Abstract
Aedes aegypti and Aedes albopictus are competent natural and laboratory vectors for numerous arthropod-borne viruses (arboviruses), many of which pose global public health concerns. Efficiently imbibing a blood meal from an artificial membrane feeder, Ae. aegypti is an easy feeder: ∼96% success. Alternatively, Ae. albopictus is known to be a difficult feeder imbibing poorly: ∼20% success. Adult female mosquitoes were grouped in cohorts of 50, proffered a bovine blood meal, and challenged with experimental variables, and feeding success was documented. Controls included Ae. aegypti and the artificial glass membrane feeder: topside presentation (upside-down feeding position only). Variables included lambskin versus bovine collagen sausage membranes, presence or absence of gentle motion, filial generations, and large or small blood packets positioned differently: horizontal presentation (right side-up or nose-up feeding position) and vertical presentation (nose-up feeding position only). Both species preferred sausage casings, and ultrastructural analysis revealed that sausage casings had a textured gripping surface not observed on lambskin membranes. Neither filial generations nor gentle motion improved feeding; however, a 32%–46% increase in blood feeding was observed when Ae. albopictus fed on large horizontal and large or small vertical blood packets. Upside-down feeding of Ae. albopictus with a blood suspension of Sindbis virus heat resistant (SVHR) and the original isolate (AR339) resulted in virus dissemination of 10% and 50%, respectively. Use of bovine collagen sausage membranes in a vertical feeding position will increase the number of engorged females, thereby substantially increasing the number of arbovirus-exposed organisms in the laboratory. Differences in blooding success in response to feeding position further separates the behavior attributes of two Aedine species. Blood meal presentation facilitates gravity and we suggest this is a deciding factor in the feeding success of Ae. albopictus.
Key Words: Blood feeder, Feeding position, Mosquitoes, Sausage membrane, Sindbis virus
Introduction
Aedes aegypti, the Yellow Fever mosquito, and Aedes albopictus, the Asian tiger mosquito, are common container-breeding species encountered worldwide. Both species are found in the United States and coexist as domestic mosquitoes in many urban areas of Florida (Alto et al. 2003). First detected in Harris County, Texas, in 1985 (Sprenger and Wuithiranyagool 1986), Ae. albopictus continued to spread and in 2001 was found breeding in California (Gratz 2004). Now residing in 866 counties in 26 states, Ae. albopictus has been dubbed the most invasive mosquito in the world (Andreadis 2009). This invasiveness is closely related to the decline and displacement of Ae. aegypti in rural and suburban areas (O'Meara et al. 1995, Alto et al. 2003, Gratz 2004) and because of these geographical changes, Ae. albopictus is speculated to become the principle vector of arboviruses in the United States (Gratz 2004).
Ae. albopictus is a competent laboratory vector for at least 22 arboviruses (Andreadis 2009), many of which are emerging and re-emerging global health concerns (Moore and Mitchell 1997, Delatte et al. 2010). Vector competence for Dengue, Yellow Fever, Rift Valley fever, and Eastern Equine Encephalitis viruses has been demonstrated (Moore and Mitchell 1997). Considered an opportunistic feeder when compared with Ae. aegypti (Ponlawat and Harrington 2005), females take advantage of accessible blood meals, utilize a broad range of target hosts, and feed on mammals and avian hosts to remain reproductively active (Moore and Mitchell 1997, Richards et al. 2006). Opportunistic feeding behavior fuels the rapid, widespread invasiveness of Ae. albopictus, and choosing a less preferential host (Davies 1990) can often serve to bridge arbovirus transmission between humans and other animals (Gad et al. 1999, Richards et al. 2006). Ae. albopictus also participates in interrupted feeding, whereby the mosquito travels to consume multiple blood meals at a time (Hodgson et al. 2001, Delatte et al. 2010). Broad geographic distribution, virus susceptibility, opportunistic feeding, wide host preference, and interrupted feeding, taken together, directly promote arbovirus transmission.
