Abstract
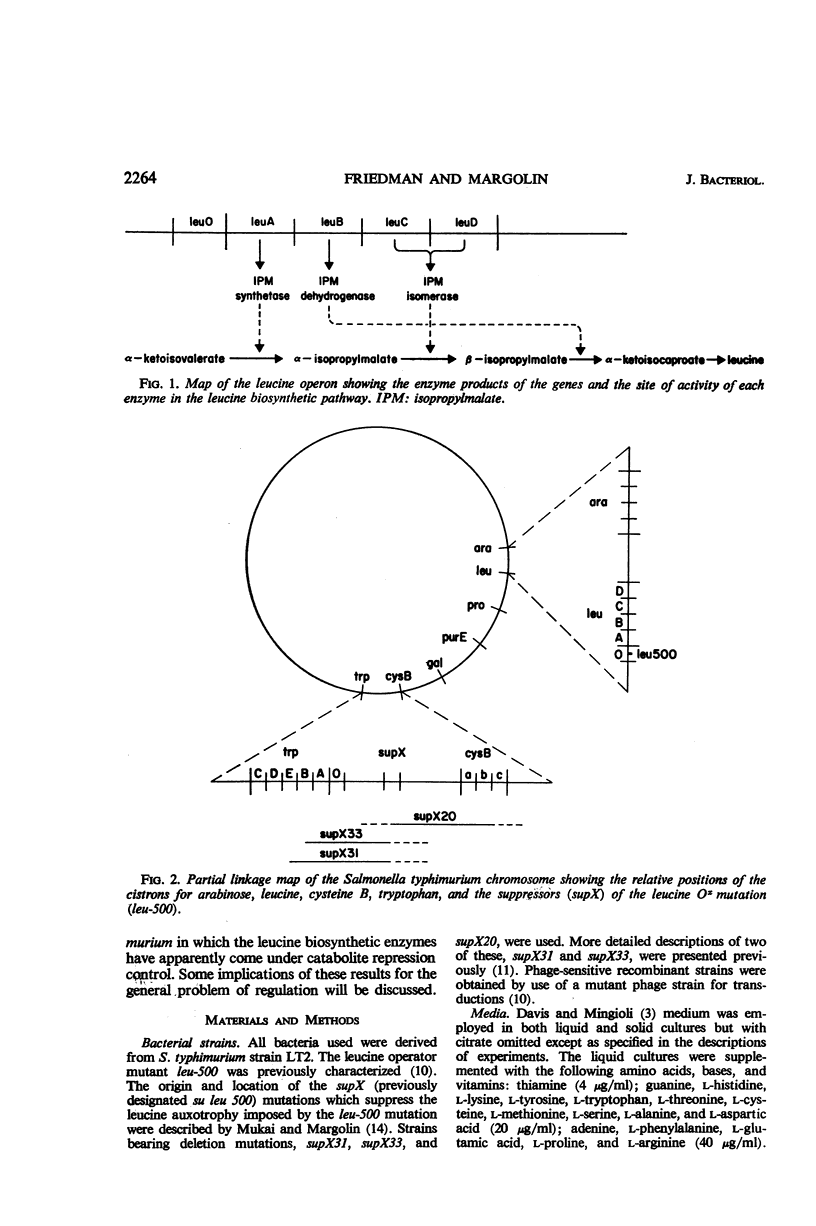
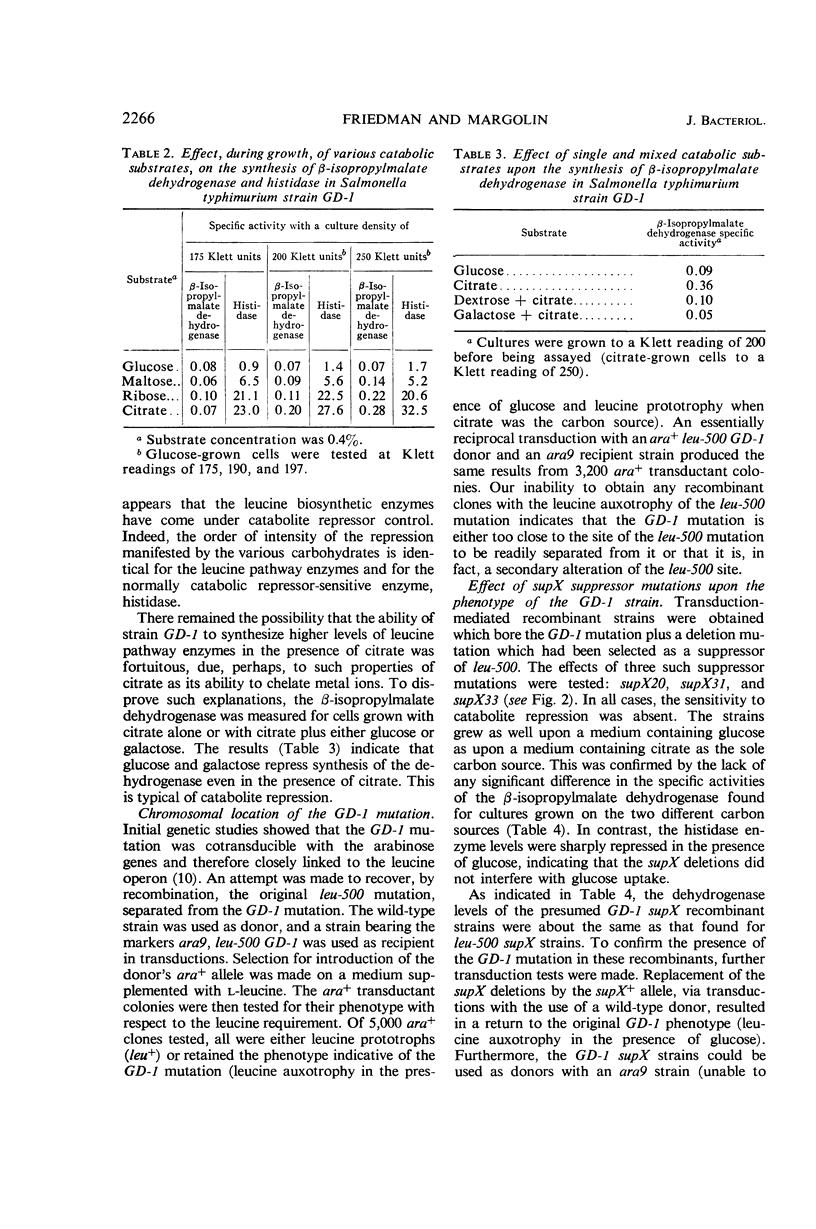
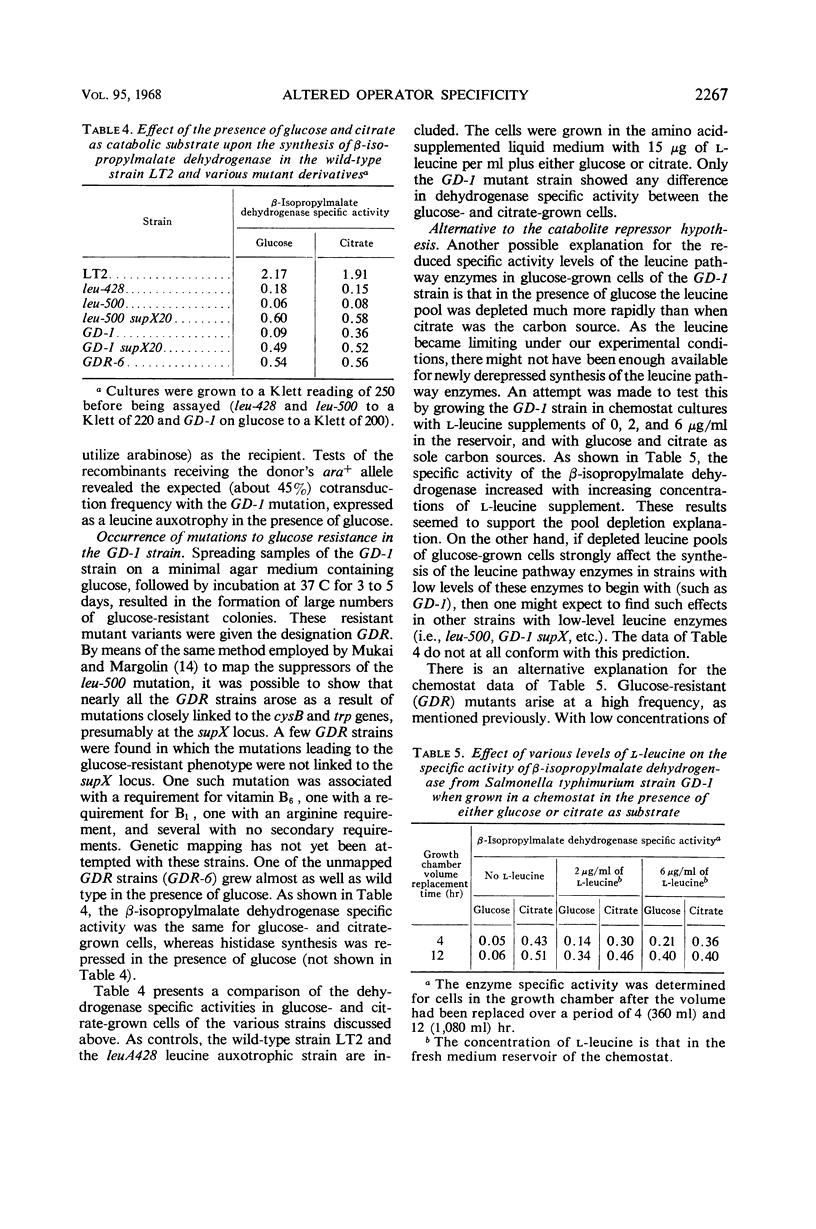
A mutation, GD-1, in the leucine operon imposed unusual growth characteristics upon a leucine auxotrophic strain bearing the leucine operator mutation, leu-500. The strain with the GD-1 mutation was able to grow on a minimal salts medium when citrate was the sole carbon source, but required leucine when glucose was present. Tests with a large number of carbohydrates suggest that in the strain bearing the GD-1 mutation the leucine biosynthetic enzymes are under catabolite repressor control. Recombination studies indicate that the GD-1 mutation is a secondary alteration of the leucine operator at or very close to the site of the leu-500 mutation. Mutations at the supX locus (previously termed su leu 500 and located on the chromosome between the cysteine B and tryptophan gene clusters) result in elimination of the catabolite repression effect. The data are interpreted as an indication that the GD-1 and leu-500 mutations alter the leucine