Abstract
Objective
High smoking rates in adults with attention deficit hyperactivity disorder (ADHD) and nicotine’s amelioration of ADHD suggest that effective ADHD treatment might facilitate abstinence in smokers with ADHD. The present study evaluated if using osmotic release methylphenidate (OROS-MPH) to treat ADHD enhances response to smoking cessation treatment in smokers with ADHD.
Method
A randomized, double-blind, placebo-controlled, 11-week trial with a one month follow-up conducted at six clinical sites between December 2005 and January 2008. Adults (18–55), meeting DSM-IV criteria for ADHD and interested in quitting smoking were randomized to OROS-MPH titrated to 72 mg/day (n=127) or placebo (n=128). All participants received brief weekly individual smoking cessation counseling for 11 weeks and 21 mg/day nicotine patch starting on the smoking quit day (day 27) through study week 11. Outcome measures included prolonged smoking abstinence and DSM-IV ADHD Rating Scale (ADHD-RS) score.
Results
Of 255 randomized, 204 (80%) completed the trial. Prolonged abstinence rates, 43.3% and 42.2%, for the OROS-MPH and placebo groups, respectively, did not differ significantly (odds ratio, 1.1; 95% confidence interval, 0.63 – 1.79; p=0.81). OROS-MPH, relative to placebo, evidenced a greater reduction in DSM-IV ADHD-RS score (p<0.0001) and in cigarettes per day during the post-quit phase (p=.016). OROS-MPH, relative to placebo, increased blood pressure and heart rate to a statistically, but not clinically, significant degree; medication discontinuation did not differ significantly between treatments.
Conclusions
ADHD treatment did not improve smoking cessation success; OROS-MPH, relative to placebo, effectively treated ADHD and was safe and generally well tolerated in this healthy sample of adult ADHD smokers.
Keywords: Smoking cessation, ADHD, OROS-MPH, Clinical Trial, Double-blind
INTRODUCTION
Each year, cigarette smoking accounts for an estimated 438,000 deaths and $92 billion in productivity losses in the United States.1 Recent efforts to combat cigarette smoking have included not only the direct treatment of nicotine dependence but also co-occurring conditions that may make individuals vulnerable to the persistence of cigarette smoking.2 Research suggests that cigarette smoking is significantly more common in children3 and adults with attention deficit hyperactivity disorder (ADHD) compared to the general population and/or controls.4,5 Moreover, ADHD is associated with earlier onset of cigarette smoking,3 more severe nicotine dependence,6 and more difficulty quitting smoking.4 It has been suggested that this greater incidence of smoking might reflect an attempt at self-medication of ADHD symptoms.7
Adult ADHD, which has a prevalence rate of approximately 4.4%8, is characterized by symptoms of inattention, impulsivity, and hyperactivity and is associated with significant impairment in nearly every area of functioning, including poorer performance in educational and occupational settings, higher rates of divorce/separation, and higher rates of other psychiatric and substance use disorders.9 While adult ADHD is one of the most common mental health disorders of adulthood,10 treatment rates remain low.11 Research indicates that nicotine is effective in decreasing ADHD symptoms7, 12, 13 and, in fact, several nicotinergic agents are currently being evaluated as ADHD treatments.14 Some data also suggest that treating ADHD with stimulants protects against the development of later cigarette smoking.15 If smokers with ADHD are, in fact, using nicotine in an attempt to ameliorate their symptoms, then successfully treating their ADHD should facilitate successful smoking cessation.
Psychostimulants are the mainstay pharmacologic treatment for ADHD. More than 200 randomized controlled trials,16,17 along with decades of clinical experience, have established the safety and efficacy of methylphenidate (MPH) in treating ADHD.18 In recent years, a long-acting formulation of MPH, osmotic release MPH (OROS-MPH), has been developed. OROS-MPH appears to be as effective as thrice-daily immediate- release MPH (IR-MPH),19 with the benefits of requiring just once-a-day dosing and a lower abuse liability.20 While OROS-MPH is effective in treating ADHD in adolescents21 and adults,22, 23 there is no compelling evidence to suggest that it would be useful in managing nicotine withdrawal, which includes changes in mood, appetite, and increased cigarette craving, and typically accompanies the early stages of smoking abstinence. In contrast, while nicotine has been found to increase attention, it may be less effective in increasing response inhibition.24 Response inhibition, a core component of ADHD,24 appears to be more difficult during early smoking abstinence for individuals with ADHD compared to those without ADHD.25 This suggests that the use of nicotine replacement therapy alone may be insufficient for managing the re-emergence of ADHD symptoms following smoking cessation. Based on the available literature, we hypothesized that using OROS-MPH to treat ADHD in conjunction with smoking cessation treatment would facilitate successful smoking cessation in smokers with ADHD.
The present trial was an initial evaluation of the efficacy and safety of OROS-MPH with smoking cessation treatment, specifically nicotine patch and counseling, relative to placebo with smoking cessation treatment, in initiating and maintaining abstinence in adult smokers with ADHD. The safety evaluation focused on two primary areas of concern. The first involved possible increases in cardiovascular side effects associated with providing a stimulant to individuals already using a stimulant (i.e., nicotine); this evaluation is timely given concerns about the degree to which stimulants may be associated with adverse cardiovascular events in both children and adults.26 The second concern involved the possibility that OROS-MPH might actually increase, rather than decrease, smoking, a concern raised by laboratory studies finding that IR-MPH significantly increases smoking in adult non-ADHD smokers who were not trying to quit smoking;27, 28 based on these findings, the investigators suggested that clinicians consider using non-stimulants to treat ADHD in smokers.28 In the present trial, it was predicted that OROS-MPH with smoking cessation treatment, relative to placebo with smoking cessation treatment, would be safe and would significantly increase smoking abstinence in smokers with ADHD.
METHODS
Study Design
This randomized, intent-to-treat, double-blind, placebo-controlled trial included an 11- week treatment phase during which efficacy outcomes were measured and a one-month follow-up assessment to collect additional safety data. Typically, smoking cessation trials include 6 and 12 month assessments of smoking abstinence. However, this trial was the first to assess the effect of OROS-MPH on smoking outcomes in adults with ADHD and, thus, was focused on more immediate effects. To evaluate whether ADHD treatment enhanced smoking cessation success, the present trial included an extended pre-quit phase to allow OROS-MPH to effectively ameliorate ADHD symptoms prior to the quit-smoking day. Specifically, the treatment period consisted of two phases: the pre-quit phase, comprised of the first 26 days of treatment and the post-quit phase, starting on day 27 (the designated quit-smoking day), through the end of the treatment period. This trial was conducted by the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) between December 2005 and January 2008. Six study sites, located in Cambridge, Massachusetts, Columbus, Ohio, New York City, New York (2 sites), Portland, Oregon, and Rochester, Minnesota, recruited participants. Two study sites were substance abuse community treatment programs that had participated in previous CTN trials; four additional sites were recruited for the present trial--two ADHD clinics and two smoking cessation clinics. All participants were given a thorough explanation of the study, including possible side effects, and signed an informed consent form that was approved by the Institutional Review Boards of the participating sites.
