Abstract
Purpose
We evaluated the effectiveness of second-line maximum androgen blockade (MAB) with an alternative antiandrogen in patients who relapsed after initial MAB.
Materials and Methods
We retrospectively analyzed 47 patients with prostate cancer who relapsed after initial MAB, including surgical or medical castration combined with antiandrogens, from January 1998 to December 2009. When the serum prostate-specific antigen (PSA) level was increased on three consecutive occasions, we discontinued the antiandrogen and then administered an alternative antiandrogen. Seven patients were assessed for antiandrogen withdrawal syndrome (AWS). The effect of the second-line MAB was evaluated by the serum PSA level, and response was subdivided into ≥50% and <50% PSA reductions from the baseline PSA at the start of second-line MAB.
Results
PSA reduction was observed in 32 patients (68.1%). Among them, 23 (48.9%) achieved ≥50% PSA reductions with a mean response duration of 13.4±5.4 months. Nine (19.2%) patients reached <50% PSA reductions with a mean response duration of 12.2±6.2 months. The time to nadir PSA level after first-line MAB in the ≥50% PSA reduction group, <50% PSA reduction group, and PSA elevation group was 15.6±12.9 months, 11.8±6.0 months, and 8±6.5 months, respectively. That is to say, it was significantly longer in the responder groups (p=0.038).
Conclusions
Second-line MAB using an alternative antiandrogen is an effective treatment option before cytotoxic chemotherapy in patients who relapse after initial MAB.
Keywords: Androgen antagonists, Prostate-specific antigen, Prostatic neoplasms
INTRODUCTION
Almost all prostate cancers express androgen receptor, and the growth and survival of prostate cancer initially depends on continued activation of the androgen receptor by androgens. Therefore, androgen deprivation therapy (ADT) has been a mainstay of treatment against prostate cancer [1,2]. The use of ADT has been increasing steadily in all stages of prostate cancer: as primary therapy for those with localized disease or who are unfit for radical local treatment, as an adjunct to radiotherapy or surgery for those with locally advanced (high-risk) disease, as treatment for biochemical relapse after failure of localized therapy, and as treatment for men with metastatic disease, with and without symptoms [2,3].
Although the majority of patients with prostate cancer initially respond to ADT, almost all patients ultimately develop tumor progression despite castrate levels of testosterone, which is often referred to as castration-resistant prostate cancer (CRPC) [4-7]. Treatment options for CRPC remain limited, and the prognosis of patients with CRPC is dismal, with a median survival of 12 to 18 months [5]. For management of these patients, antiandrogen withdrawal, second-line ADT (alternative antiandrogen, estrogen and ketoconazole), and cytotoxic chemotherapy may be considered [2,8].
Despite advances in cytotoxic chemotherapy, secondline ADT is often used after the development of CRPC because many patients with CRPC remain sensitive to secondary hormonal manipulations. Second-line ADT either lowers the androgen levels further or directly antagonizes the androgen receptor in prostate cancer cells [1,7]. Several investigators have reported the usefulness of alternative ADT for treating resistance to first-line ADT [1,9-15].
Therefore, we analyzed the clinical effects of an alternative antiandrogen for second-line maximum androgen blockade (MAB) in patients with relapsing prostate cancer who received first-line MAB in Korea.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 47 patients with histologically confirmed prostate cancer who relapsed after initial MAB from January 1998 to December 2009.
For first-line therapy, all patients were initially treated with MAB consisting of either bilateral orchiectomy or medical castration with luteinizing hormone-releasing hormone (LHRH) agonist (3.6 mg goserelin acetate or 3.75 mg leuprorelin acetate every 4 weeks) plus antiandrogen (50 mg bicalutamide or 200 mg cyproterone acetate daily). The selection of the antiandrogen drug was left to the individual doctor's discretion.
Serum prostate-specific antigen (PSA) levels were measured by using a chemiluminescent enzyme immunoassay at least once every 4 or 8 weeks. Clinical staging was evaluated according to the 1997 TNM classification.