operator with respect to its specificity of response to repressors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., UMBARGER H. E., GROSS S. R. THE BIOSYNTHESIS OF LEUCINE. III. THE CONVERSION OF ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE TO ALPHA-KETOISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1053–1058. doi: 10.1021/bi00905a024. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W. Induction by arginine of enzymes of arginine biosynthesis in Escherichia coli B. Proc Natl Acad Sci U S A. 1961 Jul 15;47:961–971. doi: 10.1073/pnas.47.7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. Inhibitory effect of glucose on enzyme formation. Nature. 1956 Oct 13;178(4537):801–802. doi: 10.1038/178801b0. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. The effect of glucose on the induced biosynthesis of bacterial enzymes in the presence and absence of inducing agents. Biochim Biophys Acta. 1956 Aug;21(2):324–334. doi: 10.1016/0006-3002(56)90016-6. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin P., Bauerle R. H. Determinants for regulation and initiation of expression of tryptophan genes. Cold Spring Harb Symp Quant Biol. 1966;31:311–320. doi: 10.1101/sqb.1966.031.01.041. [DOI] [PubMed] [Google Scholar]
- Margolin P., Mukai F. H. A model for mRNA transcription suggested by some characteristics of 2-aminopurine mutagenesis in Salmonella. Proc Natl Acad Sci U S A. 1966 Feb;55(2):282–289. doi: 10.1073/pnas.55.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F. H., Margolin P. ANALYSIS OF UNLINKED SUPPRESSORS OF AN O degrees MUTATION IN SALMONELLA. Proc Natl Acad Sci U S A. 1963 Jul;50(1):140–148. doi: 10.1073/pnas.50.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMALEY R. F., BERNLOHR R. W. APPARENT INDUCTION OF ORNITHINE TRANSCARBAMYLASE AND ARGINASE BY ARGININE IN BACILLUS LICHENIFORMIS. J Mol Biol. 1965 Apr;11:842–844. doi: 10.1016/s0022-2836(65)80041-9. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D., LEDERBERG J. Genetic exchange in Salmonella. J Bacteriol. 1952 Nov;64(5):679–699. doi: 10.1128/jb.64.5.679-699.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]



