Abstract
Tristetraprolin (TTP) is a well-characterized, zinc finger-containing, RNA-binding protein. TTP targets tumor necrosis factor α for degradation via the 3′ untranslated region (3′UTR). Although AU-rich elements (AREs) in the 3′UTR of interleukin-6 (IL-6) mRNA dictate mRNA degradation, the role of TTP in the post-transcriptional regulation of IL-6 gene expression is unclear. Here we used TTP-deficient mice to test the hypothesis that IL-6 expression is influenced by TTP. Genetic and siRNA-mediated knockdown of TTP resulted in increased IL-6 production and overexpression of TTP had the reverse effect. IL-6 and tumor necrosis factor α production were elevated after injection of IL-1β in TTP-deficient mice. Further, embryonic fibroblasts from these mice (mouse embryonic fibroblasts) exhibited greater IL-6 mRNA expression and longer half-life than wild-type mouse embryonic fibroblasts. Overexpression of TTP reduced IL-6 3′UTR luciferase reporter activity in an ARE-dependent manner. Proximal and distal regions of the 3′UTR acted synergistically to produce the full repression of TTP. Mutation-based luciferase assays show that ARE2, ARE3, and ARE4 are required for TTP-mediated repression. The constitutively activated p38-MK2 pathway abrogated TTP-mediated repression of IL-6 3′UTR reporter activity. RNA immunoprecipitation assay indicated that the deficiency of p38α resulted in the increased affinity of TTP to IL-6 mRNA. Taken together, we propose that TTP downregulates IL-6 gene expression at the post-transcriptional level by targeting ARE elements in the 3′UTR region.
Introduction
Tristetraprolin (TTP), also known as ZFP36, TIS11, G0S24, and NUP475, is a member of a small family of tandem CCCH zinc finger proteins. TTP has been shown to bind to a variety of AU-rich sequences found in the 3′ untranslated region (3′UTR) of transcripts, including sequences from tumor necrosis factor α (TNFα), granulocyte macrophage-colony stimulating factor (GM-CSF), and interleukin (IL)-2 (Patil and Kirkwood 2007; Khabar 2010). The TTP knockout mouse exhibits a profound inflammatory syndrome with erosive arthritis, autoimmunity, and myeloid hyperplasia, which are reversed with administration of anti-TNFα antibody (Varnum and others 1991; Taylor and others 1996). Overexpression of TTP promoted the decay of reporter transcripts that contained AU-rich sequences from TNFα (Lai and others 1999). In this context, TTP can be thought of as an anti-inflammatory protein. As a point of therapeutic potential, our lab group has previously shown that TTP overexpressed in vivo attenuated lipopolysaccaride-induced inflammation and bone destruction in a periodontal disease model (Patil and others 2008).
IL-6 is a multifunctional cytokine produced by a vast variety of cell types, including lymphocytes, macrophages, fibroblasts, synovial cells, endothelial cells, glial cells, and keratinocytes. IL-6 expression is induced by a variety of stimuli, including IL-1, TNF, platelet-derived growth factor, and lipopolysaccaride. There is significant evidence that IL-6 may play an important role in various diseases, including inflammation and malignancies (Nishimoto 2010), including evidence that IL-6 is constitutively overexpressed in synovial tissues of rheumatoid patients (Hirano and others 2001). The role of IL-6 in human malignancy is most clearly established in multiple myeloma (Yoshio-Hoshino and others 2007; Adachi and others 2008). Monoclonal antibodies enhance the effectiveness of chemotherapy in this disease (Kastritis and others 2009). There is also in vitro evidence that IL-6 can act as an autocrine growth factor in a number of human epithelial malignancies, including renal, lung, and prostate cancer (Waldner and Neurath 2008; Kasuga and others 2001). To clarify the mechanism involved in the abnormal expression of IL-6, it is important to investigate the mechanism of IL-6 gene expression under physiological conditions.
An adenine and uridine (AU)-rich element (ARE) in the 3′UTR of cytokine transcripts is an important determinant of post-transcriptional mRNA control. A hallmark of AREs is the pentamer AUUUA that occurs either alone or clustered. TNFα, GM-CSF, and IL-3 AREs are typical class II AREs with a core AUUUA motif cluster (Khabar 2005, 2007, 2010). The AREs of c-myc and c-fos are prototypes of class I, containing 1 to 3 scattered copies of the AUUUA motif, whereas another group of cytokine transcripts including IL-2, IL-4, and IL-6 contain class I–like AREs. This fact is highlighted in the IL-6 transcripts that contain 5 AUUUA pentamers where none are clustered together.
In recent years, significant information has accumulated regarding the role of 3′UTR of proinflammatory genes as targets of mitogen-activated protein kinase (MAPK) p38 pathway, and many of the effects appear to be mediated by its substrate, MAPK-activated protein kinase 2 (MK2) (Kotlyarov and Gaestel 2002; Stoecklin and others 2004; Sandler and Stoecklin 2008). It has been shown that p38 MAPK/MK2 cascade is involved in regulating mRNA stability via 3′-UTRs of TNFα, IL-8, GM-CSF, COX-2, and VEGF mRNA (Sandler and Stoecklin 2008). In macrophages the 3′UTR of IL-6 is the downstream target of MK2, which is an essential component of the mechanism that regulates mRNA stability. For the IL-1β-induced IL-6 biosynthesis, the involvement of p38 in vivo at the post-transcriptional level has only recently been explored (Patil and others 2004; Zhao and others 2008).