If disease was determined to be relapsed (increasing PSA on 3 successive occasions) after initial MAB, the first-line antiandrogen was stopped. In 7 patients, the patients were assessed for antiandrogen withdrawal syndrome (AWS) and an alternative antiandrogen (switching from bicalutamide to cyproterone acetate or from cyproterone acetate to bicalutamide) was then started. In 40 patients, second-line MAB therapy with an alternative antiandrogen was started without evaluation of AWS. AWS was defined as a decrease in PSA levels of greater than 50% after the stoppage of initial antiandrogen. In all cases, LHRH agonist administration was continued to maintain medical castration after failure of first-line MAB.
We investigated age, initial serum PSA level, Gleason score, clinical stage, and AWS response. We also analyzed serum PSA level (baseline and nadir), response to ADT, time to nadir PSA, and response duration for both first- and second-line MAB.
With first-line MAB, a complete response (CR) was defined as a decrease in PSA levels to less than 4 ng/ml, whereas a partial response (PR) was considered to be a decrease in PSA levels of greater than 50% with a concentration not less than 4 ng/ml.
For all patients in this study, the duration of response was defined as the period from the start of ADT to increasing PSA on 3 consecutive occasions.
We classified the patients as responders (subdivided into 2 groups: ≥50% and <50% PSA reductions) and nonresponders (PSA elevation) in second-line MAB. We then compared these 3 groups with respect to the above-mentioned factors to clarify the clinical differences.
The method of statistical analysis used was ANOVA and chi-square test (with Fisher's exact test) using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered to be statistically significant.
RESULTS
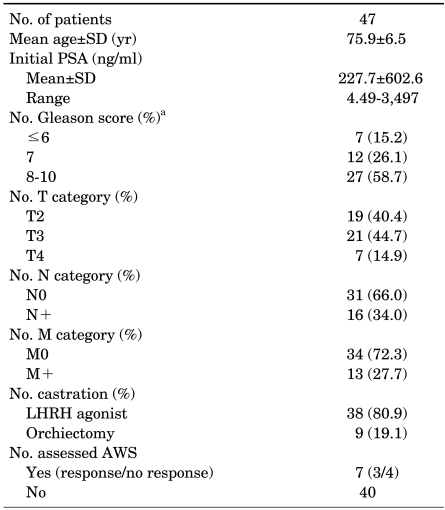
Patient characteristics are presented in Table 1. The age distribution ranged from 62 to 87 years with a mean age of 75.9±6.5 years. PSA before treatment was 227.7±602.6 ng/ml (range, 4.49-3,497 ng/ml). Gleason scores were less than 7 in 7 patients (15.2%), 7 in 12 patients (26.1%), and more than 8 in 27 patients (58.7%). We could not determine the Gleason score in one patient with mucinous prostate adenocarcinoma. For clinical stage, greater than T3 disease, regional lymph node metastases, and distant metastases were found in 28 (59.6%) patients, 16 (34.0%) patients, and 13 (27.7%) patients, respectively. In first-line MAB, orchiectomy was performed in 9 patients (19.1%) and LHRH agonists were administered in 38 patients (80.9%). AWS was assessed in 7 patients and observed in 3 (42.9%) patients with a mean duration of 2.7 months.
TABLE 1.
Patient characteristics
AWS: antiandrogen withdrawal syndrome, LHRH: luteinizing hormone-releasing hormone, PSA: prostate-specific antigen, a: a Gleason score could not be determined in one patient with mucinous prostate adenocarcinoma.
