Abstract
Transmission of West Nile virus (WNV) on mainland California poses an ongoing threat to the island scrub-jay (ISSJ, Aphelocoma insularis), a species that occurs only on Santa Cruz Island, California, and whose total population numbers <5000. Our report describes the surveillance and management efforts conducted since 2006 that are designed to understand and mitigate for the consequences of WNV introduction into the ISSJ population. We suspect that WNV would most likely be introduced to the island via the movement of infected birds from the mainland. However, antibody testing of >750 migrating and resident birds on the island from 2006 to 2009 indicated that WNV had not become established by the end of 2009. Several species of competent mosquito vectors were collected at very low abundance on the island, including the important mainland vectors Culex tarsalis and Culex quinquefasciatus. However, the island was generally cooler than areas of mainland California that experienced intense WNV transmission, and these lower temperatures may have reduced the likelihood of WNV becoming established because they do not support efficient virus replication in mosquitoes. A vaccination program was initiated in 2008 to create a rescue population of ISSJ that would be more likely to survive a catastrophic outbreak. To further that goal, we recommend managers vaccinate >100 ISSJ each year as part of ongoing research and monitoring efforts.
Key Words: Birds, Mosquito(es), Surveillance, West Nile, Conservation
Introduction
The island scrub-jay (ISSJ, Aphelocoma insularis) is North America's sole island-endemic bird species. It is currently restricted to Santa Cruz Island, ∼30 km off-shore of Santa Barbara, California (Curry and Delaney 2002, Delaney and Wayne 2005). Because the introduction of novel pathogens can decimate island avifauna (Wikelski et al. 2004), conservationists have been concerned about the threat posed to ISSJ by West Nile virus (WNV), since the virus spread to the western United States (Boyce et al. 2004). WNV first arrived in southern California in 2003 (Reisen et al. 2004) and has since killed tens of thousands of birds across the state, with especially high mortality occurring among species of Corvidae, for example, western scrub-jay (WESJ, Aphelocoma californica), American crow (Corvus brachyrhinos), and yellow-billed magpie (Pica nuttali) (Crosbie et al. 2008, Wheeler et al. 2009).
We consider the ISSJ to be at high risk for a catastrophic population decline because of its highly restricted insular range, small population size (<5000 birds; Sillett et al. unpublished), and because corvid species closely related to the ISSJ are known to be highly susceptible to WNV. In particular, Wheeler et al. (2009) found that WESJ were significantly impacted by the arrival of WNV into California, and we expect that ISSJ will respond similarly to WNV infection because they are closely related to WESJ. Indeed, Wheeler et al. (2010) used the more abundant WESJ as a model for ISSJ in an experimental challenge study to evaluate the efficacy of three different WNV vaccines: (1) Fort Dodge West Nile-Innovator® DNA equine vaccine (Overland Park, KS), (2) pCBWN, an experimental DNA plasmid vaccine (Chang et al. 2007), and (3) the Merial Recombitek® West Nile virus equine vaccine (Duluth, GA).
The current report describes surveillance and proactive management actions undertaken since 2006 to detect, as well as prepare and mitigate for, the introduction and establishment of WNV in ISSJ on Santa Cruz Island. Our goals were to implement a surveillance program to detect WNV introduction and transmission, identify potential mosquito vectors and the likelihood for mosquito-borne transmission, and evaluate the feasibility of vaccinating >100 ISSJ each year (∼2% of the population). The rationale for vaccinating free-ranging ISSJ is that a vaccinated rescue population could increase the likelihood of species persistence should an outbreak of WNV have catastrophic population consequences. The Wheeler et al. (2010) experimental vaccine-challenge study with WESJ was conducted concurrently with this field study and provides detailed information about the safety and efficacy of the vaccines discussed in this article.
Materials and Methods
Study area
Santa Cruz Island is the largest of the California Channel Islands, and is co-owned and managed by The Nature Conservancy and the United States National Park Service. The 255-km2 island experiences a mediterranean-type climate of cool winters and warm dry summers, and is characterized by two rugged mountain ranges flanking a narrow central valley. The island supports an array of native vertebrates, including reptiles, rodents, island fox (Urocyon littoralis), spotted skunk (Spilogale gracilis), and many species of resident and migratory birds. The highest densities of ISSJ generally occur in the island's central valley, where oak woodland and chaparral vegetation is prevalent (Sillett et al. unpublished). Also within that area of core ISSJ habitat is a University of California field research station (33°59′ 49″N; 119°43′ 33″W), which serves as a base for ongoing research and monitoring.
