alcohol use in the presence of mood symptoms is corroborated by D:Olsson et al. (2005) large, gender-balanced study of adolescent drinking and the 5-HTTLPR. This study found that within risky environments, each additional S allele lowered the risk of anxiety and drinking problems. Together, these results help elucidate the relative contribution of gender and age factors on the effects of this polymorphism regarding alcohol outcomes.
Strengths of the current study include the careful phenotyping and a sample of young hazardous drinkers. Limitations include the cross-sectional design and the relatively small sample (n = 72). While the sample of young heavy drinkers (AUDIT ≥ 8) can be seen as an asset of the study, it also limits the generalizability of the findings to heavy-drinking young adults. Additional study limitations include the temporal coverage of the assessments, as the BDI-II covered a 2-week period while the TLFB comprised a 30-day period. Moreover, the relatively low levels of depressive symptoms in this sample may also limit its generalizability to clinical levels of depression and suggest that further studies in clinical samples are warranted.
On balance, the present findings provide evidence for a genetic contribution of the serotonin transporter polymorphism to alcohol use and alcohol problems among individuals reporting higher levels of depressive symptoms. These results suggest that the LL genotype may confer risk for alcohol problems in the context of co-occurring psychiatric symptoms, which is consistent with, and extends, several recent studies reporting a significant association between the LL genotype and alcohol problems in the presence of environmental stressors (Laucht et al., 2009) and comorbid internalizing psychopathology (Olsson et al., 2005). Refining the environmental and psychological moderators of the association between genes and clinical outcomes is critical to reconciling mixed findings in psychiatric genetics, including studies of alcoholism vulnerability.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would also like to thank Pauline Chin, Eliza Hart, Andia Heydari, James Ashenhurst, and Christina Pedley for their contribution to data collection and data management for this project. This study was supported by seed funds from the Department of Psychology at the University of California Los Angeles to Dr. Lara Ray.
References
- Allen J., Litten R. Z., Lee A. (1998). What you need to know: detecting alcohol problems in general medical practice. Singapore Med. J. 39, 38–41 [PubMed] [Google Scholar]
- Armeli S., Conner T. S., Covault J., Tennen H., Kranzler H. R. (2008). A serotonin transporter gene polymorphism (5-HTTLPR), drinking-to-cope motivation, and negative life events among college students. J. Stud. Alcohol Drugs 69, 814–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr C. S., Newman T. K., Lindell S., Shannon C., Champoux M., Lesch K. P., Suomi S. J., Goldman D., Higley J. D. (2004). Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch. Gen. Psychiatry 61, 1146–1152 10.1001/archpsyc.61.11.1146 [DOI] [PubMed] [Google Scholar]
- Bleich S., Bonsch D., Rauh J., Bayerlein K., Fiszer R., Frieling H., Hillemacher T. (2007). Association of the long allele of the 5-HTTLPR polymorphism with compulsive craving in alcohol dependence. Alcohol Alcohol. 42, 509–512 [DOI] [PubMed] [Google Scholar]
- Brody G. H., Beach S. R., Philibert R. A., Chen Y. F., Lei M. K., Murry V. M., Brown A. C. (2009). Parenting moderates a genetic vulnerability factor in longitudinal increases in youths’ substance use. J. Consult. Clin. Psychol. 77, 1–11 10.1037/a0012996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A. F., Schmid B., Blomeyer D., Becker K., Treutlein J., Zimmermann U. S., Jennen-Steinmetz C., Schmidt M. H., Esser G., Banaschewski T., Rietschel M., Schumann G., Laucht M. (2009). Impact of age at first drink on vulnerability to alcohol-related problems: testing the marker hypothesis in a prospective study of young adults. J. Psychiatr. Res. 43, 1205–1212 10.1016/j.jpsychires.