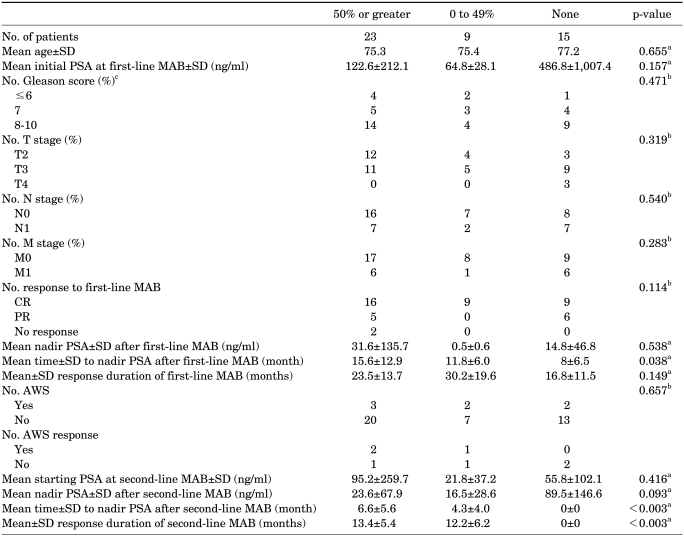
Clinical factors in responders and nonresponders to second-line MAB are compared in Table 2. Of the 47 patients treated with second-line MAB, 32 (68.1%) were considered to be responders. Twenty-three (48.9%) achieved ≥50% PSA reductions (mean response duration: 13.4±5.4 months) and 9 (19.2%) reached <50% PSA reductions (mean response duration: 12.2±6.2 months). Fifteen (31.9%) of the 47 patients had an increase in the PSA level with second-line MAB and were considered to be nonresponders.
TABLE 2.
Comparisons of clinical factors in responders and nonresponders to second-line MAB
AWS: antiandrogen withdrawal syndrome, CR: complete response, MAB: maximum androgen blockade, PR: partial response, PSA: prostate-specific antigen, a: ANOVA, b: chi-square test (with Fisher's exact test), c: a Gleason score could not be determined in one patient with mucinous prostate adenocarcinoma.
There were no statistically significant differences between these groups except that the time to nadir PSA level after first-line MAB of the second-line responders was longer than that of the nonresponders (p=0.038). The time to nadir PSA level after first-line MAB was 15.6±12.9 months, 11.8±6.0 months, and 8±6.5 months in the ≥50% PSA reduction group, <50% PSA reduction group, and PSA elevation group, respectively.
DISCUSSION
Prostate cancer is the most common cancer in men in the United States and the incidence of this disease is rapidly increasing in Korea [5,16]. Traditionally, androgen deprivation was achieved by bilateral orchiectomy and oral estrogen [17]. The goal with ADT is to reach castrate levels of testosterone. From this point of view, elimination of the production of gonadal testosterone can be insufficient because a small amount of testosterone is produced in the adrenal glands and contributes to prostate cancer progression. Huggins and Scott demonstrated the secondary benefit of a bilateral adrenalectomy in a castrated man [18]. Their finding of a clinical benefit from eliminating all androgens produced in the body was the first demonstration of the benefit of MAB [4]. MAB has progressed with the discovery of LHRHs, the development of LHRH agonists, and the discovery of antiandrogens [4]. From the most recent systematic reviews and meta-analyses, it appears that at a follow-up of 5 years, MAB with antiandrogens provides a small but statistically significant survival advantage (<5%) when compared with LHRH monotherapy [6,19,20].
Initially, ADT is effective against prostate cancer. But nearly all patients develop tumor progression despite castrate levels of testosterone [4-8,21]. This clinical condition has been described as androgen-independent or hormone-refractory prostate cancer (HRPC). However, these previously used terms have largely been replaced with the term CRPC, with the awareness of the persistent androgen receptor signaling activity despite castrate serum testosterone levels [5]. It is important to differentiate CRPC from true HRPC. Although CRPC responds to secondary hormonal manipulations, true HRPC resists all hormonal measures [6].
No clear-cut recommendation can be made for patients with PSA elevation after first-line MAB. In the 2010 version of the European Association of Urology (EAU) guidelines, it is recommended to continue ADT with LHRH agonists, despite PSA progression; to stop antiandrogen therapy once PSA progression is documented after first-line MAB; and then to assess AWS [6]. AWS was initially documented in patients who discontinued flutamide upon the development of CRPC [6,22]. In many previous studies, response was observed within 2 to 6 weeks after antiandrogen withdrawal and ≥50% PSA reduction was achieved in 15% to 30% of patients. But the response duration is short, with a median duration of 3.5 to 5 months [6,10,11,14,22,23]. In the present study, 7 of 47 patients were evaluated for AWS. AWS was observed in 3 of 7 (42.9%) patients with a mean response duration of 2.7 months. Because there is no standard treatment for patients with disease progression after first-line MAB, not all of those enrolled but only a small number of patients were assessed for AWS. Therefore, our results are not consistent with the analysis of previous reports related to AWS.