The mechanism by which TNFα mRNA is targeted by TTP has been intensely studied (Lai and others 1999; Rigby and others 2005). However, the role of TTP in IL-6 regulation has not been well studied since the TTP-null mouse does not exhibit spontaneously elevated levels of IL-6. The role of TTP in post-transcriptional regulation of IL-6 was the focus of the present study. Our data support the idea that IL-6 mRNA is highly expressed in IL-1β-stimulated, TTP-deficient mouse embryonic fibroblasts (MEFs), and has a longer half-life than wild-type MEFs through a mechanism that requires multiple AREs within the 3′UTR. This occurs in a p38-MK2-dependent manner since an increased affinity of TTP to IL-6 mRNA is observed in a p38α-null background. Taken together, we propose that TTP downregulates IL-6 gene expression at the post-transcriptional level by targeting the 3′UTR.
Materials and Methods
Mice
TTP−/− mice were generated as previously described (Taylor and others 1996). Genotyping of offspring was performed by polymerase chain reaction (PCR) analysis of tail DNA using primers as described (Taylor and others 1996). All mice were maintained in autoclaved microisolator cages in a barrier facility. All animal care and experiments were in accordance with the University of Michigan institutional guidelines for animal use.
Cell culture
MEFs were derived from wild-type, p38α−/−, and TTP−/− mice. The establishment of MEFs has been previously described (Zhao and others 2008). Cells were grown in Dulbecco's minimal essential medium supplemented with 10% fetal calf serum, 2 mM glutamine, 100 U of penicillin per mL, and 100 mg of streptomycin per mL, and incubated at 37°C in 5% CO2. The MEFs were transfected using Lipofectamine Plus Reagent (Invitrogen) according to the manufacturer's protocol. Cells were stimulated with IL-1β (1 ng/mL) for the indicated times.
Reagents
IL-6 enzyme-linked immunosorbent assay system and recombinant mouse IL-1β were purchased from R&D Systems. The dual-Luciferase Reporter Assay System was purchased from Promega. SB203580 and actinomycin D were from Calbiochem and Invitrogen, respectively. Assays-on-Demand Gene Expression Products (mIL-6 and mGAPDH) and TaqMan Universal PCR Master Mix were from Applied Biosystems.
Plasmids
pcDNA3-p38α, -p38AF, -MKK3E, and -MKK3E were gifts from J. Han (Scripps Institute, La Jolla, CA). 3′-UTR of mIL-6 was amplified by PCR using primers terminating in XbaI recognition sequences. The establishment of full-length pGL3 IL-6 3′UTR, truncations, and mutants has been described previously (Zhao and others 2008).
Luciferase assay
Luciferase activity was determined using a luciferase assay system, following the manufacturer's protocol (Promega Corp.). Briefly, cell monolayers in 12-well plates were removed by scraping into 200 μL of reporter lysis buffer. Cells were vortexed and cellular debris removed by centrifugation (30 s at 12,000 ×g). Luciferase activity was measured using an LMax II 384 (Molecular Devices). A Renilla luciferase reporter vector (pEF-1 R-Luc) was included in every experiment as a transfection efficiency control. Relative luciferase activity was determined and normalized to Renilla luciferase activity as previously described (Zhao and others 2008).
Real-time quantitative reverse transcription-polymerase chain reaction
IL-6 mRNA expression was analyzed by reverse transcription (RT)-PCR and Real-time PCR. First-strand cDNA was synthesized from RNA (600 ng) using SuperScript® III Reverse Transcriptase (Invitrogen). First-strand cDNA was used for PCR with specific oligonucleotide primers for mIL-6 (forward, 5′ATGAAGTTCCTCTCTGCAAGAGACT3′; reverse, 5′CACTAGGTTTGCCGAGTAGATCTC3′)and Real-time PCR with primers designed by Applied Biosystems (mIL-6, mm00446190; mGAPDH, mm99999915).
RNAi against mouse TTP in MEF cells
MEFs were transfected with 100 nM siRNA using Lipofectamine 2000. After 24 h, cells were re-plated and transfected again for another 24 h. Scrambled control siRNAs were purchased from Ambion.
Immunoprecipitation of RNP complexes and RT-PCR
Immunoprecipitation of endogenous RNA-protein complexes was described previously. Cytoplasmic lysates, prepared from wild-type and p38−/− MEFs, were incubated (16 h, 4°C) with 100 μL of a 50% (vol/vol) suspension of protein A-Sepharose beads precoated with 30ug each of rabbit IgG1 or rabbit anti-TTP (Santa Cruz Biotechnology). The beads were washed 5 times with cytoplasmic lysis buffer (20 mM Tris-Hcl [pH7.5], 100 mM KCl, 5 mM MgCl2, 0.3% IGEPAL CA-630, and RNASEOUT [1,000U/mL]). RNA was extracted using phenol and chloroform and precipitated in ethanol. The RNA isolated from immunoprecipitaion (IP) material was reverse transcribed using oligo(dT) primer. Amplicon primers were used for murine IL-6 (accession no. NM_031168.1; forward 5′-atgaagttcctctctgcaagagact-3′ and reverse 5′-cactaggtttgccgagtagatctc-3′) and murine GAPDH (accession no. NM_008084; forward 5′-caccatggagaaggccgggg-3′ and reverse 5′-gacggacacattgggggtag-3′). PCR products were run on a 1% agarose gel containing 0.5 μg/mL ethidium bromide.