Participants
Recruitment methods included advertising, letters to clinic patients, and direct community promotions, such as networking with community professionals. Eligible participants were interested in quitting smoking and were between 18 and 55 years of age and in good physical health as determined by a medical history, electrocardiogram, and vital signs. The vital signs criterion cut-off was 135/85 mmHg for blood pressure and 90 bpm for heart rate (HR) for the first 143 participants randomized into the trial. However, based on data and safety monitoring board (DSMB) recommendations following the 2006 OROS-MPH labeling changes,29 the criterion was made more restrictive for participants 40 or older, with cut-offs being 130/80 mmHg and/or a HR > 88 bpm for the remainder of the trial. Participants were required to meet DSM-IV criteria for ADHD as assessed by the Adult Clinical Diagnostic Scale version 1.2,30 to have a DSM-IV ADHD Rating Scale (ADHD-RS)31 total score >22, to smoke at least 10 cigarettes per day (CPD), to have a Carbon Monoxide (CO) level ≥8 ppm, and to have smoked cigarettes for at least 3 months. Candidates were excluded if they were a significant suicidal/homicidal risk, had used tobacco products other than cigarettes in the past week, had a positive urine screen for an illicit drug, or met DSM-IV criteria for: current abuse or dependence for any psychoactive substance other than nicotine; current major depression; any anxiety disorder except specific phobias; antisocial personality disorder; or a lifetime diagnosis of bipolar disorder or psychosis. Other exclusion criteria included a history of narrow angle glaucoma or seizure disorder, tics, or a family history of Tourette’s syndrome. Individuals were also excluded if they had been treated for ADHD with psychomotor stimulants or had used smoking cessation counseling programs or medications within the last 30 days, if they were currently taking a medication that could adversely interact with OROS-MPH, had a known allergy to OROS-MPH, or had been a non-responder to a reasonable course of MPH treatment. Women were ineligible if they were pregnant or breastfeeding or unwilling to use an adequate method of birth control.
Procedures
An overview of study procedures is provided in Figure 1. Randomization of participants to OROS-MPH or matching placebo was in a 1:1 ratio, stratified by site, and completed by computer at a centralized location. For OROS-MPH, the starting dose of 18 mg/day was escalated during the first two study weeks to a maximum of 72 mg/day or to the highest dose tolerated; OROS-MPH/placebo was taken through the week 11 visit. A trained interventionist provided each participant with a weekly 10 minute smoking cessation counseling session during study weeks 1–11. The manual used was an unpublished manual, “Smoke Free and Living It,©” developed by the Mayo Clinic Nicotine Research Program for use in clinical trials. Interventionists received training on the manual and were certified after a review of a successful mock session. All therapy sessions were videotaped to monitor adherence; of the 163 sessions rated, 156 (95.7%) were rated as adherent. All participants received transdermal nicotine patches (Habitrol, Novartis, Parsippany, NJ) and were instructed to wear a patch daily for 24 hours beginning on the target quit date (study day 27). Participants were provided with 21-mg patches for use through week 11; for tapering, participants were provided with 14-mg patches for study weeks 12 and 13, and 7-mg patches for study week 14. During the 11-week active trial participants were scheduled to attend one research visit per week. Study participants received $25 per research visit; at the week 11 visit, participants received an additional $25 because of the larger assessment burden associated with the visit.
Figure 1.
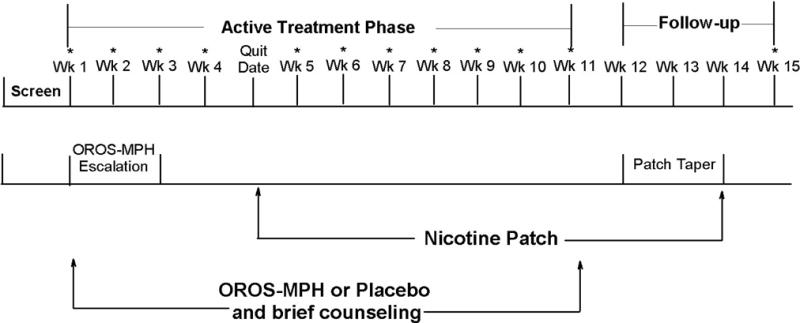
Study Schema
Measures
Self-report of cigarette use, assessed using time-line follow-back (TLFB) assessment,32,33 and CO measurement were obtained weekly. Consistent with the recommendations of the Society for Research on Nicotine and Tobacco, 34 the primary outcome measure was prolonged abstinence defined as a self-report of tobacco abstinence without treatment failure (defined as smoking each day for seven consecutive days or having smoked at least one day of each week in two consecutive weeks) during study weeks 7 – 10; the selection of a 4-week period is consistent with FDA standards for approving smoking cessation medications.34 Secondary smoking outcome measures included point-prevalence abstinence at week 10, defined as self-report of not smoking in the previous seven days, confirmed by a CO level <8 ppm,35 and self-reported CPD. ADHD outcome measures included DSM-IV ADHD-RS total score,31 assessed with prompts for the interviewer,36 and ADHD severity assessed by the Clinical Global Impression (CGI) severity scale.37 Rater training in ADHD measures was conducted according to accepted methodology38 prior to study initiation. Both ADHD assessments were obtained weekly during the pre-quit phase and bi-weekly during study weeks 7–11. Safety measures included weekly vital signs and adverse event (AE) assessments. Medication adherence was assessed weekly via self-report and pill count; nicotine patch adherence was assessed weekly via self-report and patch count (weeks 5–11 only).
