Abstract
Background
The mechanism for loss of myeloid dendritic cells (mDCs) from the circulation in HIV-infected individuals and its relationship to disease progression is not understood.
Methods
A longitudinal analysis of the mDC response in blood and lymph nodes during the first 12 weeks of infection was performed in a cohort of SIVmac251-infected rhesus macaques with different disease outcomes.
Results
Monkeys that rapidly progressed to disease or had long-term stable infection had significant losses or increases, respectively, in blood mDCs that were inversely correlated with virus load at set-point. The loss of mDCs from progressor animals was associated with evidence of an increase in CCR7-CCL19 dependent mDC recruitment to lymph nodes and an increase in mDC apoptosis.
Conclusions
mDC recruitment to and death within inflamed lymph nodes may contribute to disease progression in SIV infection, whereas mobilization without increased recruitment to lymph nodes may promote disease control.
Keywords: nonhuman primate, AIDS, cell dynamics, innate immunity
Introduction
Dendritic cells (DCs) are a heterogeneous family of innate immune system cells that secrete antiviral cytokines and induce adaptive immune responses to viral infections including HIV [33]. It has long been appreciated that DCs are lost from the blood of individuals with progressive HIV infection associated with an increase in virus load, whereas long-term non-progressors that control infection have relatively normal levels of blood DCs [1, 4, 12, 19, 22, 26, 31, 39, 44, 49]. Similarly, depletion of blood DCs has been described both in acute and end-stage SIV infection of rhesus macaques [9, 10]. These data support the long held notion that DCs are beneficial in HIV infection and that their loss compromises viral immunity and promotes disease progression [46]. However, recent comparative studies have demonstrated that SIV infection of African nonhuman primate species, which is generally nonpathogenic, is characterized by rapid resolution of innate immune responses, whereas progressive infection of rhesus macaques is characterized by persistent innate immune activation [7, 29, 34]. These and other studies [42, 43] suggest that chronic activation of innate immune cells plays a role in AIDS pathogenesis and have led to the hypothesis that the DC response may be pathologic depending on the stage of infection [6, 21, 28]. These contrasting roles of DCs either in facilitating control of virus infection or promoting disease progression have yet to be resolved and remain an important issue in the field.
While several recent studies have focused on the plasmacytoid DC (pDC) response in blood and tissues [10, 11, 27, 36, 41, 42], relatively little is known about the dynamics of the myeloid DC (mDC) response to HIV and SIV infection. A critical issue is the fate of mDCs that are lost from blood during progressive infection. In vitro studies with HIV and purified DC populations support the hypothesis that mDCs are activated via interaction with activated pDCs which leads to their recruitment to lymph nodes through increased chemotaxis [23]. However, findings relating to mDC in lymph nodes during HIV infection are inconsistent, with both accumulation and substantial loss being reported [5, 17, 32, 37]. mDCs are depleted from lymph nodes in rhesus macaques with end-stage SIV infection [9, 56], but data in the acute and chronic stages of infection in this tissue compartment are lacking. What is needed is a comprehensive study of the kinetics of the mDC response to infection in blood and tissues over time, but reports of this nature are absent from the literature. These studies are difficult to undertake in HIV-infected humans but are feasible and justifiable in nonhuman primates with SIV infection.
We have previously reported on an adenovirus-based immunotherapy study in rhesus macaques infected with SIVmac251 that was effective at boosting the strength of virus-specific T cell immunity but which had no discernable impact on virus load or disease outcome [48]. In a recent follow-up study [53] we found that monkeys in this cohort had differential survival and disease that was independent of immunotherapy but correlated with virus load at set-point, prior to any therapeutic intervention. Here we describe the dynamics of the mDC response to SIV infection up to virus set-point in this cohort that has allowed us to better define the relationship between mDC and disease progression and control.
Materials and methods
Animals, virus infection and therapy
21 adult Indian-origin rhesus macaques (Macaca mulatta) used in this study were enrolled in an immunotherapy trial the majority of which has been reported elsewhere [48]. Animals were infected by intravenous inoculation with 1,000 TCID50 of uncloned, pathogenic SIVmac251. Animals were given two intervals of antiretroviral therapy (ART) beginning at week 12, and intramuscular injections of adenovirus serotype 5 and 35-based vaccines beginning at week16, as detailed elsewhere [48]. Analyses described here were done at or before 12 weeks post infection, prior to any therapeutic interventions.