In nature, a blood meal is directly essential for energy and reproduction, and indirectly responsible for arbovirus transmission. In the laboratory, blood feeding is necessary for colony maintenance and for virus–host investigations. Intrathoracic inoculation is an effective way to infect insects (Rosen and Gubler 1974); however, because this reliable method bypasses gut barriers (Kramer et al. 1981), study of virus dissemination is precluded. Numerous techniques for blood meal ingestion have been published (Jones and Potter 1972, Gerberg et al. 1994, Higgs and Beaty 1996, Mourya et al. 2000, Tseng 2003). Ae. aegypti, an easy feeder, is known to imbibe successfully on artificial apparatuses, whereas Ae. albopictus, a difficult feeder, shows resistance preferring to feed on live animals. Alto and colleagues (2003) determined that Ae. aegypti and Ae. albopictus consistently fed more successfully from restrained chickens compared with an artificial (silicon) membrane system. Although live, small viremic hosts are preferable, a glass membrane feeder is a valuable alternative for oral inoculation of arboviruses (Rutledge et al. 1964).
This report offers modifications to blood meal delivery that increase the feeding success of a recalcitrant feeder, Ae. albopictus. Ae. aegypti and the glass membrane feeder served as controls and variables included lambskin versus bovine collagen sausage membranes, filial generation, presence or absence of gentle motion, large or small blood packets, and varied feeding positions. Recommended modifications can be incorporated in any laboratory equipped to work with hematophagous insects and will provide the researcher with a larger number of colony and/or infectious blood fed mosquitoes with which to work.
Materials and Methods
Hatching and rearing of mosquitoes
Ae. aegypti (Orlando) and Ae. albopictus (Skuse) were hatched, reared, and maintained in the University of North Florida insectary maintained at 25.5°C ± 0.5°C, 70%–80% relative humidity, and 16:8 (light:dark) photoperiod as previously described (Bowers et al. 1995). Female and male adults were housed together for 5 days in white bucket cages topped with mosquito netting, supplied ad lib with honey-soaked cellucotton, and water-saturated cotton. Since both species mate quickly without need for forced-copulation techniques (Akey and Jones 1968), 5 days in mixed-gender cages should provide ample time for mating. Un-blooded females day 5–7 postemergence were separated from males 48 h before feeding experiments and all trials were conducted in triplicate.
Artificial blood feeding
Fifty females were caged together for 48 h, deprived of carbohydrates for 24 h, and proffered defibrinated bovine blood (Colorado Serum Inc.) at 37°C in artificial feeders for 1 h. Mosquitoes were placed on a chill plate, separated to calculate percent fed, and engorged females were returned to cages and supplied fresh carbohydrate and water sources. Eggs collected on oviposition sites were stored in humidity chambers in insectary conditions (Clements 1992, Gerberg et al. 1994).
Traditional artificial glass membrane feeder
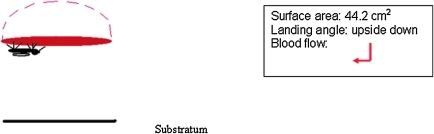
Membranes were secured to glass membrane feeders (Rutledge et al. 1964) with rubber bands, filled with 10 mL blood, and connected to a water bath pump set at 37°C to simulate human body temperature. Water pumped through the water-jacketed feeder created a gentle motion, and feeders were placed on top of screened cages for 1 h (Model 1; upside-down feeding only).
Model 1.
Mosquito feeding position is upside-down only on traditional glass membrane feeder. The angle of blood flow is indicated by a red arrow.
Blood feeding variables
1. Filial Generation. Two Ae. albopictus, parental lines were initiated: P1 and P2. Mosquitoes were fed using a glass membrane feeder with lambskin membrane (Trojan®; Brand Condoms). Blooded females were isolated; eggs were collected and hatched in attempts to select for a membrane-friendly colony. This was repeated for the F1 and F2 generations.
2. Lambskin Membranes versus Bovine Collagen Sausage Casings on the Glass Feeder. Lambskin membranes were rinsed three times in tap water for a total of 30 min and cut-to-fit feeder. Bovine collagen sausage casings (The Sausage Maker Inc.) were wetted with tap water to soften and cut into 12-cm-long strips.