Data Analysis
All analyses were completed on the intent-to-treat (ITT) sample using SAS, Version 9.1.3.39 Statistical tests were conducted at a 5% Type I error rate (two-sided) for all measures. Outcomes measured at multiple time points were analyzed using Generalized Estimating Equation models (GEE). Dichotomous efficacy measures were analyzed using logistic regression. Given the smoking quit date’s substantial impact on CPD, analyses for CPD evaluated treatment effects for the pre-quit and post-quit phases as well as for the entire treatment phase. Analyses for cardiovascular effects included baseline as a covariate. All modeling included treatment, week, a treatment by week interaction effect, and site effects. Site effects were evaluated for significance and dropped from the model if non-significant (p>.10). A significant treatment by week interaction effect suggests a significant difference in slope over time between the OROS-MPH and placebo groups and, in the absence of a treatment by week interaction effect, a significant treatment effect suggests an absolute difference between the two groups; thus, both effects are of interest. AEs were coded using the medical dictionary for regulatory activities (MedDRA®) and tabulated by body system and preferred term, seriousness, and relationship to study medication. Fisher’s mid-p-value was used to examine differences between treatments in AE frequency. For the smoking abstinence measures, days for which data were missing were coded as smoking days; missing data for other measures were not imputed.
RESULTS
Participants and Disposition
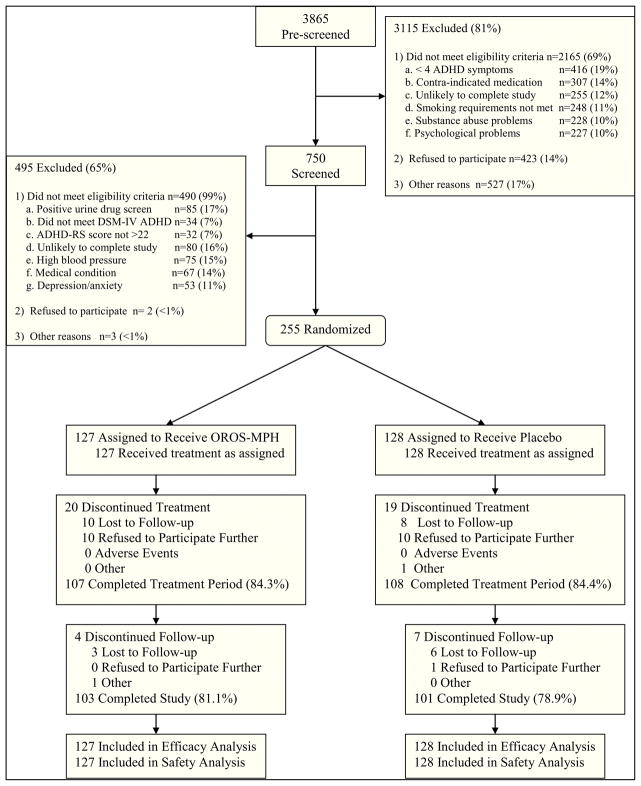
As shown in Figure 2, 3865 candidates were pre-screened, 750 were consented and screened, and 255 were randomized to OROS-MPH or placebo. Approximately 84% of participants completed the 11-week active treatment period, with no treatment group differences on completion rate or reasons for non-completion. Of non-completers, 46% were lost to follow-up, 30% reported practical problems (e.g., work schedule, transportation, etc.), 13% reported not needing further treatment, 10% withdrew consent, and 1 participant was administratively discharged. No participant discontinued the study due to an AE. Demographic and baseline characteristics did not differ significantly between the groups. As can be seen in Table 1, the sample was approximately 56% male and 82% Caucasian, and participants were 38 years of age on average. The sample had moderately severe ADHD, as assessed by the DSM-IV ADHD-RS total score, had a medium level of nicotine dependence as assessed by the Fagerström score, and, on average, smoked a pack of cigarettes per day. Medication adherence was high and did not differ significantly between treatment groups (see Table 2).
Figure 2.
Participant Disposition
Table 1.
Participant Demographic and Baseline Characteristics
| OROS-MPH (N=127) | Placebo (N=128) | |
|---|---|---|
| Age (Years) | 38.1 (10.4) | 37.5 (9.6) |
| Sex (% Male) | 60.6 | 52.3 |
| Race/Ethnicity (%) | ||
| African-American | 4.8 | 7.0 |
| Caucasian | 86.4 | 78.9 |
| Asian | 1.6 | 1.6 |
| Native American/Alaskan | 0.8 | 0 |
| Other | 3.2 | 4.7 |
| Mixed race | 3.2 | 7.8 |
| Hispanic | 7.9 | 6.3 |
| Marital Status (%) | ||
| Married | 40.5 | 27.8 |
| Separated/Divorced/Widowed | 17.4 | 25.4 |
| Never Married | 42.1 | 46.8 |
| Education (Years) | 14.4 (2.4) | 14.5 (2.4) |
| Employed Full/Part time (%) | 94.4 | 89.7 |
| Lifetime Psychiatric Comorbidity (%) | ||
| Major depression | 32.3 | 35.9 |
| Bipolar disorder | 0.0 | 0.0 |
| Anxiety disorder | 34.6 | 32.8 |
| Substance use disorder | 63.0 | 58.6 |
| DSM-IV ADHD Rating Scale Total Score | 36.0 (7.1) | 36.7 (7.5) |
| Adult ADHD subtype (%) | ||
| Inattentive | 25.2 | 23.4 |
| Hyperactive-Impulsive | 2.4 | 0.8 |
| Combined | 72.4 | 75.8 |
| Smoking history | ||
| Fagerström score | 5.6 (2.1) | 5.4 (2.3) |
| No. of Smoking years | 19.9 (10.0) | 19.5 (9.3) |
| No. of cigarettes/day | 19.8 (8.1) | 19.8 (7.5) |
| No. of past quit attempts | 7.5 (10.3) | 6.4 (9.8) |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Table 2.
Summary of Medication Adherence and Tolerability
| OROS-MPH (n=127) | Placebo (n=128) | P Value | |
|---|---|---|---|
| Medication Adherence | |||
| Percentage of OROS-MPH/Placebo pills taken: | |||
| Self-report a | 93.1 (13.5) | 93.1 (13.3) | 0.41 |
| Pill count b | 95.8 (14.6) | 95.2 (20.0) | 0.77 |
| Percentage of nicotine patches taken: | |||
| Self-report a | 84.6 (26.5) | 78.9 (32.9) | 0.60 |
| Patch count b | 74.8 (29.6) | 77.1 (27.4) | 0.79 |
| Medication Tolerability | |||
| Tolerability of maximum OROS-MPH/Placebo dose: | |||
| Reached maximum n (%) | 109 (85.8) | 111 (86.7) | 0.84 |
| Sustained dose at maximum n (%) | 80 (63.0) | 88 (68.8) | 0.33 |
| Sustained dose 21 mg/day for nicotine patch n (%) | 96 (75.6) | 88 (68.8) | 0.22 |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Self-reported adherence was calculated by dividing the number of pills/patches taken by the number prescribed and multiplying by 100;
For pill/patch count, adherence was calculated by taking the number of pills/patches dispensed minus the number returned or reported lost divided by the number of pills/patches prescribed to be taken and multiplying by 100. In cases where participants failed to return their medication bottles/patches, those bottles/patches were excluded from the analysis.