Based on prospective randomized phase 3 trials, docetaxel at 75 mg/m2 at 3-wk intervals in combination with prednisone represents the cytotoxic regimen of choice in men with CRPC resulting in a median survival benefit of 3 months and a significant improvement of pain and quality of life when compared with mitoxantrone [6,24,25]. But most cytotoxic chemotherapy produces severe systemic side effects and reduces quality of life. Therefore, the antiandrogen may be switched to an alternative antiandrogen in patients who relapse after initial MAB.
The effectiveness of alternative antiandrogens has been demonstrated through various studies [1,9-15]. Miyake et al reported that second-line ADT (flutamide) resulted in a reduction of the PSA level in 25 of 55 patients (45%), the median (range) duration of the PSA response was 6 (1-13) months after the start of second-line therapy [13]. In the study by Suzuki et al, after switching to an alternative antiandrogen (from bicalutamide to flutamide and from flutamide to bicalutamide), ≥50% and <50% PSA reductions were observed in 35.8% and 25.4% of patients, respectively, and the overall response duration was more than 202 days [11]. Nakabayashi et al also reported that nilutamide appeared to have activity as a secondary ADT, and 8 of 45 patients (40%) achieved ≥50% PSA reductions with a median (range) time to progression of 4.4 (range, 0.31-44.7) months [14]. In the present study, after second-line MAB, there was a PSA reduction in 32 of the 47 (68.1%) patients and 23 of the 47 (48.9%) patients achieved ≥50% PSA reduction. The mean duration of PSA response was 13.4±5.4 months in the ≥50% PSA reduction group and 12.2±6.2 months in the <50% PSA reduction group. The mean duration of PSA response in our studies was longer than in previous reports. This result may be because of the relatively small study population (sample size) with three patients showing a very long response duration (21, 25, and 29 months) and early switching to an alternative antiandrogen by frequent PSA follow-up.
In view of the clinical characteristics of responders to second-line MAB, various predictive factors have been suggested through previous studies. Suzuki et al reported that the significant clinical factors that predicted the response to second-line ADT were clinical stage, M category, and the response to first-line MAB therapy [11]. Nishimura et al concluded that patients who had a longer response duration to first-line MAB had a significantly greater response to second-line MAB [12]. Miyake et al reported that patients without bone metastases or those whose disease progressed >1 year after first-line therapy had a significantly higher incidence of PSA response to second-line therapy [13]. Compared with previous studies, our study showed that the significant clinical factor predicting response to second-line MAB was the time to nadir PSA level after first-line MAB.
This study had several limitations. First, our study was retrospective and had a relatively small study population. Furthermore, only 7 of 47 patients (14.9%) were assessed for AWS. For these reasons, the mean response duration of second-line MAB and the predictive factors of good responsiveness to second-line MAB differed from previous studies. Second, we could not evaluate patient survival, such as cause-specific survival and overall survival. Large, prospective, randomized clinical trials including the analysis of survival benefit of alternative antiandrogen therapy should be conducted.
CONCLUSIONS
Our results demonstrate PSA reduction in 68.1% of patients who relapse after initial MAB, with a response duration of 13 months, and confirm previous findings that second-line MAB using an alternative antiandrogen is effective. Also, our results show that the time to nadir PSA level after first-line MAB predicts responsiveness to second-line MAB.
Second-line MAB using an alternative antiandrogen is an attractive therapeutic option and can be used to delay time to cytotoxic chemotherapy. Further investigation is necessary to clarify the clinical factors that predict responsiveness to second-line MAB and to assess whether alternative antiandrogen therapy contributes to survival benefit.
Footnotes
The authors have nothing to disclose.