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Caspi A. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT Gene. Science 301 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- Caspi A., Hariri A. R., Holmes A., Uher R., Moffitt T. E. (2010). Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 167, 509–527 10.1176/appi.ajp.2010.09101452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Polich J. (2009). Binge drinking in young adults: data, definitions, and determinants. Psychol. Bull. 135, 142–156 10.1037/a0014414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Tennen H., Armeli S., Conner T. S., Herman A. I., Cillessen A. H., Kranzler H. R. (2007). Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol. Psychiatry 61, 609–616 10.1016/j.biopsych.2006.05.018 [DOI] [PubMed] [Google Scholar]
- Feinn R., Nellissery M., Kranzler H. R. (2005). Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 133B, 79–84 10.1002/ajmg.b.30132 [DOI] [PubMed] [Google Scholar]
- Grant B. F., Dawson D. A., Stinson F. S., Chou S. P., Dufour M. C., Pickering R. P. (2004). The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 74, 223–234 10.1016/j.drugalcdep.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Harwood H. (2000). Updating Estimates of the Economic costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism [Google Scholar]
- Hasin D. S., Stinson F. S., Ogburn E., Grant B. F. (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry 64, 830–842 10.1001/archpsyc.64.7.830 [DOI] [PubMed] [Google Scholar]
- Heils A., Mossner R., Lesch K. P. (1997). The human serotonin transporter gene polymorphism – basic research and clinical implications. J. Neural Transm. 104, 1005–1014 10.1007/BF01273314 [DOI] [PubMed] [Google Scholar]
- Heinz A., Goldman D., Gallinat J., Schumann G., Puls I. (2004). Pharmacogenetic insights to monoaminergic dysfunction in alcohol dependence. Psychopharmacology (Berl.) 174, 561–570 10.1007/s00213-004-1903-x [DOI] [PubMed] [Google Scholar]
- Heinz A., Jones D. W., Mazzanti C., Goldman D., Ragan P., Hommer D., Linnoila M., Weinberger D. R. (2000). A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol. Psychiatry 47, 643–649 10.1016/S0006-3223(99)00171-7 [DOI] [PubMed] [Google Scholar]
- Hinckers A. S., Laucht M., Schmidt M. H., Mann K. F., Schumann G., Schuckit M. A., Heinz A. (2006). Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol. Psychiatry 60, 282–287 10.1016/j.biopsych.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Hingson R. W., Zha W. (2009). Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics 123, 1477–1484 10.1542/peds.2008-2176 [DOI] [PubMed] [Google Scholar]
- Hu X., Oroszi G., Chun J., Smith T. L., Goldman D., Schuckit M. A. (2005). An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol. Clin. Exp. Res. 29, 8–16 10.1097/01.ALC.0000150008.68473.62 [DOI] [PubMed] [Google Scholar]
- Johnson B. A. (2000). Serotonergic agents and alcoholism treatment: rebirth of the subtype concept – an hypothesis. Alcohol. Clin. Exp. Res. 24, 1597–1601 10.1111/j.1530-0277.2000.tb02048.x [DOI] [PubMed] [Google Scholar]
- Johnson B. A. (2004). Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs 18, 1105–1118 10.2165/00023210-200418150-00005 [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Neale M. C., Heath A. C., Kessler R. C., Eaves L. J. (1994). A twin-family study of alcoholism in women. Am. J. Psychiatry 151, 707–715 [DOI] [PubMed] [Google Scholar]
- Kuo P. H., Gardner C. O., Kendler K. S., Prescott C. A. (2006). The temporal relationship of the onsets of alcohol dependence and major depression: using a genetically informative study design. Psychol. Med. 36, 1153–1162 10.1017/S0033291706007860 [DOI] [PubMed] [Google Scholar]
- Kweon Y. S., Lee H. K., Lee C. T., Lee K. U., Pae C. U. (2005). Association of the serotonin transporter gene polymorphism with Korean male alcoholics. J. Psychiatr. Res. 39, 371–376 10.1016/j.jpsychires.2004.10.005 [DOI] [PubMed] [Google Scholar]