Smoking Outcomes
The treatment groups did not differ significantly on the primary outcome measure of prolonged abstinence, with rates of 43.3% in the OROS-MPH, and 42.2% in the placebo group (X2=0.06, df=1, p=0.81). There was a similar lack of treatment effect on point-prevalence abstinence, with rates of 39.4% in the OROS-MPH, and 38.3%, in the placebo group (X2=0.08, df=1, p=0.78). CPD analyses revealed one statistically significant effect, a treatment × week interaction effect for the post-quit phase (X2=5.85, df=1, p=0.016), which reflected fewer CPD in the OROS-MPH, relative to the placebo, group (Figure 3). It should be noted that this difference in CPD was quite small, equating to approximately one CPD.
Figure 3.
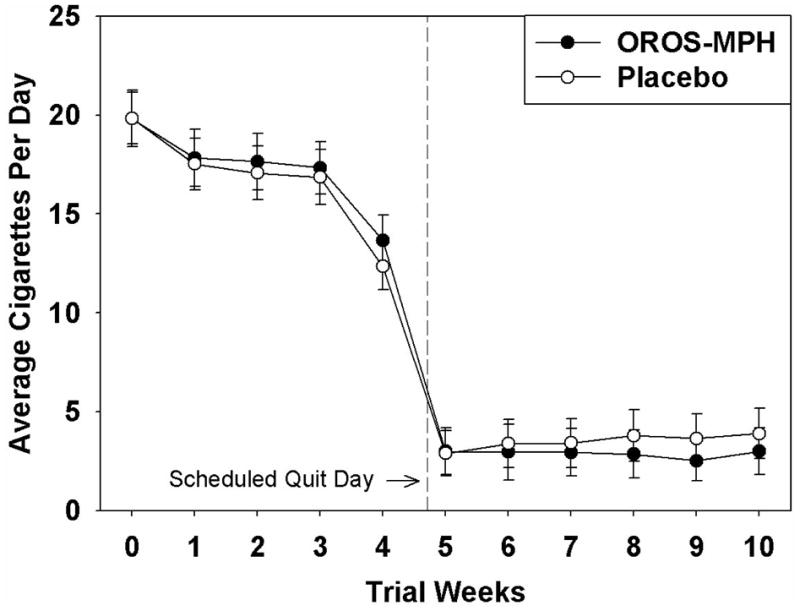
Cigarettes per day as a function of treatment group and time
ADHD Outcomes
OROS-MPH, relative to placebo, participants had a significantly greater decrease in ADHD symptom severity as assessed by both the DSM-IV ADHD-RS total score (X2=15.93, df=1, p<0.0001; see Figure 4) and the CGI severity scale (X2=6.97, df=1, p=0.008). Prior clinical trials for adult ADHD have reported treatment response rates defined as a 30% or greater decrease on the primary ADHD assessment and a change on the CGI improvement scale. The CGI improvement scale was not included in the present trial; thus a treatment responder was defined as a participant whose week-11 DSM-IV ADHD-RS total score was reduced by 30% or more compared to baseline, and who had a one point or greater reduction in week-11 CGI severity scale score relative to baseline. Of the 255 randomized participants, 215 participants, 107 OROS-MPH and 108 placebo, had week-11 data and, thus, could be classified as a responder or non-responder. In these 215 participants, the treatment response rate was 71% in the OROS-MPH group and 44% in the placebo group, a statistically significant difference (X2=15.56, df=1, p<0.001).
Figure 4.
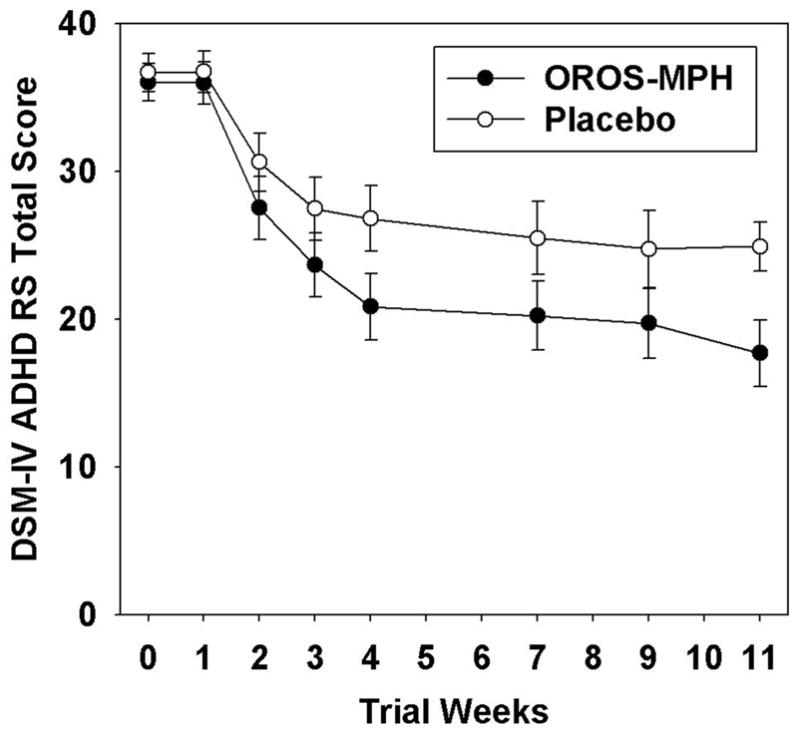
DSM-IV ADHD-RS total score as a function of treatment group and time
Smoking Outcomes as a Function of ADHD Outcomes
As would be expected, not all OROS-MPH participants were treatment responders. If, as would be suggested by the self-medication hypothesis, smoking cessation would be more successful in individuals in whom ADHD has been effectively treated, then it would be expected that the smoking behavior of treatment responders will differ significantly from those of non-responders. To determine whether ADHD treatment response during the pre-quit phase improved smoking outcomes, responder analyses were completed for which a responder was defined as a participant whose week-4 DSM-IV ADHD-RS total score was reduced by 30% or more compared to baseline, and who had a one point or greater reduction in week-4 CGI severity scale score relative to baseline. Of the 255 randomized participants, 229 participants, 114 OROS-MPH and 115 placebo, had week-4 data and, thus, could be classified as a responder or non-responder; the 26 participants with missing week-4 data were excluded from the responder analyses. The average week 4 dose was 3.54 pills (SD= 0.95) per day for the OROS-MPH group (i.e., an average of 63.72 mg/day) and 3.72 pills (SD= 0.73) per day for the placebo group. Seventy-one (i.e., 62%) and 39 (i.e., 34%) of OROS-MPH and placebo participants, respectively, were treatment responders at week 4. To test the effect of responder status on smoking outcome, the models used in the ITT analyses were modified to include the responder and the responder × treatment interaction effects. The results revealed no significant responder × treatment interaction effects for prolonged abstinence (X2=0.15, df=1, p=0.70), point-prevalence abstinence (X2=0.15, df=1, p=0.70), and post-quit CPD (X2=2.28, df=1, p=0.13).