Enumeration and characterization of cells by flow cytometry
Identification of mDCs was done by flow cytometric analysis following antibody staining of peripheral blood mononuclear cells (PBMCs) and lymph node cell suspensions as described [53]. mDCs were identified as cells lacking expression of lineage markers CD3 (antibody clone SP34-2), CD20 (2H7) and CD14 (M5E2) and expressing HLA-DR (G46-6) and CD11c (S-HCL-3) [8, 9]. mDCs were analyzed for activation by labeling with cross-reactive antibodies to CCR7 (150503), CD40 (5C3), CD80 (L307.4) and CD86 (FUN-1). Apoptosis was determined by labeling fixed and permeabilized cells with antibody to active caspase-3 (C92-605), as described [53]. The absolute number of blood CD4+ T cells was determined using a precise volume of blood stained with antibodies to CD3 and CD4 in the absence of any wash step in tubes that contained a known number of fluorescent beads to provide internal calibration (TruCOUNT, BD Biosciences), as described [48]. The number of mDCs was calculated based on the ratio of mDCs to CD4+ T cells in PBMC at the same time point. All flow cytometric analysis was performed on an LSR II cytometer with FACSDiva software (BD Bioscience).
Detection of chemokine expression in tissues
Lymph nodes were harvested at 12 weeks post infection and total RNA was isolated from cell suspensions. Real time PCR analysis of CCL19, CCL21 and β-glucuronidase was done using primers and probes from Taqman human gene expression arrays as described [53].
Results
Changes in blood mDCs but not CD4+ T cells at set-point are predictive of disease outcome
Animals in this cohort were distinguished on the basis of disease progression, with one group of 11 monkeys remaining healthy until elective sacrifice at a mean of 60 weeks post infection (‘stable’ group) and the other group of 10 animals succumbing to AIDS-defining illnesses at a mean of 32 weeks (‘progressor’ group)[53]. This differential outcome was independent of immunotherapy and was inversely proportional to virus loads at set-point, defined as the mean plasma virus titers from weeks 8–12 post infection, consistent with previous reports [50, 52]. When we monitored mDC numbers in blood in these groups of animals over the first 12 weeks of infection, we found a divergent response. While both stable and progressor animals had an initial decline in circulating mDCs at week 2 post infection, progressor animals had a continued decline to week 12, whereas stable animals had a significant increase in the number of blood mDCs over this period [53]. When individual animals were evaluated over time we found that mDCs dropped to 30% of pre-infection levels in some progressor animals (mean=60%) but increased to nearly 500% in some stable animals (mean=206%). This change in the number of mDCs over the first 12 weeks of infection had an inverse relationship with virus load (P = .0054, r = −.6264). In contrast, changes in CD4+ T cell counts over this time were not predictive of ultimate disease outcome [53]. These data suggest that a divergent mDC response in blood during primary infection predicts whether SIV infected animals go on to control infection or to progress rapidly to disease (Fig. 1).
Figure 1.
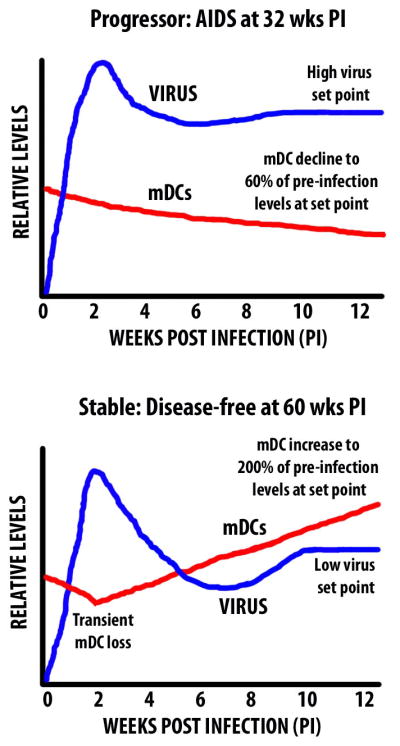
Relationship between virus load and mDCs in blood in the early stages of SIV infection of monkeys with different disease outcomes.