3. Large and Small Sausage Packets. Prewarmed (37°C) blood was added to large sausage casings (32 mm diameter) filled with 50 mL and small sausage casings (22 mm diameter) filled with 15 mL. Dialysis clamps at both end insured that blood packets were held closed and taunt.
4. Gentle Motion. Agitation intended to simulate a living organism was introduced by placing a magnetic stir bar (octagonal, 5/8 inch length by 5/16 inch diameter; Fisher Scientific) inside each sausage blood packet. One packet was laid horizontal on the cage floor, each cage was placed on a stir plate, and stirring was set at a constant low speed.
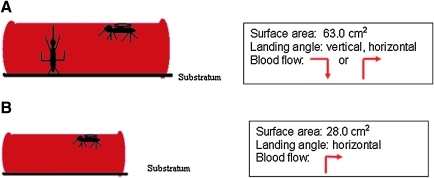
5. Position of Feeder. Feeding position was assayed by placing blood packets horizontal on the floor of cages (Model 2A large and Model 2B small; upright horizontal or nose-up feeding position) or hanging vertically through the mosquito netting (Model 3; nose-up feeding position only). Feeding success was compared with the control traditional feeder (Model 1; upside-down feeding position).
Model 2.
(A) Large and (B) Small. Mosquito feeding is right side-up or nose up on the large horizontal sausage packet and right side-up only on the small horizontal sausage packet. The angle of blood flow is indicated by a red arrow.
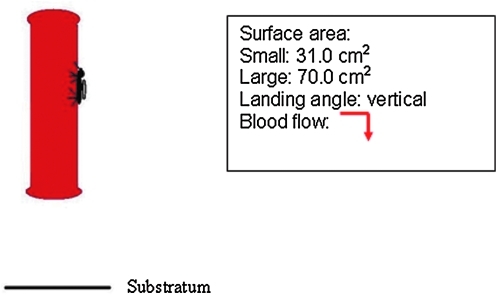
Model 3.
Mosquito feeding position is nose-up only on large or small vertical sausage packets. The angle of blood flow is indicated by a red arrow.
Ultrastructural analysis of membrane surface
Lambskin sausage membranes were washed and wetted with tap water as described above. Membrane sources were cut into 2 cm2 swatches and placed on aluminum stubs with double-sided carbon adhesive tape. Specimens were observed in a FEI Quanta 200 environmental scanning electron microscope under hard vacuum, operated at 2 kV and a spot size of 1. Specimens were viewed at magnifications of 46×, 300×, and 1600× and labeled with micron markers, and digital images were captured.
Infectious blood meal
Females were proffered Sindbis virus (SIN) infectious blood via an artificial glass feeder using lambskin membranes. Two variants of SIN virus, Sindbis virus heat resistant (SVHR) and the original isolate AR339 (ATCC), were added at a titer of ×107 and ×108 pfu/mL, respectively. After one-hour exposure to the viremic blood meal, fully engorged mosquitoes were isolated and incubated at insectary conditions for 10 days.
Leg assay of infectious virus
One leg from each individual was collected at day 10 postvirus exposure, placed in a prelubricated conical vial (Sigma Aldrich) with glass beads (2 mm; Fisher), and triturated on a vortex for 2 min in 3% fetal calf serum in phosphate-buffered saline buffer at 7.4 pH (Bowers et al. 1995). Supernatant was adsorbed on preconfluent BHK-21 cells grown in six-well plates for 1 h at room temperature on a rocker at low speed. Fresh minimal essential cell medium was added to all culture plates, and incubated at 37°C and 5% CO2. Detection of cytopathic effect at 24 or 48 h incubation indicated virus dissemination.
Results
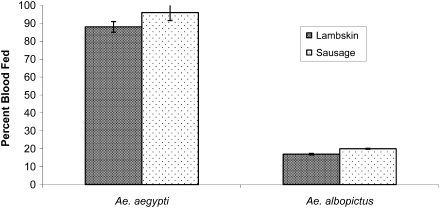
Comparison of blood feeding success between Ae. aegypti and Ae. albopictus using a glass membrane feeder fitted with either lambskin or sausage membrane and gentle pump motion is observed (Fig. 1). Ae. aegypti fed an average of 88% ± 3% using lambskin and 96% ± 4.5% using sausage membrane, and Ae. albopictus fed an average of 17% ± 0.44% using lambskin and 20% ± 0.33% using sausage membrane. In the upside-down feeding position (Model 1), Ae. aegypti blood fed significantly better than Ae. albopictus, and both species fed better on sausage membranes compared to lambskin.