Surveillance
Surveillance for WNV from 2006 to 2009 consisted of capture, sampling, and serologic testing of ISSJ and other resident and migrant bird species (Table 1). The first samples (n = 39) were collected in 2006 from wild turkeys (Melagris gallopavo) captured in the island's central valley. These turkeys served as sentinels for prior WNV transmission on the island since they live for several years, and they are relatively resistant to WNV (Swayne et al. 2000), much like sentinel chickens monitored for WNV transmission in mainland California. In 2007 and 2008, ISSJ were sampled along with several other resident (n = 12) and migrating species (n = 25) (Table 2). Most captures were conducted during the fall migration period (September–November), following the most likely period of peak WNV transmission. The goals of 2007 and 2008 sampling were to detect enzootic transmission (seropositive resident birds) and to investigate whether WNV may have been introduced to the island via birds flying from the mainland (seropositive migrants). In 2009, ISSJ (n = 117) were sampled at different times of the year, and sera samples from 10 resident island foxes were also tested.
Table 1.
Summary of West Nile Virus Surveillance of Birds and Mammals on Santa Cruz Island, California, from 2006 to 2009
| Date sampled | Species | No. of individuals sampled |
|---|---|---|
| December 2006 | Birds–wild turkey | 39 |
| October 2007 | Birds–multiple speciesa | 222 |
| October 2007 | Island scrub-jay | 68 |
| October–December 2008 | Birds–multiple speciesa | 243 |
| October–December 2008 | Island scrub-jay | 54 |
| June 2009 | Island scrub-jay | 47 |
| September–October 2009 | Island scrub-jay | 70 |
| 2009 | Island fox | 10 |
All samples were seronegative by enzyme immunoassay.
Excluding island scrub-jay, bird species and numbers sampled are in Table 2.
Table 2.
Species and Migratory Status of Birds Captured on Santa Cruz Island, California in 2007 and 2008
| Common name | Scientific name | No. sampled 2007 | No. sampled 2008 | Island resident or migratory |
|---|---|---|---|---|
| Bewick's wren | Thryomanes bewickii | 7 | 7 | R |
| Bushtit | Psaltriparus minimus | 1 | R | |
| Brown-headed cowbird | Molothrus ater | 2 | M | |
| Cassin's vireo | Vireo cassinii | 2 | M | |
| California quail | Callipepla californica | 3 | R | |
| Chipping sparrow | Spizella passerina | 2 | M | |
| Common yellowthroat | Geothlypis trichas | 3 | M | |
| Common raven | Corvus corax | 23 | R | |
| Dark-eyed junco | Junco hyemalis | 1 | M | |
| Fox sparrow | Passerella iliaca | 6 | 8 | M |
| Golden-crowned sparrow | Zonotrichia atricapilla | 21 | M | |
| Green-tailed towhee | Pipilo chlorurus | 1 | M | |
| Hermit thrush | Catharus guttatus | 5 | 33 | M |
| Hermit warbler | Dendroica occidentalis | 1 | M | |
| House finch | Carpodacus mexicanus | 3 | 11 | R |
| Hutton's vireo | Vireo huttoni | 5 | R | |
| Island scrub-jay | Aphelocoma insularis | 68 | 54 | R |
| Lesser goldfinch | Carduelis psaltria | 14 | M | |
| Lincoln's sparrow | Melospiza lincolnii | 6 | 2 | M |
| Pacific-slope flycatcher | Empidonax difficilis | 1 | R | |
| Orange-crowned warbler | Vermivora celata | 21 | R | |
| Red-winged blackbird | Agelaius phoeniceus | 1 | M | |
| Ruby-crowned kinglet | Regulus calendula | 3 | 1 | M |
| Rufous-crowned sparrow | Aimophila ruficeps | 3 | 2 | R |
| Savannah sparrow | Passerculus sandwichensis | 12 | M | |
| Song sparrow | Melospiza melodia | 17 | 29 | R |
| Spotted towhee | Pipilo maculatus | 10 | 25 | R |
| Sharp-shinned hawk | Accipiter striatus | 1 | M | |
| Tennessee warbler | Vermivora peregrina | 1 | M | |
| Townsend's warbler | Dendroica townsendi | 2 | M | |
| Unknown Empidonax | 8 | M | ||
| Warbling vireo | Vireo gilvus | 11 | 3 | M |
| White-crowned sparrow | Zonotrichia leucophrys | 42 | 33 | M |
| Wilson's warbler | Wilsonia pusilla | 2 | M | |
| Yellow warbler | Dendroica petechia | 8 | M | |
| Yellow-rumped warbler | Dendroica coronata | 9 | 3 | M |
All were found to be seronegative for antibodies to West Nile virus.