Evidence for increased mDC recruitment and death in lymph nodes in progressive SIV infection
To investigate the dynamics of the mDC response further we first analyzed the phenotype of mDCs in blood. mDCs were rapidly activated following SIV infection regardless of outcome, with CD80 and CD86 expression on blood mDCs increasing by 2 weeks in all animals [53]. However, at 12 weeks post infection there was a differential expression of the chemokine receptor CCR7 on blood mDCs in the two groups, with substantially more upregulation on mDCs of animals in the progressor but not the stable group [53]. CCR7 is the ligand for chemokines CCL19 and CCL21 and mediates DC recruitment to lymph nodes [24]. To investigate whether these ligands were upregulated in lymphoid tissues we performed quantitative analyses of mRNA. Expression of CCL19 in lymph node cell suspensions was 8-fold greater in monkeys with progressive infection relative to naive animals as determined by real time PCR, whereas expression in stable animals was unchanged. Minor differences were noted in expression of CCL21 [53]. These findings raise the possibility that the loss of mDCs from blood in progressor animals could be the result of enhanced recruitment to lymph nodes via CCR7-CCL19 interactions. Surprisingly, there was no difference in the proportion of mDCs in lymph nodes are week 12 post infection in either stable or progressor animals relative to uninfected monkeys, although mDCs from progressor animals had increased apoptosis based on expression of active caspase-3 [53]. The overall findings from the study are summarized in Table 1. The data are consistent with an increase in mDC recruitment from blood to inflamed lymph nodes in progressive SIV infection that is offset by an increase in cell death within tissues.
Table 1.
Changes in mDCs and chemokines at virus set-point in SIV-infected rhesus macaques with different disease outcomes1
| Parameter | Stable SIV infection2 | Progressive SIV infection3 |
|---|---|---|
| Number of mDCs in blood | Increased to ~200% of pre-infection levels4 | Decreased to 60% of pre-infection levels |
| Activation of mDCs in blood | Minor increase in CCR7; CD80/86 elevated | Major increase in CCR7; CD80/86 elevated |
| Proportion of mDCs in lymph node | Similar to naïve | Similar to naïve |
| Activation of mDCs in lymph node | CCR7, CD40, CD86, MHC class II low | CCR7, CD40, CD86, MHC class II normal |
| Expression of CCL19 mRNA in lymph node | Similar to naïve | 8-fold higher than naïve |
| Apoptosis of mDCs in lymph node | Similar to naïve | 3-fold higher than naïve |
Data adapted from [53]
Healthy at elective sacrifice at a mean of 60 weeks post infection (range 56 to 63 weeks)
Sacrificed due to AIDS-defining illness at a mean of 32 weeks post infection (range 11 to 43 weeks)
Blood mDCs in stable infection had initial decline at 2 weeks post infection
Discussion
The loss of mDCs from blood in HIV-infected individuals was first reported more than 20 years ago [39], yet the mechanism for this loss and its relationship to disease progression remain unclear. SIV infection of rhesus macaques provides a powerful model to understand mDC depletion in HIV infection, as mDCs in this species are similar to mDCs in humans [8, 14], and the pathobiology and clinical syndrome associated with SIV and HIV infection are analogous, although the disease course of SIV infection in macaques is accelerated [35]. We have used this model to monitor the mDC response to infection with a pathologic isolate of SIV in animals that have divergent disease outcomes. Our data reveal that readily discernable differences in the kinetics of the blood mDC response occur early in the course of infection that reflect whether animals will have long-term stable infection without disease or will progress relatively rapidly to AIDS. The finding that the mDC response in both blood and tissues differs from a relatively early stage as a function of disease outcome may help to explain the apparent inconsistencies in the literature regarding mDC dynamics in HIV and SIV infection (Table 2).
Table 2.