FIG. 1.
Aedes aegypti blood fed more successfully than Aedes albopictus on the traditional artificial glass membrane feeder (upside-down feeding position), and both species fed better on bovine sausage casings compared to lambskin membranes. Ae. aegypti feeding success on the lambskin membrane (88% ± 3%) improved on the sausage casing (96% ± 4.5%). Ae. albopictus feeding success on the lambskin membrane (17% ± 0.44%) improved on the sausage casing (20% ± 0.3%).
Effect of filial generations on blood feeding is documented in Table 1. Blood feeding success was acceptable (88% ± 3%) for Ae. aegypti and subsequent progeny were not analyzed. Blood feeding by Ae. albopictus increased slightly in subsequent generations, P1 = F1 (16%) to F2 (23.5%) and P2 = F1 (13.5%) to F2 (29%), but never approached the success of Ae. aegypti. Because egg production dwindled, experiments ceased at F2 generation.
Table 1.
Comparison of Filial Generation to Blood Feeding Success on a Traditional Glass Membrane (Upside-Down Feeding Position) with a Lambskin Membrane
| Generationa | Aedes aegypti percent blood fed | Aedes albopictus (P1) percent blood fed | Aedes albopictus (P2) percent blood fed |
|---|---|---|---|
| Parental | 88 | 16 | 13.5 |
| F1 | NA | 21 | 34 |
| F2 | NA | 23.5 | 29 |
Filial generation.
NA, not assayed.
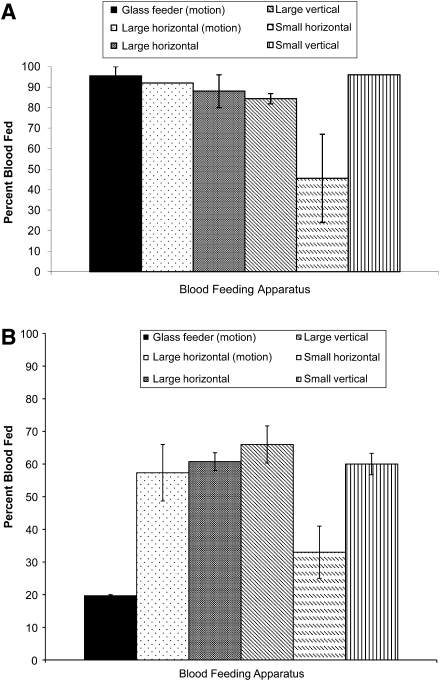
Challenging mosquitoes with different feeding opportunities resulted in changes in feeding successes. Compared to the glass feeder (Model 1; control), Ae. aegypti fed well in all groups (86% ± 2.5%–96%) except the small horizontal sausage packet (Model 2B; Fig. 2A) where feeding was significantly less (67% ± 21.5%). Ae. albopictus fed significantly better in all groups (57% ± 8.6%–66% ± 5.7%; Fig. 2B) compared to the control (17%–20%) except the small horizontal blood packet (Model 2B; 33% ± 8.0%).
FIG. 2.
Analysis of blood feeding success in response to gentle motion, blood meal packet surface area, and position. Bovine sausage casings and bovine blood were used for all groups. Motion did not significantly improve feeding success. (A) Ae. aegypti fed successfully on all feeders compared with the glass feeder (control), except the small horizontal blood packet. (B) Ae. albopictus fed significantly better on all feeders compared to the glass feeder (control). While feeding success on the small horizontal blood packet was enhanced over the glass feeder, it was significantly less compared with all other experimental trials.