Birds were captured by box traps, mist nets, bownets, and other methods by personnel from the Smithsonian Migratory Bird Center, The Nature Conservancy, and the UC Davis Wildlife Health Center. Whole blood (100 μL) was placed in 900 μL of phosphate-buffered saline and frozen until testing by enzyme immunoassays (EIA) and/or plaque reduction neutralization test (PRNT) at the Center for Vectorborne Diseases (CVEC) at UC Davis (Beaty et al. 1995, Chiles and Reisen 1998, Ebel et al. 2002). All activities were conducted with approved permits from California Department of Fish and Game, United States Fish and Wildlife Service, United States Geological Survey Bird Banding Laboratory, and University of California Animal Care and Use Committees.
Mosquito vectors and temperatures
There were three field efforts to sample mosquitoes on the island and detect infection with WNV and other viruses. In November 2005, June 2006, and again in July 2009, mosquitoes were collected along an ∼3 km transect parallel to the natural stream bed along the central valley. Mosquitoes were collected with CO2 traps (Newhouse et al. 1966), gravid traps (Cummings 1992), and hand-held mechanical aspirators. Pools of mosquitoes, ranging from 1 to 50, were separated by species and sex, frozen on dry ice, and later tested for western equine encephalomyelitis virus (WEEV), Saint Louis encephalitis virus (SLEV), and WNV by a multiplex quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) at CVEC using primers and methods similar to those described by Lanciotti et al. (2000) and Shi and Kramer (2003).
The likelihood of mosquito-borne transmission was assessed by comparing mean monthly temperatures on Santa Cruz Island with mainland sites that experienced WNV activity during the 2006–2009 study period. We examined annual temperature profiles of each site to estimate the duration of the year above the minimum threshold temperature (14.3°C) required for WNV replication in mosquitoes (Reisen et al. 2006a). Sites included the central valley of Santa Cruz Island, which is the warmest part of the island and ideal ISSJ habitat; Whittier Hills, a site in Los Angeles County with intense WNV activity during the study period; and Bakersfield, a hot inland site in California's Central Valley with intense WNV transmission. Temperature data for Santa Cruz Island and Whittier Hills were obtained from the California Climate Data Archive's Remote Automated Weather Station network maintained by the Western Regional Climate Center (www.calclim.dri.edu/scaraws.html). Data for Bakersfield were not available from this source, so they were downloaded for the National Climatic Data Center's station at the Bakersfield airport available from the UC integrated pest management online weather archive (http://ipm.ucdavis.edu/weather/wxretrieve.html).
Vaccination
The safety and feasibility of vaccinating free-ranging ISSJ on the island was first tested in fall 2008 using a killed-virus equine vaccine (Fort Dodge West Nile-Innovator), whose manufacturer recommends two doses (1 mL each) given twice 3–6 weeks apart for horses, and that has been widely used to attempt to reduce morbidity and mortality from WNV in birds in zoos, wildlife rehabilitation centers, and other captive settings (Nusbaum et al. 2003, Okeson et al. 2007). We chose to initially vaccinate jays on the eastern end of the island because a number of birds there had become accustomed to being fed peanuts, and we assumed that this would facilitate trapping, recapture, and revaccination. Birds were captured, bled, marked with individually colored leg bands and a numbered aluminum USGS leg band, and given 1 mL of vaccine intramuscularly divided equally in two locations on each side of the keel. Birds were released, and 3 weeks later we recaptured, bled, and re-vaccinated as many of the birds as possible. Blood was collected and a third booster vaccine was given to those birds that could be recaptured for the third time 4 weeks later. Blood samples were collected at each capture and tested for the presence of antibodies by EIA (whole blood) and PRNT (sera) at CVEC using methods described above.