Results
TTP downregulates IL-1β-induced IL-6 production
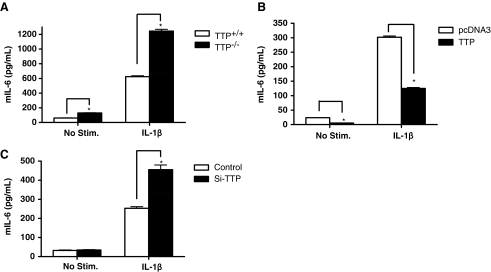
To investigate the function of TTP in vivo, primary MEFs were prepared from wild-type (TTP+/+) and TTP−/− embryos, and stimulated with IL-1β for 24 h. IL-6 production from culture supernatants was examined by enzyme-linked immunosorbent assay (Fig. 1A). Remarkably, TTP−/− MEFs produced a 2-fold increase of IL-6 compared with wild-type counterparts, in the presence or absence of IL-1β. To verify that TTP downregulates IL-6 production, TTP was downregulated by siRNA and overexpressed in MEF cells. Two different siRNAs were used to target TTP, thereby reducing TTP expression to about 50% of its original level by RT-PCR (data not shown). IL-6 production was decreased by overexpression of TTP (Fig. 1B), and increased by silencing TTP (Fig. 1C). These results indicate that TTP inhibits IL-6 production.
FIG. 1.
TTP downregulates IL-6 production. (A) MEFs were cultured in the presence of IL-1β (1 ng/mL) for 24 h. The culture supernatants were harvested, and IL-6 concentrations were measured by enzyme-linked immunosorbent assay (ELISA). (B) TTP+/+ and TTP−/− MEFs were seeded into 12-well plates in duplicate and transfected with empty vector pcDNA3, pcDNA3- TTP. After 24 h, the cells were left untreated or were stimulated with IL-1β (1 ng/mL) for 24 h. IL-6 from culture supernatants was measured by ELISA. (C) TTP−/− MEFs were transfected twice during a period of 4 days with short interfering RNAs (siRNAs) targeting TTP. Cells were treated with IL-1β (1 ng/mL) for 24 h post-transfection and IL-6 concentrations from supernatant were measured by ELISA. Data presented represent the average of duplicate experiments repeated 3 times (n=3; *P<0.05). TTP, tristetraprolin; IL, interleukin; MEF, mouse embryonic fibroblast.
TTP destabilizes endogenous IL-6 mRNA
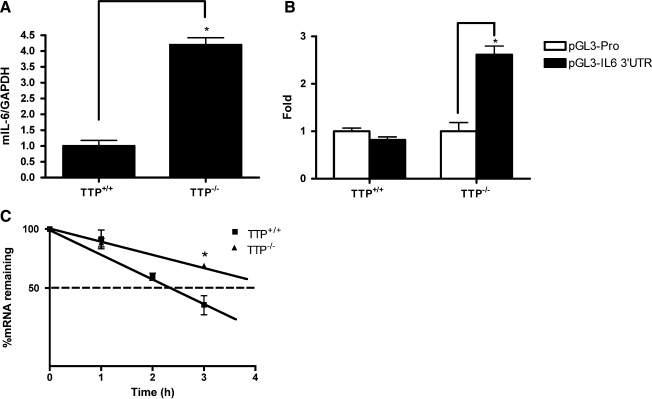
The destabilizing effect of TTP on its target mRNA is associated with the direct binding of TTP via cis-elements at the 3′UTR of the target mRNA. To investigate the mechanism by which TTP regulates IL-6 production, the IL-6 mRNA level was compared between wild-type and TTP−/− MEFs. Total mRNA of wild-type and TTP−/− MEFs were collected after 24 h of IL-1β treatment, and IL-6 mRNA levels were examined by real-time PCR. In TTP−/− MEFs, IL-6 mRNA was upregulated compared with wild-type MEFs (Fig. 2A). To examine the significance of the IL-6 3′UTR, wild-type and TTP−/− MEFs were transiently transfected with control and IL-6 3′UTR reporter constructs (Fig. 2B). TTP−/− MEFs consistently had significantly higher (P<0.05) luciferase activity than wild-type cells. To determine whether TTP regulates IL-6 mRNA turnover, we investigated the half-life of endogenous IL-6 mRNA from wild-type and TTP−/− MEFs (Fig. 2C). After 24 h of IL-1β treatment, Actinomycin D was added to MEFs and IL-6 mRNA level was measured by Real-Time PCR at indicated time points. Figure 2C shows a relatively longer half-life in TTP−/− MEFs (t1/2=5.69 h) than in wild-type cells (t1/2=2.55 h). Differences in mRNA half-life reached significance at 3 h (P=0.0347). These data indicate that IL-6 mRNA was stabilized by TTP.
FIG. 2.
TTP destabilizes endogenous IL-6 mRNA. (A) TTP+/+ and TTP−/− MEFs were treated with IL-1β (1 ng/mL) for 24 h and mRNA was harvested. IL-6 mRNA expression was measured by quantitative real-time PCR and adjusted for GAPDH. (B) TTP+/+ and TTP−/− MEFs were transiently transfected with pGL3promoter, pGL3-IL6-3′-UTR. Renilla luciferase (pEF-RLuc) vector was cotransfected as transfection efficiency control in all transient transfection experiments. Significant differences in reporter expression between TTP+/+ and TTP−/− MEFs are indicated (*P<0.05). The values represent 3 independent experiments, and each experiment was performed in duplicate. (C) TTP+/+ and TTP−/− MEFs were stimulated by IL-1β (1 ng/mL) for 24 h. Actinomycin D (10 μg/mL) was added for indicated time points, and the mRNA level of IL-6 was measured by quantitative real-time PCR. Data represent the average of 2 independent experiments (*P=0.0347). 3′UTR, 3′ untranslated region; PCR, polymerase chain reaction.