Differences in blood feeding success between Ae. aegypti and Ae. albopictus, relative to feeding position, are presented in Table 2. Both species fed better on vertical sausage packets (Model 3) compared with horizontal sausage packets (Model 2A, B). Relative to feeding on a glass membrane feeder, Ae. albopictus demonstrated an advantage in both horizontal (+32) and vertical (+46) presentations over Ae. aegypti (horizontal −19 and vertical −6). Ae. aegypti demonstrated a substantial advantage in the upside-down position (Model 1; 76%), whereas this advantage decreased in the horizontal (25%) and vertical (24%) feeding positions. Ultrastructural investigation of membrane surfaces revealed visible differences; the lambskin was smooth, the small sausage was moderately textured, and the large sausage was greatly textured (Fig. 3). Oral blood feeding of Ae. albopictus with SIN variants using the glass membrane feeder results in disseminated infections (10% with SVHR and 50% with AR339; Table 3). Virus suspensions were proffered to cohorts of 100 in the upside-down position and 10% and 20% of the mosquitoes fed, respectively.
Table 2.
Percent Blooding Success Compared to Feeding Position
| |
Percent feeding in response to feeding position |
||
|---|---|---|---|
| Species | Traditional glass membrane feeder | Vertical | Horizontal |
| Ae. aegypti | 96 | 90 (−6)a | 77 (−19) |
| Ae. albopictus | 20 | 66 (+46) | 52 (+32) |
| 76b | 24 | 25 | |
Percent difference in feeding success compared to control.
Percent difference in feeding between Ae. aegypti (control) and Ae. albopictus (handicap).
FIG. 3.
High-resolution ultrastructural analysis of membrane surface architecture found on lambskin (A), small sausage (B), and large (C) sausage sources. All SEM observations were conducted under hard vacuum at 2 kV using a spot size of 1 and images captured at 300 ×.
Table 3.
Dissemination of Sindbis Virus Aedes albopictus
| Virus titer in blood meal (pfu/mL blood) | Percent fed | Percent disseminationa |
|---|---|---|
| SVHR (×107) | 10 | 10 (1/10)b |
| AR339 (×108) | 20 | 50 (10/20) |
Dissemination at day 10 postinfection as determined by leg assay.
Number of virus-positive mosquitoes/total number mosquitoes.
Discussion
Ae. aegypti and Ae. albopictus, two related mosquitoes found worldwide, demonstrated an intraspecific difference when proffered a blood meal via a glass membrane feeder. Ae. aegypti fed significantly better than Ae. albopictus in the upside-down position. Artificial feeders are frequently used for blood feeding in the laboratory (Higgs and Beaty 1996); while some mosquito species feed successfully, others are recalcitrant, that is, Ae. albopictus. Phagostimulants such as ATP (Galun 1967), L-lactic acid (Davis and Solove 1976), and natural odor ligands from human skin (Ghaninia et al. 2008) result in varying degrees of success. A colony of Ae. albopictus was maintained by 30%–32% blood feeding success on a Para film membrane wrapped in fiberglass (Mourya et al. 2000, Tseng 2003). Ooi and colleagues (2005) investigated local membrane sources, including skin of chicken, fish, cattle, and salted sausage, and reported that Ae. albopictus fed on human blood at 50% success using chicken skin and 57% using cattle skin. We observed increased feeding success on an artificial glass feeder using bovine collagen sausage membrane compared to lambskin; Ae. aegypti improved 8% and Ae. albopictus improved 3%.
By challenging mosquitoes with several variables, we attempted to identify strategies for improving feeding success on artificial feeders. Two successive generations of Ae. albopictus were bred to select a membrane-friendly colony, but this effort was unsuccessful. Gentle motion did not significantly improve feeding and was discontinued (Fig. 2A, B). Variables such as smelly socks (Schmied et al. 2008), blood source (chicken), and cage color (black instead of white) did not enhance feeding of Ae. albopictus on glass feeders (Bowers, unpublished data). Increased success was observed after providing sausage casing blood packets in different feeding positions (Fig. 2A, B). Three models were chosen (see Models, blood meal presentation = feeding position). Model 1, traditional glass feeder topside (control) = upside-down feeding [only]; Model 2A, large horizontal = right side-up and nose-up feeding [two options]; Model 2B, small horizontal = right side-up feeding [only]; Model 3, vertical = nose-up feeding [only]. Ae. aegypti (control) fed well on Models 1, 2A, and 3 (>88%), a feeding improvement of ∼20% over Model 2B. Ae. albopictus fed significantly better on Models 2A and 3 (>57%), a feeding improvement of ∼33% over Model 1 and ∼24% over Model 2B. Feeding improvement for Ae. albopictus on Models 2A and 3 surpassed the feeding success on Para film (Tseng 2003).