Impact of SIV and HIV infection on mDCs in vivo
| Stage of infection | Tissue | Finding1 | Reference(s) |
|---|---|---|---|
| Acute | Blood | Reduced mDCs in primary HIV infection and acute SIV infection of RMs | [39, 44, 45, 53] |
| Lymph node | Evidence for mDC accumulation in HIV infection | [37] | |
| Chronic | Blood | mDC depletion is inversely correlated with virus load in HIV infection | [1, 4, 12, 19, 22, 26] |
| mDC from HIV-infected individuals have reduced allostimulatory function | [18, 38, 39] | ||
| Evidence for mDC hyperresponsiveness to stimulation in HIV infection | [45] | ||
| Increased mDC apoptosis in HIV infection | [16] | ||
| mDC loss predicts disease progression in SIV-infected RMs | [53] | ||
| mDC activation in HIV infection and SIV-infected RMs | [4, 26, 30, 53] | ||
| mDC numbers unchanged in SIV-infected Chinese RMs | [53, 54] | ||
| mDC increased in SIV-infected CMs and RMs with stable SIV infection | [40, 53] | ||
| Increased expression of B7-H1 in HIV infection and SIV-infected RMs | [51, 55] | ||
| Lymph node | Accumulation of partially activated mDCs in HIV infection and stable SIV infection of RMs | [17, 32, 53] | |
| Increased mDC apoptosis in progressive SIV infection of RMs | [53] | ||
| mDC loss in HIV infection | [5] | ||
| Increased tissue chemokine expression in progressive SIV infection of RMs | [13, 53] | ||
| Evidence for increased mDC responsiveness in SIV-infected RMs | [53] | ||
| AIDS | Blood | Loss of mDCs in simian AIDS | [9] |
| Lymph node | Apoptosis and loss of mDCs in simian AIDS | [9, 56] | |
| Impaired DC maturation in simian AIDS | [47] |
RM=rhesus macaque, CM=cynomolgus macaque
In all animals in our study SIV infection rapidly led to activation of blood mDCs based on increased expression of costimulatory molecules, consistent with previous reports in HIV infection [4, 26, 30]. However, only in progressor animals were there substantial increases in the proportion of blood mDCs expressing CCR7 together with an increase in the corresponding ligand CCL19 in lymph nodes. Increased lymph node expression of homeostatic chemokines including CCL19 has been described previously in SIV-infected lymph nodes, although a distinction was not made between stable and progressive infection [13]. Interestingly, increased expression of CCL19 and CCL21 has been detected in serum of HIV-infected patients and shown to be correlated with virus load and disease progression [15]. Whether these systemic increases in chemokines are reflective of even greater increases in lymphoid tissues in HIV-infected individuals is not known. Collectively, the data suggest that in progressive SIV and HIV infection mDC migration from blood to lymph nodes is promoted by an increase in the CCR7/CCL19 axis. Within inflamed lymph nodes, mDCs are prone to undergo apoptosis through an indirect mechanism that may involve the CD95-CD95 ligand pathway [53]. Direct infection is unlikely to be a contributing factor as negligible numbers of lymph node mDCs contain proviral DNA [10]. The lymph node in progressive infection therefore has a sink effect, continuously pulling in mDCs that rapidly die. These changes in mDC occur prior to disease progression raising the possibility of a causal relationship between mDC decline and disease development, although this has not formally been shown.
In contrast to progressive SIV infection, animals with long-term stable infection had a steady increase in blood mDCs over time that was associated with a lack of high-level expression of CCR7 and homeostatic expression of CCL19 in lymph nodes. Coupled with evidence of increased production of mDCs in bone marrow in acute infection (Wijewardana and Barratt-Boyes, unpublished data), these findings suggest that mDCs are mobilized in SIV infected monkeys with relatively low virus loads and build up in blood due to the absence of any increased recruitment to tissues. Similarly, in SIV infection of cynomolgus macaques, which is less pathogenic than the SIV/rhesus macaque model, an increase in circulating mDCs has been noted [40]. It is interesting to speculate that the increased level of mDCs in blood could be a factor in the establishment of more effective T cell responses to opportunistic infections in stable infection.
There are several outstanding questions that remain to be answered before we fully understand the nature of the mDC response during SIV and HIV infection. One consistent finding is that lymph node mDCs in HIV infection have a relatively immature phenotype with lower level expression of costimulatory molecule expression than lymph nodes from naïve individuals [17, 32]. We also found lymph node mDCs that are relatively immature with lower expression of MHC class II, CD40, CCR7 and CD86 relative to naïve monkeys, but interestingly this phenotype was restricted to animals with stable infection rather than progressive infection [53]. This restriction implies that reduced activation of lymph node mDCs may be beneficial in SIV infection. The function of immature mDCs in HIV-infected lymph nodes is not known, but there is evidence that these cells may preferentially activate regulatory T cells [32]. Such regulatory T cells could reduce the function of virus-specific T cells but could also contribute to control of immune activation which is a hallmark of nonpathogenic SIV infection [7, 29].
A second unanswered question is whether mDC recovery in progressive SIV and HIV infection can be promoted by ART. Data on the efficacy of ART in HIV infection are contradictory, with numerous reports indicating positive effects on mDC recovery [2, 4, 12, 20, 25] and still others indicating that ART has no beneficial effect on mDC dynamics [3, 22, 44]. Our own investigations into the impact of ART on mDC responses in SIV infection indicate that ART given at 12 weeks post infection promotes recovery of blood mDCs in progressor animals and stabilizes the number of blood mDCs animals that control infection [53]. These findings need to be verified but imply that ART may have differential effects on mDCs depending on disease severity.