When it became apparent that recapturing and revaccinating ISSJ required nearly a sixfold increase in effort over initial captures, it was decided to use a newly (2008) released Fort Dodge DNA equine vaccine (West Nile-Innovator® DNA). This vaccine was intended to be used as a single-dose product in horses, but each dose was formulated into a 2 mL injection. We began vaccinating birds in April–May 2009 and initially gave each bird 1 mL divided in two locations, because we were concerned about this larger volume causing muscle damage at the injection sites. Noticing no adverse effects, we increased the dose per bird to 2 mL divided in four locations. We chose to implement vaccination in the central valley region where birds were being routinely captured, banded, and monitored as part of long-term ecological studies. This allowed for easier and more frequent monitoring of vaccinated birds.
Results
Surveillance
All of the nonvaccinated birds (residents including ISSJ and migrants) and island foxes sampled from 2006 to 2009 were seronegative by EIA and/or PRNT (Tables 1 and 2). These results strongly supported the conclusion that WNV had not become established on Santa Cruz Island by the end of 2009. It also indicated that all resident birds on the island, including nonvaccinated ISSJ, were immunologically naïve and likely fully susceptible to WNV infection.
Mosquito vectors and temperatures
Limited sampling (115 trap nights over three occasions) in the island's interior valley yielded 417 mosquitoes comprising 11 species. Only 51 Culex mosquitoes were collected in total, and these included five species considered to be competent vectors of WNV: Culex tarsalis (n = 1), Culex quinquefasciatus (n = 9), Culex stigmatosoma (n = 15), Culex restuans (n = 25), and Culex thriambus (n = 1) (Goddard et al. 2002, Reisen et al. 2008). The other species collected included Anopheles fransicanus (n = 16), Aedes washinoi (n = 19), Aedes sierrensis (n = 13), Culiseta incidens (n = 305), and Culiseta inornata (n = 5). None of the pools tested positive for WNV, WEEV or SLEV by RT-PCR, or other arboviruses by plaque assay on Vero cells.
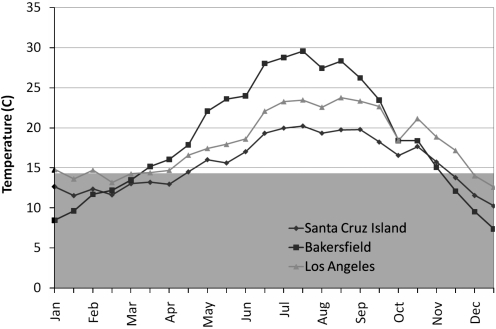
During the study period, temperatures in the central valley of Santa Cruz Island were cooler and reached the minimum replication threshold for WNV later than Los Angeles and Bakersfield (Fig. 1), both of which have had intense WNV transmission (Reisen et al. 2009, Kwan et al. 2010).
FIG. 1.
Mean half-monthly temperatures for the 2006–2009 study period at three locations in California relative to the threshold temperature (14.3°C) required for replication of West Nile virus in Culex tarsalis. The central valley of Santa Cruz Island was generally cooler than the mainland interior (Bakersfield) and mainland coastal (Los Angeles) regions, which had intense West Nile virus transmission during the study period.
Vaccination
Fifty-four ISSJ were captured during three attempts for the field trial with the Fort Dodge killed-virus vaccine. Twenty birds were vaccinated one or more times, and 34 birds served as nonvaccinated controls. Thirteen vaccinated birds were recaptured once (allowing evaluation of antibody response to a single dose of vaccine), and 5 vaccinated birds were recaptured twice (allowing evaluation of antibody response after two vaccinations). The five jays that had received two doses of killed-virus vaccine were all WNV antibody positive by PRNT80 neutralization (end point titers = 1:20, 1:40, 1:40, 1:40, and 1:80). In contrast, only 1 of the 13 jays that had received a single dose was positive by PRNT (1:20). All nonvaccinated jays were negative by PRNT on initial and subsequent recaptures, and all vaccinated and nonvaccinated jays tested negative for WNV by EIA. No overt adverse effects were detected when vaccinated birds were examined during recapture, and all vaccinated birds appeared normal when observed in the field postvaccination.