Imbibing blood depends on insect suck-and-spit activity (pharyngeal pumps), midgut integrity and capacity (stretch receptors), gripping ability (intact tarsals), feeding physiology, vertebrate host cues, and a conundrum of environmental factors (Clements 1992); we propose that gravity plays a role. In Model 1, blood was pumped downward and then flowed parallel to the substratum (upside-down feeding). In Model 2, blood was pumped upward and then flowed parallel to the substratum (right side-up feeding) or was pumped parallel to the substratum and then flowed downward (nose-up feeding) as in Model 3. Large horizontal and vertical packets (large and small) provided a nose-up feeding position, a more normal stance facilitating the downward flow of blood. Perhaps Ae. aegypti is less susceptible to the effects of gravity, whereas Ae. albopictus is more responsive. Compared with the traditional upside-down feeding position, improvement was documented in Ae. albopictus in the vertical position (+46%), but not for Ae. aegypti (−6%). The large horizontal packet (Model 2A) provided for two feeding positions and more surface area (63 cm2) than the small horizontal packet (Model 2B; 28 cm2). Small horizontal packets resulted in significantly reduced feeding success for both mosquito species and we suspect its acute radius of curvature promoted right-side up over nose-up feeding positions. The greater surface area of the large horizontal packet promoted nose-up feeding, but we suggest that because a vertical position was available that feeding was also facilitated by gravity. Differences in blood feeding success in response to feeding position further separates the behavior attributes of these two Aedine species.
Blood feeding on a vertebrate is a dangerous activity, the degree of which increases with the volume of blood consumed at the feeding site as well as the postfeeding site (Roitberg et al. 2003). Ae. aegypti imbibes 2.2–7.0 μL rabbit blood (Klowden and Lea 1979), and mosquitoes are thought to gain two to four times their body weight after blood feeding (Nayar and Sauerman 1975). An engorged mosquito faces a greater risk of fatality due to an increased body mass, and because insects usually fly right side-up, it has been suggested that they can determine gravity. Bender and Frye (2009) propose that this may not require active sensation; for example, a mosquito with a heavy abdomen will be inclined to maintain a nose-up posture. Hence, glass feeders that restrict females to an awkward upside-down feeding position are not conducive to blood meal consumption. A study by Self and colleagues (1969) examined indoor biting behavior of Culex pippins fatigans on adult Burmase males. When human subjects were positioned in a sitting manner, an average of 90.6% bites occurred below the knees with the vertical lower legs serving as the preferred biting sites. When subjects were positioned lying down, an average of 45.1% bites occurred below the knees, while the forearms and horizontal lower legs were equally attractive. These authors speculated that the biting behavior of C. p. fatigans favored areas of the human body on or near the floor and we suggest that the more numerous bites on the sitting leg is due to the vertical presentation and available nose-up feeding position.
Aside from available surface area and gravity, differences in feeding success seen in Model 1 might be accounted for by anatomical differences that permit Ae. aegypti but prevent Ae. albopictus from feeding in the upside-down position. Jones and Pilitt (1973) working with Ae. aegypti conducted an exquisite series of surgical experiments whereby they removed a small portion of the last tarsal segment from each leg to analyze the process of blood feeding. Many females tumbled or somersaulted upon landing and only fed when tilted uphill on the downward sloping forearm of a host, inferring that feeding position and tarsal gripping had a direct effect on success. Ultrastructural examination of membrane surfaces revealed a greatly textured surface seen only on the large sausage membrane, one predicted to facilitate tarsal gripping (Fig. 3). Ae. albopictus also fed poorly on the topside feeder, the only feeder placed directly on mosquito netting (Model 1). Speculating that this netting should have provided extra tarsal gripping texture, we concluded that feeding position takes priority over surface texture. Because Ae. albopictus demonstrated increased feeding success on Model 3 compared to Model 1, this species is more subject to effects of gravity and possibly more dependent on tarsal gripping than Ae. aegypti.