These studies highlight the value of the nonhuman primate model in dissecting the DC response to HIV infection and defining the DC’s role in disease control or progression. Continued studies in this model are likely to provide a better understanding of the nature and function of immature mDCs in stable SIV infection, the potential effects of ART on mDC recovery and the role of mucosal tissues in mDC recruitment and loss. The most informative approaches are likely to utilize attenuated virus strains as well as well-defined cohorts of non-progressive and progressive infection with a single pathogenic virus. Studies in nonpathogenic hosts such as the African green monkey and sooty mangabey are likely to also contribute to our understanding of DC biology in HIV infection in the near future.
Acknowledgments
This work was supported by research grant AI071777
References
- 1.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–71. [PubMed] [Google Scholar]
- 2.Almeida M, Cordero M, Almeida J, Orfao A. Persistent abnormalities in peripheral blood dendritic cells and monocytes from HIV-1-positive patients after 1 year of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;41:405–15. doi: 10.1097/01.qai.0000209896.82255.d3. [DOI] [PubMed] [Google Scholar]
- 3.Azzoni L, Chehimi J, Zhou L, Foulkes AS, June R, Maino VC, Landay A, Rinaldo C, Jacobson LP, Montaner LJ. Early and delayed benefits of HIV-1 suppression: timeline of recovery of innate immunity effector cells. Aids. 2007;21:293–305. doi: 10.1097/QAD.0b013e328012b85f. [DOI] [PubMed] [Google Scholar]
- 4.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 5.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown KN, Barratt-Boyes SM. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38:272–8. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–67. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 10.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campillo-Gimenez L, Laforge M, Fay M, Brussel A, Cumont MC, Monceaux V, Diop O, Levy Y, Hurtrel B, Zaunders J, Corbeil J, Elbim C, Estaquier J. Nonpathogenesis of simian immunodeficiency virus infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol. 2010;84:1838–46. doi: 10.1128/JVI.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chehimi J, Campbell DE, Azzoni L, Bacheller D, Papasavvas E, Jerandi G, Mounzer K, Kostman J, Trinchieri G, Montaner LJ. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 13.Choi YK, Fallert BA, Murphey-Corb MA, Reinhart TA. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood. 2003;101:1684–91. doi: 10.1182/blood-2002-08-2653. [DOI] [PubMed] [Google Scholar]
- 14.Coates PT, Barratt-Boyes SM, Zhang L, Donnenberg VS, O’Connell PJ, Logar AJ, Duncan FJ, Murphey-Corb M, Donnenberg AD, Morelli AE, Maliszewski CR, Thomson AW. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–21. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 15.Damas JK, Landro L, Fevang B, Heggelund L, Froland SS, Aukrust P. Enhanced levels of the CCR7 ligands CCL19 and CCL21 in HIV infection: correlation with viral load, disease progression and response to highly active antiretroviral therapy. Aids. 2009;23:135–8. doi: 10.1097/QAD.0b013e32831cf595. [DOI] [PubMed] [Google Scholar]
- 16.Dillon SM, Friedlander LJ, Rogers LM, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon SM, Robertson KB, Pan SC, Mawhinney S, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 19.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–6. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 20.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–52. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010 doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontaine J, Coutlee F, Tremblay C, Routy JP, Poudrier J, Roger M. HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease. J Infect Dis. 2009;199:1007–18. doi: 10.1086/597278. [DOI] [PubMed] [Google Scholar]
- 23.Fonteneau JF, Larsson M, Beignon AS, McKenna K, Dasilva I, Amara A, Liu YJ, Lifson JD, Littman DR, Bhardwaj N. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–71. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 25.Gompels M, Patterson S, Roberts MS, Macatonia SE, Pinching AJ, Knight SC. Increase in dendritic cell numbers, their function and the proportion uninfected during AZT therapy. Clin Exp Immunol. 1998;112:347–53. doi: 10.1046/j.1365-2249.1998.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–66. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 27.Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, Silvestri G, Muller-Trutwin M, Vasile-Pandrea I, Apetrei C, Hirsch V, Lifson J, Brenchley JM, Estes JD. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–91. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–8. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones GJ, Watera C, Patterson S, Rutebemberwa A, Kaleebu P, Whitworth JA, Gotch FM, Gilmour JW. Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients. Aids. 2001;15:1657–63. doi: 10.1097/00002030-200109070-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, Meyer L, Goujard C, Lebon P, Sinet M, Hosmalin A. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis. 2005;192:303–10. doi: 10.1086/430931. [DOI] [PubMed] [Google Scholar]