Adverse Effects
The occurrence of treatment emergent adverse events (TEAEs) was significantly higher in the OROS-MPH, relative to placebo, group for both TEAEs in general as well as TEAEs rated as related to study medication (Table 3). TEAEs reported at significantly higher rates in the OROS-MPH group included dyspepsia, decreased appetite, heart rate increase, and palpitations. Consistent with OROS-MPH participant report of decreased appetite, the OROS-MPH participants lost an average of 2.2 pounds (SD=11.1) between baseline and week 11 while the placebo participants gained an average of 2.1 pounds (SD=8.5) during the same time-frame (X2=42.91, df=1, p<.0001). The week 11 body mass index (BMI) was comparable for the two groups, 27.67 (SD=5.81) for OROS-MPH and 27.57 (SD=6.81) for placebo, suggesting that the weight loss in the OROS-MPH group was not clinically significant. The two serious adverse events (SAEs) reported during the trial were in the OROS-MPH group, but were considered unrelated to the study medication. One entailed an accident-related hospitalization and the other an overnight hospitalization for worsening depression in a participant who had switched to a new antidepressant while in the trial; the dose of the new medication was “sub-optimal” according to the emergency room staff and the participant’s antidepressant dose was consequently increased to a more optimal level. The OROS-MPH and placebo groups did not differ significantly on the number of participants discontinued from study medication due to TEAEs. Tolerability for OROS-MPH/placebo and the nicotine patch did not differ significantly between treatments (Table 2).
Table 3.
Summary of Treatment Emergent Adverse Events
| TEAE, n (%) | OROS-MPH (n=127) | Placebo (n=128) | P Value |
|---|---|---|---|
| Any TEAEsa | 122 (96.1) | 112 (87.5) | 0.01 |
| TEAEs related to study medicationb | 111 (87.4) | 95 (74.2) | 0.01 |
| Any serious TEAE | 2 (1.6) | 0 (0.0) | 0.25 |
| Discontinued medication due to TEAEs | 7 (5.5) | 2 (1.5) | 0.10 |
| Most frequent TEAEs c by MedDRA®d preferred term | |||
| Psychiatric disorders | |||
| Nervousness | 28 (22.0) | 21 (16.4) | 0.24 |
| Anxiety | 24 (18.9) | 18 (14.1) | 0.28 |
| Insomnia | 22 (17.3) | 17 (13.3) | 0.34 |
| Abnormal dreams | 9 (7.1) | 9 (7.0) | 0.90 |
| Initial insomnia | 9 (7.1) | 5 (3.9) | 0.23 |
| Depression | 7 (5.5) | 2 (1.6) | 0.07 |
| Nervous system disorders | |||
| Headache | 35 (27.6) | 28 (21.9) | 0.28 |
| Dizziness | 8 (6.3) | 5 (3.9) | 0.33 |
| Psychomotor hyperactivity | 9 (7.1) | 1 (0.8) | 0.01 |
| Gastrointestinal disorders | |||
| Nausea | 18 (14.2) | 10 (7.8) | 0.09 |
| Dry mouth | 15 (11.8) | 7 (5.5) | 0.06 |
| Dyspepsia | 9 (7.1) | 1 (0.8) | 0.01 |
| Musculoskeletal/connective tissue disorders | |||
| Back pain | 7 (5.5) | 6 (4.7) | 0.68 |
| Musculoskeletal pain | 7 (5.5) | 2 (1.6) | 0.07 |
| Nasopharyngitis | 20 (15.7) | 14 (10.9) | 0.24 |
| Fatigue | 15 (11.8) | 12 (9.4) | 0.48 |
| Cough | 11 (8.7) | 6 (4.7) | 0.18 |
| Decreased appetite | 23 (18.1) | 7 (5.5) | 0.00 |
| Rash | 10 (7.9) | 4 (3.1) | 0.08 |
| Heart rate increase | 9 (7.1) | 1 (0.8) | 0.01 |
| Palpitations | 9 (7.1) | 1 (0.8) | 0.01 |
Note. TEAE = Treatment Emergent Adverse Event;
TEAEs are adverse events defined as a new illness, or an exacerbation of a pre-existing condition, with onset date post-randomization;
TEAE rated as possibly, probably or definitely related to treatment;
Reported by >5% of OROS-MPH group and at a greater rate than by the placebo group;
MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA).
Cardiovascular Effects
There was a significant treatment main effect for systolic blood pressure (SBP; X2=5.22, df=1, p<.05), diastolic blood pressure (DBP; X2=12.13, df=1, p<.001) and HR (X2=10.56, df=1, p<.01), which reflected greater increases in the OROS-MPH group. While statistically significant, the average change in blood pressure and heart rate was minimal for the OROS-MPH group as a whole (see Table 4). Evaluation of clinically elevated SBP, DBP, and HR post-randomization values revealed no significant treatment group differences. For SBP, 21 (16.7%) OROS-MPH and 12 (9.6%) placebo participants experienced a maximum SBP of 140 mmHg or greater (X2=2.74, df=1, p=0.10). For DBP, 26 (20.6%) OROS-MPH and 15 (12.0%) placebo participants experienced a maximum DBP of 90 mmHg or greater (X2=3.42, df=1, p=.06). For HR, 26 (20.6%) OROS-MPH and 19 (15.2%) placebo participants experienced a maximum heart rate of 100 bpm or greater (X2=1.26, df=1, p=0.26).
Table 4.