- Laucht M., Treutlein J., Schmid B., Blomeyer D., Becker K., Buchmann A. F., Schmidt M. H., Esser G., Jennen-Steinmetz C., Rietschel M., Zimmermann U. S., Banaschewski T. (2009). Impact of psychosocial adversity on alcohol intake in young adults: moderation by the LL genotype of the serotonin transporter polymorphism. Biol. Psychiatry 66, 102–109 10.1016/j.biopsych.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Martens M. P., Neighbors C., Dams-O’Connor K., Lee C. M., Larimer M. E. (2007). The factor structure of a dichotomously scored Rutgers Alcohol Problem Index. J. Stud. Alcohol Drugs 68, 597–606 [DOI] [PubMed] [Google Scholar]
- McHugh R. K., Hofmann S. G., Asnaani A., Sawyer A. T., Otto M. W. (2010). The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 108, 1–6 10.1016/j.drugalcdep.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenakk L., Maestu J., Nordquist N., Parik J., Oreland L., Loit H. M., Harro J. (2011). Effects of the serotonin transporter (5-HTTLPR) and alpha2A-adrenoceptor (C-1291G) genotypes on substance use in children and adolescents: a longitudinal study. Psychopharmacology (Berl.) 215, 13–22 10.1007/s00213-010-2109-z [DOI] [PubMed] [Google Scholar]
- Olsson C. A., Byrnes G. B., Lotfi-Miri M., Collins V., Williamson R., Patton C., Anney R. J. (2005). Association between 5-HTTLPR genotypes and persisting patterns of anxiety and alcohol use: results from a 10-year longitudinal study of adolescent mental health. Mol. Psychiatry 10, 868–876 10.1038/sj.mp.4001677 [DOI] [PubMed] [Google Scholar]
- Philibert R. A., Sandhu H., Hollenbeck N., Gunter T., Adams W., Madan A. (2008). The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 543–549 10.1002/ajmg.b.30657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. A., Aggen S. H., Kendler K. S. (1999). Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol. Clin. Exp. Res. 23, 1136–1144 10.1111/j.1530-0277.1999.tb04029.x [DOI] [PubMed] [Google Scholar]
- Ray L. A. (2010). Stress-induced, and cue-induced craving for alcohol in heavy drinkers: preliminary evidence of genetic moderation by the OPRM1 and CRH-BP genes. Alcohol. Clin. Exp. Res. 35, 166–174 10.1111/j.1530-0277.2010.01333.x [DOI] [PubMed] [Google Scholar]
- Richter P., Werner J., Heerlein A., Kraus A., Sauer H. (1998). On the validity of the Beck depression inventory. A review. Psychopathology 31, 160–168 10.1159/000066239 [DOI] [PubMed] [Google Scholar]
- Rowe C. L., Liddle H. A., Greenbaum P. E., Henderson C. E. (2004). Impact of psychiatric comorbidity on treatment of adolescent drug abusers. J. Subst. Abuse Treat. 26, 129–140 10.1016/S0740-5472(03)00166-1 [DOI] [PubMed] [Google Scholar]
- Schuckit M. A. (2009). An overview of genetic influences in alcoholism. J. Subst. Abuse Treat. 36, S5–S14 [PubMed] [Google Scholar]
- Schuckit M. A., Smith T. L. (2006). An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. J. Stud. Alcohol 67, 215–227 [DOI] [PubMed] [Google Scholar]
- Sihvola E., Rose R. J., Dick D. M., Pulkkinen L., Marttunen M., Kaprio J. (2008). Early-onset depressive disorders predict the use of addictive substances in adolescence: a prospective study of adolescent Finnish twins. Addiction 103, 2045–2053 10.1111/j.1360-0443.2008.02363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J. D., Merikangas K. R. (2000). The comorbidity of depression and substance use disorders. Clin. Psychol. Rev. 20, 173–189 10.1016/S0272-7358(99)00026-4 [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Brown S. A., Myers M. G., Granholm E. (1999). The role of neurocognitive abilities in coping with adolescent relapse to alcohol and drug use. J. Stud. Alcohol 60, 500–508 [DOI] [PubMed] [Google Scholar]
- Vakili S., Sobell L. C., Sobell M. B., Simco E. R., Agrawal S. (2008). Using the Timeline Followback to determine time windows representative of annual alcohol consumption with problem drinkers. Addict. Behav. 33, 1123–1130 10.1016/j.addbeh.2008.03.009 [DOI] [PubMed] [Google Scholar]
- White H. R., Labouvie E. W. (1989). Towards the assessment of adolescent problem drinking. J. Stud. Alcohol 50, 30–37 [DOI] [PubMed] [Google Scholar]