Behavioral differences between Ae. aegypti and Ae. albopictus and how they relate to virus transmission are still unclear (Ponlawat and Harrington 2005). Using Model 3, an increase of 46% feeding (Tables 2 and 3) could increase the number of blood fed mosquitoes from 10 to 460 or from 20 to 920: extrapolating to potentially 46 infected with SVHR and 460 infected with AR339. Bovine collagen sausage casings provide an effective method to deliver an infectious blood meal, and small sausage casings are valuable because less stock virus is needed for calculating a high titer in 15 mL total volume. Casings can be filled with any specific type of blood source and titer of arbovirus, are commercially available, are more cost effective than lambskin, more stable than Para film, and are effective at feeding a recalcitrant vector, Ae. albopictus. Any laboratory equipped to work with hematophagous insects should use long strips of large bovine collagen sausage casings positioned at a vertical presentation to increase the number of colony and/or potential number of arbovirus-infected females available for investigation.
Acknowledgments
The research described was supported by a grant (no. R15A1060654) from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease (NIAID) or the National Institutes of Health (NIH). We thank Prof. Jay Huebner, Department of Physics, University of North Florida, for research availability of the environmental scanning electron microscope. We thank David Wilson for his expertise in image and graphic presentation.
Disclosure Statement
Research was funded by NIH NIAID; no competing financial interests exist.
References
- Akey DH. Jones JC. Sexual responses of adult male Aedes aegypti using the forced-copulation technique. Biol Bull. 1968;135:445–453. doi: 10.2307/1539707. [DOI] [PubMed] [Google Scholar]
- Alto BW. Lounibos LP. Juliano SA. Age-dependent blood feeding of Aedes aegypti and Aedes albopictus on artificial and living hosts. J Am Mosq Control Assoc. 2003;19:347–352. [PubMed] [Google Scholar]
- Andreadis TG. Failure of Aedes albopictus to overwinter following introduction and seasonal establishment at a tire recycling plant in the northeastern USA. J Am Mosq Control Assoc. 2009;25:25–31. doi: 10.2987/08-5813.1. [DOI] [PubMed] [Google Scholar]
- Bender JA. Frye MA. Invertebrate solutions for sensing gravity. Curr Biol. 2009;19:186–190. doi: 10.1016/j.cub.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Bowers DF. Abell B. Brown DT. Replication and tissue tropism of the alphavirus Sindbis in the mosquito Aedes albopictus. Virology. 1995;212:1–12. doi: 10.1006/viro.1995.1447. [DOI] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes: Development, Nutrition, and Reproduction. Vol. 1. New York, NY: CABI Publishing; 1992. [Google Scholar]
- Davies CR. Interrupted feeding of blood-sucking insects: causes and effects. Parasitol Today. 1990;6:19–22. doi: 10.1016/0169-4758(90)90387-j. [DOI] [PubMed] [Google Scholar]
- Davis EE. Sokolove PG. Lactic acid-sensitive receptors on the antennae of the mosquito, Ae. aegypti. J Comp Physiol. 1976;A105:43–54. [Google Scholar]
- Delatte H. Desvars A. Bouetard A. Bord A, et al. Blood feeding behaviour of Aedes albopictus, a vector of Chikungunya on La Reuion. Vector Borne Zoonot Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- Gad AM. Farid HA. Ramzy RR. Riad MB, et al. Host feeding of mosquitoes (Diptera: Culicidae) associated with the recurrence of Rift Valley Fever in Egypt. J Med Entomol. 1999;36:709–714. doi: 10.1093/jmedent/36.6.709. [DOI] [PubMed] [Google Scholar]
- Galun R. Feeding stimuli and artificial feeding. Bull World Health Organ. 1967;36:590–593. [PMC free article] [PubMed] [Google Scholar]
- Gerberg EJ. Barnard DR. Ward RA. Manual for Mosquito Rearing and Experimental Techniques. Lake Charles, LA: American Mosquito Control Association; 1994. [Google Scholar]