Changes in Blood Pressure and Pulse as a function of Treatment Group and Time
| Time | Systolic blood pressure (mm Hg)
|
Diastolic blood pressure (mm Hg)
|
Heart rate (bpm)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OROS-MPH
|
Placebo
|
OROS-MPH
|
Placebo
|
OROS-MPH
|
Placebo
|
|||||||
| n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | |
| Baseline | 127 | 119.1 (9.6) | 128 | 115.4 (10.8) | 127 | 74.5 (6.5) | 128 | 72.8 (7.0) | 127 | 76.0 (7.4) | 128 | 75.2 (7.7) |
|
| ||||||||||||
| Week 2 | 114 | 0.6 (7.7) | 118 | −0.6 (8.5) | 114 | 1.3 (5.5) | 118 | 0.4 (6.9) | 114 | 6.3 (9.0) | 118 | 2.4 (8.8) |
| Week 4 | 111 | 0.8 (8.8) | 113 | −1.8 (8.2) | 111 | 1.5 (6.2) | 113 | −1.2 (5.1) | 111 | 5.8 (10.8) | 113 | 2.6 (8.1) |
| Week 6 | 109 | 0.4 (9.7) | 102 | −1.2 (7.9) | 109 | 0.1 (6.7) | 102 | −1.1 (6.0) | 109 | 2.8 (10.2) | 102 | 0.1 (8.0) |
| Week 8 | 102 | −0.7 (10.0) | 94 | −1.4 (8.8) | 102 | 1.0 (7.1) | 94 | −1.3 (6.2) | 102 | 3.6 (8.9) | 94 | 0.6 (8.4) |
| Week 11 | 107 | 1.8 (10.0) | 109 | 0.1 (8.3) | 107 | 1.4 (7.0) | 109 | −0.8 (6.2) | 107 | 2.2 (10.2) | 109 | 0.6 (8.4) |
| Week 15+ | 104 | 1.6 (10.3) | 101 | 0.4 (8.9) | 104 | 0.6 (7.2) | 101 | 0.1 (7.1) | 104 | 1.8 (10.8) | 101 | 2.4 (11.0) |
One-month follow-up.
DISCUSSION
The results from this 11-week randomized, double-blind, placebo-controlled trial suggest that using OROS-MPH, relative to placebo, to treat ADHD did not improve smoking outcomes in adult smokers with ADHD participating in smoking cessation treatment (i.e., nicotine patch and counseling). There might be several reasons for this lack of significant effect. First, all participants were provided with the nicotine patch, which has been found to be effective for both smoking cessation40 and for ameliorating inattention symptoms;7,12,13 thus, the lack of OROS-MPH effect might reflect a ceiling effect. The abstinence rates--43% for prolonged abstinence and 39% for point-prevalence abstinence--would appear to be sufficiently low, however, to allow the demonstration of a treatment effect if one were present. Alternatively, the lack of effect might, in fact, reflect a true lack of medication effect. While the self-medication hypothesis might account for the observed differences between ADHD and non-ADHD individuals in smoking onset3 as well as the potential protective effects of stimulant medication in preventing the onset of smoking in individuals with ADHD,15 it might be that, once an individual becomes nicotine dependent, the dependence itself is the primary factor determining outcome as opposed to the factors that initially led to dependence.
Past research suggests that smokers with ADHD have more difficulty quitting smoking compared to smokers without ADHD.4,41 The present trial did not include a non-ADHD comparison group and, thus, we are unable to directly address the relative efficacy of the nicotine patch and counseling in our ADHD sample relative to a non-ADHD sample. However, to provide a context for our results, it is helpful to compare the present abstinence rates to those from nicotine patch trials completed in study samples not selected on ADHD status. A meta-analysis evaluating the efficacy of the nicotine patch revealed that the abstinence rate, generally defined as point-prevalence abstinence, for trials using the nicotine patch for 8 weeks or less was 37.9% on average.42 This rate is comparable to the point prevalence abstinence rate in the present trial which was 39% after 6 weeks of nicotine patch treatment. It is thus possible that the ADHD participants in the present trial did not experience greater difficulty in quitting smoking when treated with nicotine patch and counseling relative to non-ADHD individuals.
From a public health perspective, the key finding from the present study was that OROS-MPH, relative to placebo, was effective and safe in treating ADHD in adult smokers. In the present trial, 71% of OROS-MPH participants had a clinically meaningful decrease in symptoms (i.e., a decrease of at least 30%), a rate comparable to that reported for prior MPH trials conducted with samples not selected for smoking. The 44% treatment response rate in the placebo group was somewhat high but is consistent with the 39% placebo response rate reported by Biederman et al.22 Thus, neither smoking nor smoking cessation treatment appeared to decrease the effectiveness of OROS-MPH in treating ADHD.
The safety profile of OROS-MPH in the present trial, in which it was used in combination with the nicotine patch in smokers, is similar to that observed in two large clinical trials of OROS-MPH in adults with ADHD.22,23 Similar to the findings from these prior trials, OROS-MPH, relative to placebo, was associated with statistically significant, but relatively small, increases in blood pressure and heart rate. In the present study, there was no significant treatment group difference for the number of participants experiencing a clinically significant elevation of heart rate while there was a non-significant trend for more OROS-MPH participants to have clinically significant elevations in blood pressure; patients on OROS-MPH should have their blood pressure and heart rate monitored as recommended in the prescribing information for OROS-MPH. The TEAEs occurring at a significantly greater rate in OROS-MPH (decreased appetite, dyspepsia, psychomotor hyperactivity, heart rate increase, and heart palpitations) have been associated with OROS-MPH in previous trials.22,23 In the present trial, 5.5% of OROS-MPH, and 1.5% of placebo participants were permanently discontinued from OROS-MPH/placebo, rates comparable to that of Medori et al. 23 and lower than that of Biederman et al.22 The latter difference is likely due to higher doses used by Biederman et al.22 (1.3 mg/kg/day). The present findings also indicate that the 72mg/day OROS-MPH dose was generally well tolerated and that OROS-MPH did not significantly affect tolerability of the 21 mg/day nicotine patch. In comparing the present safety results to those of prior trials,22,23 it is important to note that the vital signs eligibility criterion utilized in the present trial was likely more restrictive than the criterion used in prior trials and, thus, may underestimate risks that might arise if OROS-MPH were used in smokers with higher baseline blood pressure and heart rate.