After it was recognized that it was not feasible to routinely capture jays for revaccination, the newly released Fort Dodge DNA vaccine (West Nile-Innovator DNA) was used in the central valley. The first nine birds that were captured were given a half dose of vaccine (1 mL) to assess for any adverse reactions to the vaccine itself. When no adverse reactions were noted, subsequent birds (n = 77) were given the full 2 mL dose recommended for horses. No adverse reactions were noted with this higher dose, and vaccinated birds that were observed in the days and weeks after vaccination appeared to behave normally. None of the 86 DNA-vaccinated birds were positive for WNV antibodies on initial capture, and the 10 vaccinated birds that were recaptured and retested remained seronegative by EIA and PRNT.
Discussion
WNV transmission has been detected repeatedly on mainland California since 2003, with epidemics in the Los Angeles area during 2004 and 2008 (Kwan et al. 2010). However, tests on sera from >750 birds collected from 2006 to 2009 indicated that WNV had not become established on Santa Cruz Island by the end of 2009, and that these birds did not have a history of prior WNV exposure (Tables 1 and 2). The two most likely portals of entry for WNV to the island are immigration of infected birds and introduction of infected mosquitoes. Although Cx. tarsalis can fly up to 6 km/night (Reisen and Lothrop 1995), and other species have been collected at high altitudes along storm fronts (Sellers 1980, Kay and Farrow 2000), we suspect that infected mosquitoes from the mainland would be more likely to reach the island by boat or airplane than by direct flight. However, we hypothesized that birds posed an even greater risk than mosquitoes for introducing the virus because thousands of birds migrate to the island in the fall and spring, whereas boat and airplane traffic is generally limited (small aircraft charters originating from Ventura County land on the island 1–2 times per week; a commercial pedestrian ferry services the island 1–2 times per day; ∼20,000 private recreational boats visit the island in a year, but not all of those visits involve passengers coming ashore [TNC, unpublished]). However, we found no evidence that previously infected birds had flown the 30 km distance from the mainland to the island, since WNV antibodies were not detected among 25 species of migrating birds sampled in 2007 and 2008. The absence of antibodies in resident birds sampled from 2006 to 2009 also supports the conclusion that WNV has not been successfully introduced by infected birds or mosquitoes.
Several species of WNV-competent mosquito vectors (Culex spp.) were collected on the island, including the important mainland vectors Cx. tarsalis and Cx. quinquefasciatus (Goddard et al. 2002). These potential vector species were collected in very low numbers (<1 female per trap night) along the central valley riparian area, where oak trees were common and ISSJ were most abundant. The most abundant mosquito in our collections was Cs. incidens, and this species has been shown to be a poor vector for WNV (Reisen et al. 2006b). Overall, our collections yielded relatively low numbers of vector species compared to mainland areas with active WNV transmission such as Kern County (Reisen et al. 2009) or Los Angeles (Kwan et al. 2010). However, we note that more systematic and repeated sampling of mosquitoes within and across years will be necessary to characterize WNV transmission risk based on vector abundance.
Despite the prevalence of WNV on coastal mainland southern California, we are unaware of WNV being reported from any of the California Channel Islands. We hypothesize that slightly cooler maritime climates help buffer the islands from the virus. Sustained WNV replication in mosquitoes requires temperatures that exceed minimum thresholds (Reisen et al. 2006a). Even at the highest mean temperatures observed on Santa Cruz Island during this study, WNV would require an incubation period of ∼3 weeks in the mosquito before transmission could occur (Reisen et al. 2006a), meaning that transmission on the island would be relatively inefficient, especially with the apparent paucity of competent mosquito vectors. The island is quite topographically heterogeneous, however, with large temperature variations across short distances, so there may be important variation in transmission risk across the island. Increases in temperature expected with climate change (Cayan et al. 2008) would also increase the likelihood of enzootic transmission should the virus be introduced.
Vaccination of free-ranging birds allowed us to evaluate the safety and feasibility of creating a rescue population that would be more likely to survive a catastrophic WNV outbreak. Based on the absence of observed adverse effects, both of the vaccines (killed-virus and DNA) we tested in the field appeared safe for use in this species. Although 2.0 mL is a large volume to inject intramuscularly into a 125 g bird, the relative safety of the Fort Dodge DNA vaccine was confirmed in the vaccination trial conducted using the WESJ as a surrogate for the ISSJ (Wheeler et al. 2010). Likewise, an experimental pCBWN plasmid DNA vaccine that has been used in California Condors (Chang et al. 2007) was also found to be relatively safe in the trial with WESJ. In contrast, a recombinant canary pox virus vaccine (Merial Recombitek) caused potentially debilitating lesions at the vaccination site in WESJs, even when used at the recommended equine dose of 1 mL (Wheeler et al. 2010). We had considered using the Merial Recombitek vaccine on Santa Cruz Island because of its lower volume and the anecdotal reports of its frequent use in zoos and other captive avian collections. However, we rejected it in favor of the Fort Dodge DNA vaccine so as to not introduce the strain of canary-pox that is contained in the Merial vaccine to the avifauna of Santa Cruz Island. The findings of Wheeler et al. (2010) validated this decision, and we encourage others to carefully consider which WNV vaccine is safest and most appropriate for use in captive and free-ranging birds.