References
- 1.Sharifi N, Dahut WL, Figg WD. Secondary hormonal therapy for prostate cancer: what lies on the horizon? BJU Int. 2008;101:271–274. doi: 10.1111/j.1464-410X.2007.07236.x. [DOI] [PubMed] [Google Scholar]
- 2.Seruga B, Tannock IF. The changing face of hormonal therapy for prostate cancer. Ann Oncol. 2008;19(Suppl 7):vii79–vii85. doi: 10.1093/annonc/mdn477. [DOI] [PubMed] [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010;17:R305–R315. doi: 10.1677/ERC-10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chodak GW. Maximum androgen blockade: a clinical Update. Rev Urol. 2005;7(suppl 5):S13–S17. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim SI. Current treatment strategies for castration-resistant prostate cancer. Korean J Urol. 2011;52:157–165. doi: 10.4111/kju.2011.52.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59:572–583. doi: 10.1016/j.eururo.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Van Allen EM, Ryan CJ. Novel secondary hormonal therapy in advanced prostate cancer: an update. Curr Opin Urol. 2009;19:315–321. doi: 10.1097/MOU.0b013e328329b73a. [DOI] [PubMed] [Google Scholar]
- 8.Han KS, Cho KS, Lee SH, Hong SJ. Estramustine phosphate based chemotherapy for hormone refractory prostate cancer. Korean J Urol. 2007;48:684–690. [Google Scholar]
- 9.Okegawa T, Nutahara K, Higashihara E. Alternative antiandrogen therapy in patients with castration-resistant prostate cancer: a single-center experience. Int J Urol. 2010;17:950–955. doi: 10.1111/j.1442-2042.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 10.Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Okihara K, Miyake H, Fujisawa M, Miyoshi S, Matsumoto T, et al. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol. 2008;180:921–927. doi: 10.1016/j.juro.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura K, Arichi N, Tokugawa S, Yoshioka I, Kishikawa H, Ichikawa Y. Effects of flutamide as a second-line agent for maximum androgen blockade of hormone refractory prostate cancer. Int J Urol. 2007;14:264–267. doi: 10.1111/j.1442-2042.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyake H, Hara I, Eto H. Clinical outcome of maximum androgen blockade using flutamide as second-line hormonal therapy for hormone-refractory prostate cancer. BJU Int. 2005;96:791–795. doi: 10.1111/j.1464-410X.2005.05766.x. [DOI] [PubMed] [Google Scholar]
- 14.Nakabayashi M, Regan MM, Lifsey D, Kantoff PW, Taplin ME, Sartor O, et al. Efficacy of nilutamide as secondary hormonal therapy in androgen-independent prostate cancer. BJU Int. 2005;96:783–786. doi: 10.1111/j.1464-410X.2005.05714.x. [DOI] [PubMed] [Google Scholar]
- 15.Kojima S, Suzuki H, Akakura K, Shimbo M, Ichikawa T, Ito H. Alternative antiandrogens to treat prostate cancer relapse after initial hormone therapy. J Urol. 2004;171:679–683. doi: 10.1097/01.ju.0000106190.32540.6c. [DOI] [PubMed] [Google Scholar]
- 16.Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009;41:122–131. doi: 10.4143/crt.2009.41.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huggins C, Hodges CV. Studies on prostate cancer, I. the effect of castration, of estrogen and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 18.Huggins C, Scott WW. Bilateral adrenalectomy in prostatic cancer; clinical features and urinary excretion of 17-ketosteroids and estrogen. Ann Surg. 1945;122:1031–1041. [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt B, Wilt TJ, Schellhammer PF, DeMasi V, Sartor O, Crawford ED, et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology. 2001;57:727–732. doi: 10.1016/s0090-4295(00)01086-4. [DOI] [PubMed] [Google Scholar]
- 20.Moul JW. Twenty years of controversy surrounding combined androgen blockade for advanced prostate cancer. Cancer. 2009;115:3376–3378. doi: 10.1002/cncr.24393. [DOI] [PubMed] [Google Scholar]
- 21.Park SC, Choi HY, Kim CS, Hong SJ, Kim WJ, Lee SE, et al. Predictive variables of the progression to androgen independent prostate cancer after combined androgen blockade. Korean J Urol. 2007;48:408–415. [Google Scholar]
- 22.Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607–609. doi: 10.1016/s0022-5347(17)36163-3. [DOI] [PubMed] [Google Scholar]
- 23.Sartor AO, Tangen CM, Hussain MH, Eisenberger MA, Parab M, Fontana JA, et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426) Cancer. 2008;112:2393–2400. doi: 10.1002/cncr.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 25.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]