Activation of the p38-MAPK pathway abrogates TTP-induced downregulation in IL-6 3′UTR luciferase activity
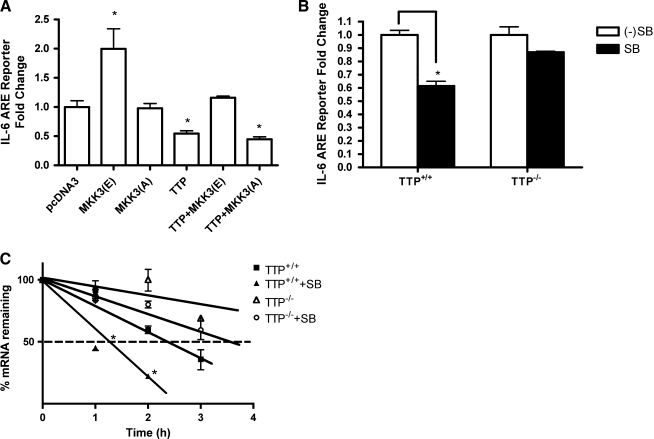
Activation of the p38-MAPK pathway has previously been reported to inhibit TTP and thereby stabilizes ARE-mRNA (Stoecklin and others 2004). Constitutive activation of p38 by MKK3(E) induced a 2-fold increase of luciferase activity in RAW macrophages, whereas the dominant negative MEKK(A) showed no change (Fig. 3A). Overexpression of TTP reduced the reporter-gene activity by 50% compared with the control (pcDNA3). This reduction was abrogated by co-transfection with constitutively active MEKK3(E) but not MEKK(A). These results indicate that activation of the p38 MAPK pathway blocks the TTP-mediated downregulation of IL-6 3′UTR.
FIG. 3.
p38-MAPK pathway is involved in TTP effect on IL-6 3′UTR. (A) Raw cells were transiently co-transfected with pcDNA3, MKK3(E), MKK3(A), and/or TTP with pGL3-IL6-3′-UTR. (B) TTP+/+ and TTP−/− MEFs were transiently transfected with pGL3-IL6-3′-UTR. Cells were stimulated by IL-1β (1 ng/mL) for 24 h with or without p38 inhibitor (SB). (C) TTP+/+ and TTP−/− MEFs were stimulated by IL-1β (1 ng/mL) for 24 h with or without SB inhibitor. Actinomycin D (10 μg/mL) was added, and the mRNA level of IL-6 was measured by quantitative real-time PCR. Data represent the average of 3 independent experiments. *P =0.014 at 2 h and P=0.0229 at 3 h between TTP+/+ and TTP−/− MEFs treated with SB inhibitor.
To further investigate the role of p38 in TTP regulation, TTP+/+ and TTP−/− MEFs were transiently transfected with pGL3 promoter or pGL3-IL6-3′-UTR luciferase reporter genes, and Renilla luciferase (pEF-RLuc) vector as a control for transfection efficiency. In wild-type cells expressing TTP, the p38 inhibitor significantly decreased IL6 reporter activity, whereas in TTP−/− MEFs this effect of p38 was not observed (Fig. 3B). These findings are consistent with a role for p38 in TTP-mediated IL-6 3′UTR luciferase activity. To investigate whether p38 regulates TTP-mediated IL-6 mRNA stability, TTP+/+ and TTP−/− MEFs were stimulated by IL-1β for 24 h followed by incubation with Actinomycin D to arrest ongoing mRNA transcription. The mRNA level of IL-6 was measured by quantitative real-time PCR. Compared with TTP−/− MEFs, TTP+/+ showed reduced stability of IL-6 mRNA, which was amplified in the presence of the p38 inhibitor (Fig. 3C). The half-life of TTP+/+ MEFs is 2.5 h, TTP−/− MEFs is 5.8 h, TTP+/++SB is 1.4 h, and TTP−/− MEFs+SB is 3.6 h. There is a significant difference between TTP+/+ and TTP−/− MEFs, and between TTP+/+ MEFs and TTP+/+ MEFs +SB, but no significant difference between TTP KO and TTP KO+SB. Together, these results show that p38MAPK inhibits TTP-induced downregulation of IL-6 mRNA.
The AU-rich 3′UTR of IL-6 mRNA is a target of TTP
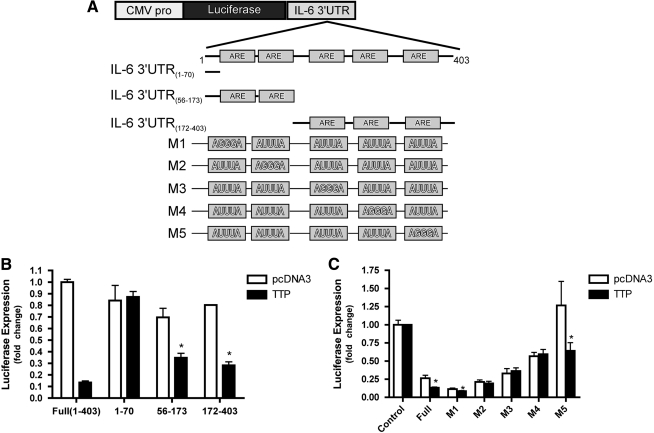
The 3′UTR of IL-6 contains 5 AREs. Since AREs in the 3′-UTR of many cytokine mRNAs are responsible for both mRNA stability and translational control, we examined whether TTP targets the 3′-UTR of IL-6. The full-length 3′UTR of IL-6 was inserted downstream of the luciferase reporter-gene (Zhao and others 2008). Additionally, a series of luciferase reporter gene constructs, containing the various regions of IL-6 3′UTR and the motifs mutated from AUUUA to AGGGA, were generated (Zhao and others 2008).
pGL3-IL-6 3′UTR and control plasmid were transiently transfected into wild-type and TTP−/− MEFs (Fig. 4A). Luciferase activity of the reporter gene with IL-6 3′UTR was increased more than 2-fold in TTP−/− MEFs compared with wild-type MEFs. Overexpression of TTP in wild-type MEFs resulted in a dramatic decrease (∼90%) of luciferase expression of pGL3-IL-6 3′UTR. The luciferase activity of both pGL3-IL-6 3′UTR(56–173) and pGL3-IL-6 3′UTR (172–403) was only reduced ∼50% of control, whereas the decreased expression was abrogated in pGL3-IL-6 3′UTR(1–70), which includes none of the AREs. These findings suggested that TTP represses IL-6 3′UTR luciferase activity in an ARE-dependent manner, and both proximal and distal regions are required for the full reduction.