- Ghaninia M. Larsson M. Hansson BS. Ignell R. Natural odor ligands for olfactory receptor neurons of the female mosquito Aedes aegypti: use of gas chromatography-linked single sensillum recordings. J Exp Biol. 2008;211:3020–3027. doi: 10.1242/jeb.016360. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Higgs S. Beaty BJ. Rearing and containment of mosquito vectors. In: Beaty BJ, editor; Marquardt WC, editor. The Biology of Disease Vectors. Niwot, CO: University Press of Colorado; 1996. pp. 595–605. [Google Scholar]
- Hodgson JC. Spielman A. Komar N. Krahforst CF, et al. Interrupted blood-feeding by Culiseta melanura (Diptera: Culicidae) on European starlings. J Med Entomol. 2001;38:59–66. doi: 10.1603/0022-2585-38.1.59. [DOI] [PubMed] [Google Scholar]
- Jones HJ. Potter HW., Jr A six-position artificial feeding apparatus for Culicoides variipennis. Mosq News. 1972;32:520–528. [Google Scholar]
- Jones JC. Pilitt DR. Blood-feeding behavior of adult Aedes aegypti mosquitoes. Biol Bull. 1973;145:127–139. doi: 10.2307/1540353. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Lea AO. Effect of defensive host behavior on the blood meal size and feeding success of natural populations of mosquitoes (Diptera: Culicidae) J Med Entomol. 1979;15:514–517. doi: 10.1093/jmedent/15.5-6.514. [DOI] [PubMed] [Google Scholar]
- Kramer LD. Hardy JL. Presser SB. Houk EJ. Dissemination barriers for western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low virus dose. Am J Trop Med Hyg. 1981;30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- Moore CG. Mitchell CJ. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg Infect Dis. 1997;3:329–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT. Gokhale MD. Barde PV. Padbidri VS. A simple artificial membrane-feeding method for mosquitoes. Trans R Society Trop Med Hyg. 2000;94:460. doi: 10.1016/s0035-9203(00)90141-x. [DOI] [PubMed] [Google Scholar]
- Nayar JK. Sauerman DM. The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 2. Utilization of a blood meal for survival. J Med Entomol. 1975;12:99–102. doi: 10.1093/jmedent/12.1.99. [DOI] [PubMed] [Google Scholar]
- O'Meara GF. Evans LF., Jr. Gettman AD. Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Ooi CP. Rohani A. Zamree I. Chua WS. Effectiveness of several locally available membranes used for artificial feeding of Aedes albopictus Skuse. Trop Biomed. 2005;22:69–71. [PubMed] [Google Scholar]
- Ponlawat A. Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- Richards SL. Ponnusamy L. Unnasch TR. Hassan HK, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitberg BD. Mondor EB. Tyerman JGA. Pouncing spider, flying mosquito: blood acquisition increases predation risk in mosquitoes. Behav Ecol. 2003;14:736–740. [Google Scholar]
- Rosen L. Gubler D. The use of mosquitoes to detect and propagate Dengue viruses. Am J Trop Med Hyg. 1974;23:1153–1160. doi: 10.4269/ajtmh.1974.23.1153. [DOI] [PubMed] [Google Scholar]
- Rutledge LC. Ward RA. Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq News. 1964;24:407–419. [Google Scholar]
- Schmied WH. Takken W. Killeen GF. Knols BG, et al. Evaluation of two counterflow traps for testing behavior-mediating compounds for malaria vector Anopheles gambiae s.s. under semi-field conditions in Tanzania. Malaria J. 2008;7:230–239. doi: 10.1186/1475-2875-7-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self LS. Abdulcader MHM. Tun MM. Preferred biting sites of Culex pipiens fatigans on adult Burmese males. Bull World Health Organ. 1969;40:324–327. [PMC free article] [PubMed] [Google Scholar]
- Sprenger D. Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc. 1986;2:217–219. [PubMed] [Google Scholar]
- Tseng M. A simple parafilm M-based method for blood-feeding Aedes aegypti and Aede albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:588–589. doi: 10.1603/0022-2585-40.4.588. [DOI] [PubMed] [Google Scholar]