Wheeler et al. (2010) demonstrated that single-dose DNA vaccines significantly reduced WNV viremia levels and increased survival of vaccinated WESJ. However, vaccination did not provide complete protection or decrease peak viremia below the 105 plaque forming unit per mL threshold required for most mosquito infection (Komar et al. 2003). Despite these limitations, we conclude that vaccination of ISSJ would very likely increase survival of a significant proportion of vaccinated birds, and survivors would likely develop sterilizing immunity after natural infection. Based on our capture and vaccination efforts in 2008 and 2009, we conclude that >100 ISSJ, or about 2% of the population, could be vaccinated once each year with only a modest effort, especially since ISSJ are routinely captured and banded for ongoing ecological studies. This approach would not be aimed at reducing or preventing WNV transmission at the population level as suggested by Kilpatrick et al. (2010). Rather, the goal would be to increase the number of individual birds that survive and reproduce in the face of an outbreak and subsequent yearly enzootic transmission, similar to the intent of vaccinating free-ranging California Condors (Gymnogyps californianus) (Chang et al. 2007). We believe this individual-animal approach is appropriate considering the restricted geographic distribution and small population size of ISSJ. However, population models should be developed to evaluate and optimize the impacts of vaccination on population persistence.
We recommend the use of either the pCBWN or Fort Dodge DNA vaccines in free-ranging ISSJ. Unfortunately, the Fort Dodge West Nile-Innovator DNA vaccine was removed from the commercial market in 2010 after we completed this study, and the pCBWN experimental vaccine is only available in limited quantities. Clearly, additional work is needed to identify WNV vaccines that are safe and effective in a variety of bird species and to confirm that results obtained with WESJ can be extrapolated to ISSJ. Opportunistic recaptures of WNV-vaccinated ISSJ will provide some information regarding the development of serum neutralizing antibody responses, but recaptures will be limited and there is no evidence that antibody titers can be used to predict survival in vaccinated birds (Wheeler et al. 2010). Experimental challenge trials with ISSJ could provide more definitive results. The significant lesions observed in WESJ given the Merial Recombitek vaccine also provides a strong argument for conducting additional controlled studies of vaccine safety and efficacy. While there is an inherent conservation dilemma in sacrificing ISSJ for the sake of increased scientific certainty, it is also important that managers understand the degree to which investment in vaccination actually reduces the extinction risk of this species.
Acknowledgments
We thank The Nature Conservancy, the UC Natural Reserve System's Santa Cruz Island field station, Channel Islands National Park, our many talented assistants in the field, and our many collaborators in the ISSJ Conservation Initiative, especially L. Laughrin, K. Faulkner, and C. Collins. Bird sera and mosquitoes were tested under BSL3 conditions at the UC Davis CVECs laboratory under the direction of Ying Fang. This research was funded, in part, by The Nature Conservancy, The US Fish and Wildlife Service, Channel Islands National Park, and NIAID NIH Grant RO1-AI55607, using American Recovery and Reinvestment Act support.
Disclosure Statement
No competing financial interests exist.