Evaluation of the second key safety concern, that OROS-MPH might increase, rather than decrease, smoking, revealed that both OROS-MPH and placebo participants decreased their CPD during both the pre- and post-quit phases of the trial and that this decrease was to a statistically greater degree in the OROS-MPH, relative to placebo, group during the post-quit phase. This finding is not consistent with laboratory findings that IR-MPH significantly increases smoking in adult smokers without ADHD who are not trying to quit smoking.27, 28 Several critical differences between the laboratory studies and the present study, including the MPH formulation and study population, might account for the discrepant findings. In addition, it should be noted that the effects of acute dosing observed in laboratory studies do not necessarily predict a medication’s effects when used in on-going treatment. A prime example of the differing effects associated with acute and chronic dosing is bupropion, which was found to increase smoking in a laboratory study43, but which is an FDA-approved smoking cessation treatment.44 The results of the present trial suggest that the clinical use of OROS-MPH will not increase the daily smoking rate in adult smokers with ADHD who are interested in quitting smoking.
The present study had several strengths. First, this trial utilized a randomized, double-blind, placebo-controlled design, the gold standard in clinical trials. Second, study retention was high, with 84.3% of participants completing the 11-week active study phase. Third, compliance with taking OROS-MPH/placebo was high, with an average of 94% of prescribed pills taken. A final strength is that the study was conducted at sites that were geographically diverse as well as diverse in expertise, with two sites having expertise in smoking cessation trials, two sites with expertise in ADHD trials, and two sites with expertise in neither area; this diversity helps to ensure the generalizability of the results. One weakness of the present study was the somewhat restrictive eligibility criteria utilized, including the restrictive vitals signs criterion and the DSM-IV exclusions, including current abuse or dependence for a substance other than nicotine; thus, the present sample may not be representative of adult smokers with ADHD.
In conclusion, the use of OROS-MPH to treat ADHD did not significantly improve smoking outcomes in adult smokers with ADHD participating in smoking cessation treatment (i.e., nicotine patch and counseling), counter to our hypothesis. It did not worsen smoking outcomes, however, as might be predicted from human laboratory findings that IR-MPH increases smoking in smokers without ADHD.27,28 Results from the present trial suggest that OROS-MPH was safe and generally well tolerated by this sample of healthy adult ADHD smokers and effectively treated ADHD, a relatively common mental health condition, which left untreated, can result in significant impairment in nearly every area of functioning.
Acknowledgments
Funding/Support: This study was supported by the following grants from the National Institute on Drug Abuse: U10-DA015831 and K24 DA022288 to Harvard University (Dr Weiss); U10-DA013035 to New York State Psychiatric Institute (Dr. Nunes); U10-DA013046 to New York University (Dr. Rotrosen); U10-DA013036 to Oregon Health and Science University (Dr. McCarty); U10-DA013732 to the University of Cincinnati (Dr. Somoza). The study medication and matching placebo were provided by McNeil Consumer & Specialty Pharmaceuticals at no cost.
John Hughes, MD (University of Vermont) provided suggestions for the study design including the outcome measures.
The following provided additional medical support at their sites: Alvin Pelt, MD (Maryhaven); Darian Minkunas, MD and Bentson McFarland, MD PhD (Kaiser Permanente Northwest); Jane Fried, MD, Jeanne Manubay, MD, Alexander Glassman, MD, and Yvonne Singletary, RN, PhD (New York State Psychiatric Institute (NYSPI)); Richard Hurt, MD, Jon Ebbert, MD, Tim Lineberry, MD, J. Taylor Hays, MD, and Lowell Dale, MD (Mayo Clinic); Vatsal Thakkar, MD (New York University (NYU)/VA New York Harbor Healthcare System (VANYHHS)); Craig Surman, MD, and Paul Hammerness MD (MGH Pediatric Psychopharmacology).
The following individuals provided administrative support for study teams at their sites: Vivian Russell (Maryhaven); Dennis McCarty, PhD and Lynn Kunkel, MS CCRP (Oregon Health and Science University); Edward Nunes, MD, and Jennifer Lima, MPH (Columbia University and NYSPI); Richard Hurt, MD (Mayo Clinic); John Rotrosen, MD, Agatha Kulaga, MSW, and Patricia Novo, MPA (NYU/VANYHHS); Jennifer Sharpe Potter, PhD, MPH (Harvard University).
The following individuals provided training or monitoring support at their sites: Frankie Kropp, MS, Peggy Somoza MS, and Sharon Pickrel BS (Maryhaven); Joanne Weidemann, BS and Marie Shea MS (Kaiser Permanente Northwest); Catherine LoDuca, BA (NYPSI), Michelle Cordner, BA, Karen Loncto, BSN, MS, and Karen Venuto, BA (NYU/VANYHHS and NYSPI); Judy Trautman, RN and Richard Morris, BA (Mayo Clinic); David Sitt, PsyD (NYU/VANYHHS); Scott Provost, MM, MSW, and Amy Loree, BA, (MGH Pediatric Psychopharmacology).
The following individuals collected data at participating sites: Rebecca Shoemaker, RN, Jessica Rich, BA, Ann Whetzel Nevar, MPA, Stella M. Resko, MSW, PhD, Gwyn Stetler, Vicki Johnson, MS, LPCC, LICDC, and Theresa Smith (Maryhaven); Shannon Janoff MPH, Marti Summer, RN, BSN, MPA, Michelle Roberts RN FNP MSN, Micah Yarborough MA, Lynette Currie MA, Nancy Siegel, PA-C MPH, Catherine Briggs, BS, Monica Jo-Mueller RPh (Kaiser Permanente Northwest); Catherine LoDuca, BA, Jenny Masmela, BA, Victoria Salzman BA, Judith Weissman, Ph.D., Fay Stetner, MS, MPA (NYSPI); Judy Trautman, RN; Richard Morris, BA, Marianne Kosel, Kim Van Rooy, Donna Rasmussen, RN, and Sara Mason, RN (Mayo Clinic); David Shaw, BA, Alexis Brigge, BA, Lisa Reingold, BA, Erica Maya, BA, and Lauren Lynch, BA (NYU/VANYHHS); Rob Sawtelle, Allison Santry, Julia Whitley, Linsey Utzinger, Meghan Hellieson, Jennifer Park, and Lynn Sahaida (MGH Pediatric Psychopharmacology)
Footnotes
Trial Registration: Clinical Trials.gov http://www.clinicaltrials.gov; Identifier: NCT00253747
Financial Disclosures: Dr. Adler reported being a consultant to: Abbott Laboratories, Cortex Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Shire, Eli Lilly, Ortho McNeil/Jannsen/Johnson and Johnson, New River Pharmaceuticals, Cephalon, Merck, Organon, Sanofi-Aventis Pharmaceuticals, Psychogenics, and Mindsite; receiving grant support from: Abbott Laboratories, Cortex Pharmaceuticals, Bristol-Myers Squibb, Merck & Co, Novartis Pharmaceuticals, Pfizer, Shire, Eli Lilly, Ortho McNeil/Jannsen/Johnson and Johnson, New River Pharmaceuticals, and Cephalon; being on the Advisory Board for: Abbott Laboratories, Cortex Pharmaceuticals, Novartis Pharmaceuticals, Pfizer, Shire, Eli Lilly, Ortho McNeil/Jannsen/Johnson and Johnson, New River Pharmaceuticals, Cephalon, Merck, Organon, and Sanofi-Aventis Pharmaceuticals; being on the Speakers Bureau for: Eli Lilly and Shire; and receiving royalty payments (as inventor) from NYU for license of adult ADHD scales and training materials. Dr. Weiss reported receiving grant support from Eli Lilly and Company. The remaining authors reported no financial disclosures.