FIG. 4.
ARE of IL-6 mRNA is a target of TTP regulation. (A) Schematic of IL-6 3′UTR truncation and site-directed mutant reporter constructs (B) Wild-type MEFs were co-transfected with IL-6 3′UTR luciferase reporters [full length (1–403), Non-ARE (1–70), proximal ARE (56–173), and distal ARE (172–403)] along with pcDNA.TTP or pcDNA3 control vector for 48 h. Firefly L-Luciferase values from cell extracts were measured and normalized to full-length reporter (1–403) cotransfected with control vector. Results from 2 independent experiments, performed in duplicate, are presented. Significant differences between full-length reporter and deletion constructs are reported (*P<0.05). (C) p38α+/+ MEFs were transiently co-transfected with pcDNA.TTP or pcDNA3 along with pGL3promoter, pGL3-IL6 3′UTR, or pGL3-IL-6 3′UTR vectors containing the indicated ARE mutants. Results represent 3 independent experiments measured in duplicate. Significant differences in reporter expression between p are indicated (*P<0.05). ARE, AU-rich element.
The full-length IL6 3′UTR and all the mutants (Fig. 4A) were co-transfected with TTP or pcDNA3 in RAW cells. Overexpression of TTP reduced reporter activity of IL-6 3′UTR to 50% of parental control vector, whereas this reduction is completely lost with M2, M3, and M4. TTP reduced 26% of reporter activity with M1, whereas 50% with M5, similar to that observed with the wild-type IL6 3′UTR. These data strongly indicate that ARE5 is not required, whereas ARE2, ARE3, and ARE4 are essential for targeting TTP, and ARE1 is involved in this targeting.
p38α deficiency increased the affinity of TTP to IL-6 3′UTR
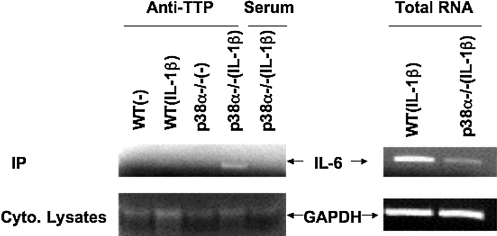
Finally, we were interested to determine whether p38α signaling could regulate the ability of TTP to bind the IL-6 3′UTR. An immunoprecipitation (IP) assay was carried out under conditions that preserved endogenous RNP associations. Following RT-PCR analysis of the IP material employing IL-6 3′UTR specific oligomers, an IL-6 product was readily detected in the IP material obtained using an anti-TTP antibody in p38α−/− MEF cells stimulated by IL-1β, whereas only residual amplification was observed in wild-type MEF cells or serum control IP (Fig. 5). RT-PCR results from total RNA showed that actually p38α−/− MEF cells produced less IL-6 mRNA than that found in IL-1β stimulated wild-type cells. These data strongly indicate that TTP increases its ability to bind IL6 3′UTR in p38α−/− MEF cells.
FIG. 5.
p38α deficiency increased the affinity of TTP to IL-6 3′UTR. p38 wild-type (WT) or p38α−/− MEFs cells were either unstimulated or stimulated with IL-1β for 4 h. Protein A agarose beads coated with either anti-TTP or control normal rabbit serum were immunoprecipitated with cell lysates from indicated MEF cells with or without IL-1β stimulation. RNA was extracted from IP products and reverse transcription (RT)-PCR was performed to evaluate cDNA amplification of IL-6 or control GAPDH. Right panel RT-PCR results are shown from preimmunopreciptated total RNA in IL-1β-stimulated WT or p38α−/− MEF cells. Representative data from 2 independently performed experiments are presented. IP, immunoprecipitaion.
Discussion
Cells of the immune system keep tight control over the production of potentially harmful cytokines by repressing their expression at the post-transcriptional level. The ARE, located in the 3′UTR of many cytokines (eg, GM-CSF, TNFα, IL-2, IL-3, and IL-6) and other proinflammatory factors (eg, COX-2 and MMP-13), plays a major role in post-transcriptional repression (Bakheet and others 2003; Khabar and others 2005; Khabar 2010). AREs regulate mRNA stability via interactions with sequence-specific RNA-BPs, which influence 2 critical steps of mRNA decay: deadenylation and/or the subsequent 3′ to 5′ exonuclease-mediated degradation. Despite the knowledge that over 20 different proteins can bind to ARE segments, only a subset of these RNA-BPs has been shown to influence the stability or translational efficiency of their target mRNAs. TTP and TTP-related proteins, BRF1 and BRF2, have a major regulatory role in controlling cytokine mRNAs (Stoecklin and others 2002; Raineri and others 2004; Lykke-Andersen and Wagner 2005). The present study expands this basic information to show that TTP regulates IL-1β-induced IL-6 expression through interactions with multiple IL-6 ARE elements in a p38 MAPK-dependent manner.