- 32.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 33.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8:350–60. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letvin NL, King NW. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3:1023–40. [PubMed] [Google Scholar]
- 36.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lore K, Sonnerborg A, Brostrom C, Goh LE, Perrin L, McDade H, Stellbrink HJ, Gazzard B, Weber R, Napolitano LA, van Kooyk Y, Andersson J. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. Aids. 2002;16:683–92. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 38.Macatonia SE, Gompels M, Pinching AJ, Patterson S, Knight SC. Antigen-presentation by macrophages but not by dendritic cells in human immunodeficiency virus (HIV) infection. Immunology. 1992;75:576–81. [PMC free article] [PubMed] [Google Scholar]
- 39.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 40.Malleret B, Karlsson I, Maneglier B, Brochard P, Delache B, Andrieu T, Muller-Trutwin M, Beaumont T, McCune JM, Banchereau J, Le Grand R, Vaslin B. Effect of SIVmac infection on plasmacytoid and CD1c+ myeloid dendritic cells in cynomolgus macaques. Immunology. 2008;124:223–33. doi: 10.1111/j.1365-2567.2007.02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malleret B, Maneglier B, Karlsson I, Lebon P, Nascimbeni M, Perie L, Brochard P, Delache B, Calvo J, Andrieu T, Spreux-Varoquaux O, Hosmalin A, Le Grand R, Vaslin B. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 42.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, Barrat FJ, Coffman RL, Staprans SI, Feinberg MB. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–87. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 43.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, Meyer L, Oksenhendler E, Sinet M, Hosmalin A. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–21. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 45.Sabado RL, O’Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–52. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Servet C, Zitvogel L, Hosmalin A. Dendritic cells in innate immune responses against HIV. Curr Mol Med. 2002;2:739–56. doi: 10.2174/1566524023361907. [DOI] [PubMed] [Google Scholar]
- 47.Soderlund J, Nilsson C, Lore K, Castanos-Velez E, Ekman M, Heiden T, Biberfeld G, Andersson J, Biberfeld P. Dichotomy between CD1a+ and CD83+ dendritic cells in lymph nodes during SIV infection of macaques. J Med Primatol. 2004;33:16–24. doi: 10.1111/j.1600-0684.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 48.Soloff AC, Liu X, Gao W, Day RD, Gambotto A, Barratt-Boyes SM. Adenovirus 5- and 35-based immunotherapy enhances the strength but not breadth or quality of immunity during chronic SIV infection. Eur J Immunol. 2009;39:2437–49. doi: 10.1002/eji.200839130. [DOI] [PubMed] [Google Scholar]
- 49.Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, Huang L, Levy JA, Liu YJ. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 50.Staprans SI, Dailey PJ, Rosenthal A, Horton C, Grant RM, Lerche N, Feinberg MB. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol. 1999;73:4829–39. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Zhang Z, Zhang S, Fu J, Yao J, Jiao Y, Wu H, Wang FS. B7-H1 up-regulation impairs myeloid DC and correlates with disease progression in chronic HIV-1 infection. Eur J Immunol. 2008;38:3226–36. doi: 10.1002/eji.200838285. [DOI] [PubMed] [Google Scholar]
- 52.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, Hu SL, Haigwood NL. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–90. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6:e1001235. doi: 10.1371/journal.ppat.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia HJ, Zhang GH, Ma JP, Dai ZX, Li SY, Han JB, Zheng YT. Dendritic cell subsets dynamics and cytokine production in SIVmac239-infected Chinese rhesus macaques. Retrovirology. 2010;7:102. doi: 10.1186/1742-4690-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 185:7340–8. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmer MI, Larregina AT, Castillo CM, Capuano S, 3rd, Falo LD, Jr, Murphey-Corb M, Reinhart TA, Barratt-Boyes SM. Disrupted homeostasis of Langerhans cells and interdigitating dendritic cells in monkeys with AIDS. Blood. 2002;99:2859–68. doi: 10.1182/blood.v99.8.2859. [DOI] [PubMed] [Google Scholar]