References
- 1.Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- 2.Hall SM. Nicotine interventions with comorbid populations. Am J Prev Med. 2007;33 (6S):S406–S413. doi: 10.1016/j.amepre.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36 (1):37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 5.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31 (6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 6.Wilens T, Vitulano M, Upadhyaya H, et al. Cigarette smoking associated with attention-deficit hyperactivity disorder. J Pediatr. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin ED, Conners CK, Sparrow E, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- 8.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163 (4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- 10.Kessler RC, Adler L, Ames M, et al. The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med. 2005;47(6):565–572. doi: 10.1097/01.jom.0000166863.33541.39. [DOI] [PubMed] [Google Scholar]
- 11.Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J of Att Dis. 2007;10 (4):335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- 12.Conners CK, Levin ED, Sparrow E, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32(1):67–73. [PubMed] [Google Scholar]
- 13.Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2001;9(1):83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: Focus on cognition. Biochem Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monuteaux MC, Spencer TJ, Faraone SV, Wilson AM, Biederman J. A randomized, placebo-controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2007;68(7):1094–1101. doi: 10.4088/jcp.v68n0718. [DOI] [PubMed] [Google Scholar]
- 16.Wilens TE, Spencer T. The stimulants revisited. In: Stubbe C, editor. Child and Adolescent Psychiatric Clinics of North America. 3. Philadelphia, PA: Saunders; 2000. pp. 573–603. [PubMed] [Google Scholar]
- 17.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165 (11):1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 18.Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38 (5):503–512. doi: 10.1097/00004583-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Wolraich ML, Greenhill LL, Pelham W, et al. Randomized, controlled trial of OROS methylphenidate once a day in children with attention deficit/hyperactivity disorder. Pediatrics. 2001;108(4):883–892. doi: 10.1542/peds.108.4.883. [DOI] [PubMed] [Google Scholar]
- 20.Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163(3):387–95. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- 21.Wilens TE, McBurnett K, Bukstein O, et al. Multisite controlled study of OROS-methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2006;160(1):82–90. doi: 10.1001/archpedi.160.1.82. [DOI] [PubMed] [Google Scholar]
- 22.Biederman J, Mick E, Surman C, et al. A randomized, placebo-controlled trial of OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;59:829–35. doi: 10.1016/j.biopsych.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Medori R, Ramos-Quiroga JA, Casas M, et al. A randomized, placebo-controlled trial of three fixed dosages of prolonged-release OROS methylphenidate in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:981–989. doi: 10.1016/j.biopsych.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Bekker EM, Böcker KBE, Van Hunsel F, van den Berg MC, Kenemans JL. Acute effects of nicotine on attention and response inhibition. Pharmacol Biochem Behav. 2005;82:539–548. doi: 10.1016/j.pbb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 25.McClernon FJ, Kollins SH, Lutz AM, et al. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl) 2008;197:95–105. doi: 10.1007/s00213-007-1009-3. [DOI] [PubMed] [Google Scholar]
- 26.Wilens TE, Prince JB, Spencer TJ, Biederman J. Stimulants and sudden death: what is a physician to do? Pediatrics. 2006;118 (3):1215–1219. doi: 10.1542/peds.2006-0942. [DOI] [PubMed] [Google Scholar]
- 27.Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PE. Methylphenidate increases cigarette smoking. Psychopharmacology. 2005;181:781–789. doi: 10.1007/s00213-005-0021-8. [DOI] [PubMed] [Google Scholar]
- 28.Vansickel AR, Stoops WW, Glaser PEA, Rush CR. A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology. 2007;193:305–313. doi: 10.1007/s00213-007-0786-z. [DOI] [PubMed] [Google Scholar]
- 29.Medscape Today. [Accessed on September 26, 2008];FDA Safety Changes: Allegra, Cymbalta, Concerta. ( http://www.medscape.com/viewarticle/550192)
- 30.Adler L, Spencer T. The Adult ADHD Clinical Diagnostic Scale (ACDS) Vol. 1.2. New York: New York University School of Medicine; 2004. [Google Scholar]
- 31.DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretations. New York, NY: The Guilford Press; 1998. [Google Scholar]
- 32.Sobell LC, Sobell MB. Timeline follow back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to Assess Alcohol Consumption. New Jersey: Humana Press, Inc; 1992. pp. 19–28. [Google Scholar]
- 33.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68 (1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 34.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 35.Hurt RD, Krook JE, Croghan IT, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21(5):914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 36.Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin N Am. 2004;27 (2):187–201. doi: 10.1016/j.psc.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.National Institute of Mental Health. CGI (Clinical Global Impression) Scale—NIMH. Psychopharmacol Bull. 1985;21:839–844. [Google Scholar]
- 38.Adler LA, Spencer T, Faraone SV, et al. Training raters to assess adult ADHD: reliability of ratings. J Atten Disord. 2005;8(3):121–126. doi: 10.1177/1087054705277168. [DOI] [PubMed] [Google Scholar]
- 39.SAS Institute Inc. SAS/STAT software, Version 9.1.3 Service Pack 4 of the SAS System for Microsoft Windows. Copyright © [2002–2003] Cary, NC, USA: SAS Institute Inc; [Google Scholar]
- 40.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2008. (1):Art. No.: CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking Cessation and Inattention or Hyperactive/Impulsivity: A Post Hoc Analysis. Nicotine Tob Res. 2008;10(12):1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiore MC, Smith SS, Jorenby DE, Baker TB. The Effectiveness of the Nicotine Patch for Smoking Cessation: A Meta-analysis. JAMA. 1994;271(24):1940–1947. [PubMed] [Google Scholar]
- 43.Cousins MS, Stamat HM, de Wit H. Acute Doses of d-amphetamine and Bupropion Increase Cigarette Smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- 44.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May, 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]