The function of TTP was elucidated through several studies using TTP-deficient mice (Varnum and others 1991). TTP−/− mice were shown to develop a generalized inflammatory condition, including an arthritic-like syndrome secondary to increased TNFα and GM-CSF levels (Taylor and others 1996). In TTP−/− mice, the increased cytokine production was shown to be a result of increased mRNA stability (Carballo and others 1998, 2000). In this study, MEF cells were established from TTP+/+ and TTP−/− mouse embryos to examine the direct role of TTP in IL-6 regulation. Consistent with previous observations, IL-6 expression is not elevated in TTP−/− MEFs until stimulated with IL-1β where significantly more IL-6 production was observed versus TTP+/+ MEFs. Similarly, data shown in this report indicate that siRNA against TTP increased inducible IL-6 expression. Recently, our research group has shown a similar effect with regard to IL-6 expression in oral cancer cells where TTP is silenced (Van Tubergen and others 2010). Nonphosphorylated TTP usually binds to mRNAs to promote rapid degradation. Overexpression studies have confirmed that TTP as well as TTP family members, BRF1 and BRF2, induce the degradation of mRNAs containing cytokine AREs (Jiang and others 2002; Stoecklin and others 2003; Sully and others 2004). Data from the present study is consistent with these observations where TTP overexpression decreases IL-1β-stimulated IL-6 expression. Consistent with TTP overexpression data, we observed that IL-1β-induced IL-6 mRNA was significantly more expressed in TTP-deficient cells with an increased half-life of the IL-6 mRNA.
The p38 MAPK pathways have been shown in a variety of systems to be an integral signaling component involved with cytokine mRNA stability (Winzen and others 1999). Previous data from our group have indicated that IL-6 regulation is highly dependent on p38 MAPK signaling in a variety of cell types (Patil and others 2004, 2006; Rossa and others 2006, 2008; Kirkwood and others 2007; Zhao and others 2008). The clinical significance of this regulation was also demonstrated through inhibition of inflammation and bone loss using small molecule inhibitors against p38 MAPK (Kirkwood and others 2007) and more recently using siRNA strategies targeting the p38 downstream kinase, MK2 (Li and others 2011). As part of these studies, we demonstrated that activation of p38 MAPK via overexpression of a constitutively active form of MAPK Kinase-3 (MKK3E) was able to induce IL-6 ARE reporter activity compared with the dominant negative form of MKK3 (MKK3A) but when TTP was co-expressed with MKK3A then TTP was able to repress the inductive capacity of MKK3E. Additionally, in TTP-deficient cells IL-6 ARE reporter activity was lost in the presence of a p38 MAPK inhibitor, suggesting that TTP effects on IL-6 are highly dependent upon the phosphorylation status of TTP. We also demonstrated that the these effects were not limited to the ARE reporter since IL-6 mRNA was not affected in TTP-deficient cells treated with a p38 MAPK inhibitor compared with wild-type cells. We conclude from these studies that the TTP directs mRNA stability of IL-6 largely in a p38 MAPK-dependent manner.
There are 5 AREs within the 3′UTR of the IL-6 mRNA that contribute toward the IL-6 mRNA stability. Previous data from our group have defined several p38 MAPK-dependent AREs within the 3′UTR of the IL-6 mRNA (Zhao and others 2008). In these studies, multiple ARE elements were found to be p38 dependent in reporter transactivation and mRNA decay studies. The present study extends this information to show that TTP can only reduce IL-6 ARE reporter activity with the reporter constructs that contain ARE cis elements within the reporter. Compared with vector control, TTP reduced ARE reporter activity in both proximal (IL-656–173) and distal (IL-6172–403) reporters but not reporters that do not contain an ARE (IL-61–70). Using site-directed mutagenesis, AREs 1–5 have been independently eliminated of each of the IL-6 ARE elements. Deletion or mutation of AUUUA motifs could alter the components of mRNA-trans-factor complex and affect the luciferase activity of reporter gene containing the IL-6 3′-UTR with mutated AUUUA motifs. Using this approach, we identified ARE1, ARE2, and ARE5 as the cis-elements regulated by p38α in IL-6–3′-UTR (Zhao and others 2008). In those studies, mutation of ARE1 and ARE2 resulted in the enhanced expression due to the deficiency of p38α phosphorylation, whereas mutation of ARE5 decreased the luciferase reporter expression in the presence of p38α, indicating that p38α is required for the ARE1 and ARE2 to repress expression and for ARE5 to enhance expression. Data from the present study indicate that ARE2, ARE3, and ARE4 are necessary for TTP to repress IL-6 3′UTR expression. Thus, although previous data suggest that ARE5 may be the most important ARE target of IL-6 regulation in a p38 MAPK-dependent manner, different ARE elements are required for TTP-mediated decay of the IL-6 mRNA. However, we cannot directly conclude this based upon the data presented here and ongoing studies are being directed toward understanding the molecular nature of ARE2, 3, and 4 and TTP interaction.
Molecular action of TTP involves phosphorylation and cytoplasmic localization. TTP binds to AREs of cytokine genes and targets them to the exosome for rapid degradation (Lai and others 1999; Chen and others 2001). p38/MK2 signaling is required for TTP phosphorylation (Carballo and others 2001; Mahtani and others 2001; Zhu and others 2001), which promotes nuclear export of TTP, an event that is partially dependent upon binding to 14–3-3 proteins, through phosphoserine residues (Johnson and others 2002). Additionally, 14–3-3 inhibits the activity of TTP by preventing TTP association with stress granules where ARE-mRNA decay occurs (Stoecklin and others 2004). Taken together, these data support the importance of TTP cytoplasmic localization and highlight the complexity of cytokine regulation and importance of spatially separate mRNA pools within the cytoplasm. To help delineate the role of TTP to interact with IL-6 3′UTR within the cytoplasm, RNA immunoprecipitation experiments were conducted. Data from these experiments suggest that in IL-1β-stimulated cells, there is a higher affinity for TTP to interact with IL-6 mRNA in the absence of p38 MAPK. Collectively, all of these data indicate the IL-6 mRNA stability is regulated by TTP through a change in affinity for the transcript that occurs in a p38 MAPK-dependent manner and involves specific AREs within the 3′UTR of the IL-6 mRNA.