References
- Beaty B. Calisher C. Shope R. Arboviruses. In: Lennette E, editor; Lennette D, editor; Lennette E, editor. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Washington: American Public Health Association; 1995. pp. 204–205. [Google Scholar]
- Boyce W. Kreuder C. Anderson R. Barker C. Potential impacts of West Nile virus on wildlife in California. 2004. www.vetmed.ucdavis.edu/whc/pdfs/wnvreport.pdf www.vetmed.ucdavis.edu/whc/pdfs/wnvreport.pdf
- Cayan DR. Maurer EP. Dettinger MD. Tyree M. Hayhoe K. Climate change scenarios for the California region. Clim Change. 2008;87:S21–S42. [Google Scholar]
- Chang GJJ. Davis BS. Stringfield C. Lutz C. Prospective immunization of the endangered California condors (Gymnogyps californianus) protects this species from lethal West Nile virus infection. Vaccine. 2007;25:2325–2330. doi: 10.1016/j.vaccine.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Chiles RE. Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- Crosbie SP. Koenig WD. Reisen WK. Kramer VL, et al. Early impact of West Nile virus on the Yellow-Billed Magpie (Pica nuttalli) Auk. 2008;125:542–550. [Google Scholar]
- Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 1992;60:170–176. [Google Scholar]
- Curry RL. Delaney KS. Island Scrub-Jay (Aphelocoma insularis) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2002. Retrieved from the Birds of North America Online. [Google Scholar]
- Delaney KS. Wayne RK. Adaptive units for conservation: population distinction and historic extinctions in the Island Scrub-Jay. Conserv Biol. 2005;19:523–533. [Google Scholar]
- Ebel GD. DuPuis AP. Nicholas D. Young D, et al. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg Infect Dis. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard LB. Roth AE. Reisen WK. Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay BH. Farrow RA. Mosquito (Diptera: Culicidae) dispersal: implications for the epidemiology of Japanese and Murray Valley encephalitis viruses in Australia. J Med Entomol. 2000;37:797–801. doi: 10.1603/0022-2585-37.6.797. [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM. Dupuis AP. Chang GJ. Kramer LD. DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonot Dis. 2010;10:377–380. doi: 10.1089/vbz.2009.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan J. Kluh S. Madon M. Reisen W. West Nile virus emergence and persistence in Los Angeles, California, 2003–2008. Am J Trop Med Hyg. 2010;83:400–412. doi: 10.4269/ajtmh.2010.10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse VF. Chamberlain RW. Johnston JG., Jr. Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News. 1966;26:30–35. [Google Scholar]
- Nusbaum KE. Wright JC. Johnston WB. Allison AB, et al. Absence of humoral response in flamingos and red-tailed hawks to experimental vaccination with a killed West Nile virus vaccine. Avian Dis. 2003;47:750–752. doi: 10.1637/7006. [DOI] [PubMed] [Google Scholar]
- Okeson DM. Llizo SY. Miller CL. Glaser AL. Antibody response of five bird species after vaccination with a killed West Nile virus vaccine. J Zoo Wildl Med. 2007;38:240–244. doi: 10.1638/1042-7260(2007)038[0240:AROFBS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Barker CM. Fang Y. Martinez VM. Does variation in Culex vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Carroll BD. Takahashi R. Fang Y, et al. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J Med Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006a;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Martinez VM. Vector competence of Culiseta incidens and Culex thriambus for West Nile virus. J Am Mosq Control Assoc. 2006b;22:662–665. doi: 10.2987/8756-971X(2006)22[662:VCOCIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Lothrop HD. Chiles RE. Madon MB, et al. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK. Lothrop HD. Population ecology and dispersal of Culex tarsalis (Diptera: Culicidae) in the Coachella Valley of California. J Med Entomol. 1995;32:490–502. doi: 10.1093/jmedent/32.4.490. [DOI] [PubMed] [Google Scholar]
- Sellers RF. Weather, host and vector—their interplay in the spread of insect-borne animal virus diseases. J Hyg (Lond) 1980;85:65–102. doi: 10.1017/s0022172400027108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi PY. Kramer LD. Molecular detection of West Nile virus RNA. Expert Rev Mol Diagn. 2003;3:357–366. doi: 10.1586/14737159.3.3.357. [DOI] [PubMed] [Google Scholar]
- Swayne DE. Beck JR. Zaki S. Pathogenicity of West Nile virus for turkeys. Avian Dis. 2000;44:932–937. [PubMed] [Google Scholar]
- Wheeler SS. Barker CM. Fang Y. Armijos MV, et al. Differential imapact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SS. Langevin S. Woods L. Carroll BD, et al. Efficacy of three vaccines in protecting Western Scrub-Jays (Aphelocoma californica) from experimental infection with West Nile virus. Vector Borne Zoonot Dis. 2011 doi: 10.1089/vbz.2010.0173. epub ahead of print; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikelski M. Foufopoulos F. Vargas H. Snell H. Galápagos birds and diseases: invasive pathogens as threats for island species. Ecol Soc. 2004;9:5. [Google Scholar]