Acknowledgments
This work was supported by Department of Defense W81XWH-05-1-0075, NIH R21DE017966, R01DE018290, R01DE018512, K02 DE019513-01, and P20RR017696.
Author Disclosure Statement
The authors disclose that they do not have any commercial associations that might cause a conflict of interest in connection with submitted article.
References
- Adachi Y. Yoshio-Hoshino N. Nishimoto N. The blockade of IL-6 signaling in rational drug design. Curr Pharm Des. 2008;14(12):1217–1224. doi: 10.2174/138161208784246072. [DOI] [PubMed] [Google Scholar]
- Bakheet T. Williams BR. Khabar KS. ARED 2.0: an update of AU-rich element mRNA database. Nucleic Acids Res. 2003;31(1):421–423. doi: 10.1093/nar/gkg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E. Cao H. Lai WS. Kennington EA. Campbell D. Blackshear PJ. Decreased sensitivity of tristetraprolin-deficient cells to p38 inhibitors suggests the involvement of tristetraprolin in the p38 signaling pathway. J Biol Chem. 2001;276(45):42580–42587. doi: 10.1074/jbc.M104953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E. Lai WS. Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281(5379):1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Carballo E. Lai WS. Blackshear PJ. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95(6):1891–1899. [PubMed] [Google Scholar]
- Chen CY. Gherzi R. Ong SE. Chan EL. Raijmakers R. Pruijn GJ. Stoecklin G. Moroni C. Mann M. Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107(4):451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- Hirano D. Nagashima M. Ogawa R. Yoshino S. Serum levels of interleukin 6 and stress related substances indicate mental stress condition in patients with rheumatoid arthritis. J Rheumatol. 2001;28(3):490–495. [PubMed] [Google Scholar]
- Jiang Y. Mehta CK. Hsu TY. Alsulaimani FF. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect Immun. 2002;70(6):3143–3148. doi: 10.1128/IAI.70.6.3143-3148.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Stehn JR. Yaffe MB. Blackwell TK. Cytoplasmic localization of tristetraprolin involves 14–3-3-dependent and -independent mechanisms. J Biol Chem. 2002;277(20):18029–18036. doi: 10.1074/jbc.M110465200. [DOI] [PubMed] [Google Scholar]
- Kastritis E. Charidimou A. Varkaris A. Dimopoulos MA. Targeted therapies in multiple myeloma. Target Oncol. 2009;4(1):23–36. doi: 10.1007/s11523-008-0102-9. [DOI] [PubMed] [Google Scholar]
- Kasuga I. Makino S. Kiyokawa H. Katoh H. Ebihara Y. Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92(9):2399–2405. doi: 10.1002/1097-0142(20011101)92:9<2399::aid-cncr1588>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Khabar KS. The AU-rich transcriptome: more than interferons and cytokines, and its role in disease. J Interferon Cytokine Res. 2005;25(1):1–10. doi: 10.1089/jir.2005.25.1. [DOI] [PubMed] [Google Scholar]
- Khabar KS. Rapid transit in the immune cells: the role of mRNA turnover regulation. J Leukoc Biol. 2007;81(6):1335–1344. doi: 10.1189/jlb.0207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KS. Post-transcriptional control during chronic inflammation and cancer: a focus on AU-rich elements. Cell Mol Life Sci. 2010;67(17):2937–2955. doi: 10.1007/s00018-010-0383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KS. Bakheet T. Williams BR. AU-rich transient response transcripts in the human genome: expressed sequence tag clustering and gene discovery approach. Genomics. 2005;85(2):165–175. doi: 10.1016/j.ygeno.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kirkwood KL. Li F. Rogers JE. Otremba J. Coatney DD. Kreider JM. D'Silva NJ. Chakravarty S. Dugar S. Higgins LS. Protter AA. Medicherla S. A p38alpha selective mitogen-activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther. 2007;320(1):56–63. doi: 10.1124/jpet.106.112466. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A. Gaestel M. Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem Soc Trans. 2002;30(Pt 6):959–963. doi: 10.1042/bst0300959. [DOI] [PubMed] [Google Scholar]
- Lai WS. Carballo E. Strum JR. Kennington EA. Phillips RS. Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19(6):4311–4323. doi: 10.1128/mcb.19.6.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q Yu H. Zinna R. Martin K. Herbert B. Liu A. Rossa C., Jr. Kirkwood KL. Silencing MAP kinase-activated protein kinase-2 arrests inflammatory bone loss. J Pharmacol Exp Ther. 2011;336(3):633–642. doi: 10.1124/jpet.110.172395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J. Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19(3):351–361. doi: 10.1101/gad.1282305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani KR. Brook M. Dean JL. Sully G. Saklatvala J. Clark AR. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol Cell Biol. 2001;21(19):6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N. Interleukin-6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010;87(4):483–487. doi: 10.1038/clpt.2009.313. [DOI] [PubMed] [Google Scholar]
- Patil C. Rossa C., Jr. Kirkwood KL. A. actinomycetemcomitans LPS induces IL-6 expression through multiple MAPK pathways in periodontal ligament fibroblasts. Oral Microbiol Immunol. 2006;21(6):392–398. doi: 10.1111/j.1399-302X.2006.00314.x. [DOI] [PubMed] [Google Scholar]
- Patil C. Zhu X. Rossa C., Jr. Kim YJ. Kirkwood KL. p38 MAPK regulates IL-1beta induced IL-6 expression through mRNA stability in osteoblasts. Immunol Invest. 2004;33(2):213–233. doi: 10.1081/imm-120034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil CS. Kirkwood KL. p38 MAPK signaling in oral-related diseases. J Dent Res. 2007;86(9):812–825. doi: 10.1177/154405910708600903. [DOI] [PubMed] [Google Scholar]
- Patil CS. Liu M. Zhao W. Coatney DD. Li F. VanTubergen EA. D'Silva NJ. Kirkwood KL. Targeting mRNA stability arrests inflammatory bone loss. Mol Ther. 2008;16(10):1657–1664. doi: 10.1038/mt.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri I. Wegmueller D. Gross B. Certa U. Moroni C. Roles of AUF1 isoforms, HuR and BRF1 in ARE-dependent mRNA turnover studied by RNA interference. Nucleic Acids Res. 2004;32(4):1279–1288. doi: 10.1093/nar/gkh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby WF. Roy K. Collins J. Rigby S. Connolly JE. Bloch DB. Brooks SA. Structure/function analysis of tristetraprolin (TTP): p38 stress-activated protein kinase and lipopolysaccharide stimulation do not alter TTP function. J Immunol. 2005;174(12):7883–7893. doi: 10.4049/jimmunol.174.12.7883. [DOI] [PubMed] [Google Scholar]
- Rossa C., Jr. Ehman K. Liu M. Patil C. Kirkwood K. MKK3/6-p38 MAPK signaling is required for IL-1β And TNF-α induced RANKL expression in bone marrow stromal cells. J Interferon Cytokine Res. 2006;26(10):719–729. doi: 10.1089/jir.2006.26.719. [DOI] [PubMed] [Google Scholar]
- Rossa C., Jr. Liu M. Kirkwood KL. A dominant function of p38 mitogen-activated protein kinase signaling in receptor activator of nuclear factor-kappaB ligand expression and osteoclastogenesis induction by Aggregatibacter actinomycetemcomitans and Escherichia coli lipopolysaccharide. J Periodontal Res. 2008;43(2):201–211. doi: 10.1111/j.1600-0765.2007.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler H. Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36(Pt 3):491–496. doi: 10.1042/BST0360491. [DOI] [PubMed] [Google Scholar]
- Stoecklin G. Colombi M. Raineri I. Leuenberger S. Mallaun M. Schmidlin M. Gross B. Lu M. Kitamura T. Moroni C. Functional cloning of BRF1, a regulator of ARE-dependent mRNA turnover. EMBO J. 2002;21(17):4709–4718. doi: 10.1093/emboj/cdf444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklin G. Gross B. Ming XF. Moroni C. A novel mechanism of tumor suppression by destabilizing AU-rich growth factor mRNA. Oncogene. 2003;22(23):3554–3561. doi: 10.1038/sj.onc.1206418. [DOI] [PubMed] [Google Scholar]
- Stoecklin G. Stubbs T. Kedersha N. Wax S. Rigby WF. Blackwell TK. Anderson P. MK2-induced tristetraprolin:14–3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23(6):1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sully G. Dean JL. Wait R. Rawlinson L. Santalucia T. Saklatvala J. Clark AR. Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1) Biochem J. 2004;377(Pt 3):629–639. doi: 10.1042/BJ20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GA. Carballo E. Lee DM. Lai WS. Thompson MJ. Patel DD. Schenkman DI. Gilkeson GS. Broxmeyer HE. Haynes BF. Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4(5):445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- Van Tubergen E. Vander Broek R. Lee J. Wolf G. Carey T. Bradford C. Prince M. Kirkwood KL. D'Silva NJ. Tristetraprolin regulates IL-6 which is correlated with tumor progression in head and neck squamous cell carcinoma. Cancer. 20102011 doi: 10.1002/cncr.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum BC. Ma QF. Chi TH. Fletcher B. Herschman HR. The TIS11 primary response gene is a member of a gene family that encodes proteins with a highly conserved sequence containing an unusual Cys-His repeat. Mol Cell Biol. 1991;11(3):1754–1758. doi: 10.1128/mcb.11.3.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldner MJ. Neurath MF. Cytokines in colitis associated cancer: potential drug targets? Inflamm Allergy Drug Targets. 2008;7(3):187–194. doi: 10.2174/187152808785748137. [DOI] [PubMed] [Google Scholar]
- Winzen R. Kracht M. Ritter B. Wilhelm A. Chen CY. Shyu AB. Muller M. Gaestel M. Resch K. Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18(18):4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshio-Hoshino N. Adachi Y. Aoki C. Pereboev A. Curiel DT. Nishimoto N. Establishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumor. Cancer Res. 2007;67(3):871–875. doi: 10.1158/0008-5472.CAN-06-3641. [DOI] [PubMed] [Google Scholar]
- Zhao W. Liu M. Kirkwood KL. p38alpha stabilizes interleukin-6 mRNA via multiple AU-rich elements. J Biol Chem. 2008;283(4):1778–1785. doi: 10.1074/jbc.M707573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W. Brauchle MA. Di Padova F. Gram H. New L. Ono K. Downey JS. Han J. Gene suppression by tristetraprolin and release by the p38 pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281(2):L499–L508. doi: 10.1152/ajplung.2001.281.2.L499. [DOI] [PubMed] [Google Scholar]