Abstract
Background & Aim
Pancreatitis-associated protein (PAP) is a secretory protein not normally expressed in the healthy pancreas but highly induced during acute pancreatitis. While PAP has been shown to be anti-bacterial and anti-apoptotic in vitro, its definitive biological function in vivo is not clear.
Methods
To elucidate the function of PAP, antisense oligodeoxyribonucleotides (AS-PAP) targeting all three isoforms of PAP were administered via intrapancreatic injections (5 mg/kg/day, two days) to rats prior to induction of pancreatitis. Severity of pancreatitis and cytokine gene expression in peripheral blood mononuclear cells (PBMC) were evaluated.
Results
Administration of AS-PAP, but not the scrambled oligodeoxyribonucleotide (SC-PAP) control, reduced pancreatitis-induced PAP expression by 55.2 ± 6.4%, 44.0 ± 8.9%, and 38.9 ± 10.7% for PAP isoforms I, II, and III, respectively, compared to saline-treated controls (P < .05 for all). Inhibition of PAP expression significantly worsened pancreatitis: Serum amylase activity, pancreas wet weight (reflecting edema), and serum C-reactive protein levels all increased in AS-PAP-treated animals compared to SC-PAP-treated controls (by 3.5-, 1.7-, and 1.7-fold, respectively; P < .05 for all). Histopathologic evaluation of pancreas revealed worsened edema, elevated leukocyte infiltration, and fat necrosis after AS-PAP treatment. Gene expressions of IL-1α/β and IL-4 were significantly higher in PBMC isolated from AS-PAP-treated rats compared to SC-PAP controls.
Conclusions
This is the first in vivo evidence indicating that PAP mediates significant protection against pancreatic injury. Our data suggest that PAP may exert its protective function by suppressing local pancreatic as well as systemic inflammation during acute pancreatitis.
Introduction
Acute pancreatitis is an acute inflammatory response to pancreatic injury and induces important changes in the expression of a number of genes in the pancreas.1, 2 Among these, the most profound change is that of the pancreatitis-associated protein (PAP) family, the expression of which is very low in the normal pancreas and becomes strongly overexpressed after even mild pancreatic inflammation.1, 3, 4
PAP is a family of secretary proteins expressed in the gastrointestinal tract and was originally isolated from the pancreatic juice of rats with acute pancreatitis.3 Currently, three PAP genes have been characterized in human5 and rat.6–8 Sequence comparisons demonstrate that they are closed related,9, 10 although differing in their expression pattern. PAP II mRNA is specifically expressed in the pancreas, whereas PAP I and III mRNA are normally expressed in the small intestine.7, 11 Results of PAP gene regulation studies suggest that PAP is an acute phase stress protein secreted from pancreas. PAP protein, not detectable in the healthy pancreas, is significantly expressed six h after induction and reaches maximal expression after 48 h.12 PAP accounts for about 5% of the secretary proteins during acute pancreatitis and can be detected in blood within 48 h of induction of acute pancreatitis.13 We have previously reported a direct relationship between severity of pancreatitis and serum levels of PAP.13
Although PAP is the most highly upregulated and abundant protein in acute pancreatitis, and has been shown to be antibacterial, anti-apoptotic, and mitogenic in vitro, little is known about PAP’s definitive biological and/or pathophysiological function in vivo. Sequence analysis of PAP reveals the presence of a carbohydrate recognition domain in the protein, suggesting that PAP I might act as a carbohydrate-binding lectin. It has been shown that PAP I can aggregate bacteria in suspension.6 Therefore, PAP may function as an endogenous anti-bacterial agent and be protective against infectious complications of pancreatitis, which can lead to severe disease with high mortality. Other in vitro studies indicate antiapoptotic and mitogenic activity of PAP in acinar cells. In addition, PAP expression is upregulated by free radicals or cytokines, and such upregulation confers cellular resistance to apoptosis.14 Previous results from our lab also show that reg III (PAP) isolated from cow is mitogenic for pancreatic-derived cells, thus implicating PAP in the proliferative response of pancreas to injury.15 An antiinflammatory effect of PAP has been found to protects the lung from leukocyte- induced injury.16 These results suggest a protective role of PAP in pancreatitis. However, there is as yet no report on the physiological function of PAP in the pancreas.
Pancreatitis is an important clinical problem, for which treatment remains largely supportive. It is our hypothesis that during acute pancreatitis, the pancreas turns on a defense mechanism that includes expression of PAP and other stress proteins that enable the survival of pancreas under conditions of acute stress. In the present study, using antisense technology, we investigated the critical role of PAP both in modulating pancreatic inflammation and in possibly protecting against subsequent injury. An antisense oligonucleotide (AS-PAP) was designed to target all three isoforms of PAP and was delivered locally via intrapancreatic injection in rats in which acute pancreatitis was later induced. To control for any nonspecific effects of treatment with AS-PAP, a similar but purposely scrambled oligonucleotide (SC-PAP) was also evaluated.
Materials and Methods
Animals
Sprague Dawley rats obtained from Harlan Sprague Dawley (Indianapolis, IN) and weighing 175–200 g at onset of studies served as subjects. They were fed standard laboratory chow, given water ad libitum, and randomly assigned to control or experimental groups.
Antisense and scrambled oligonucleotides
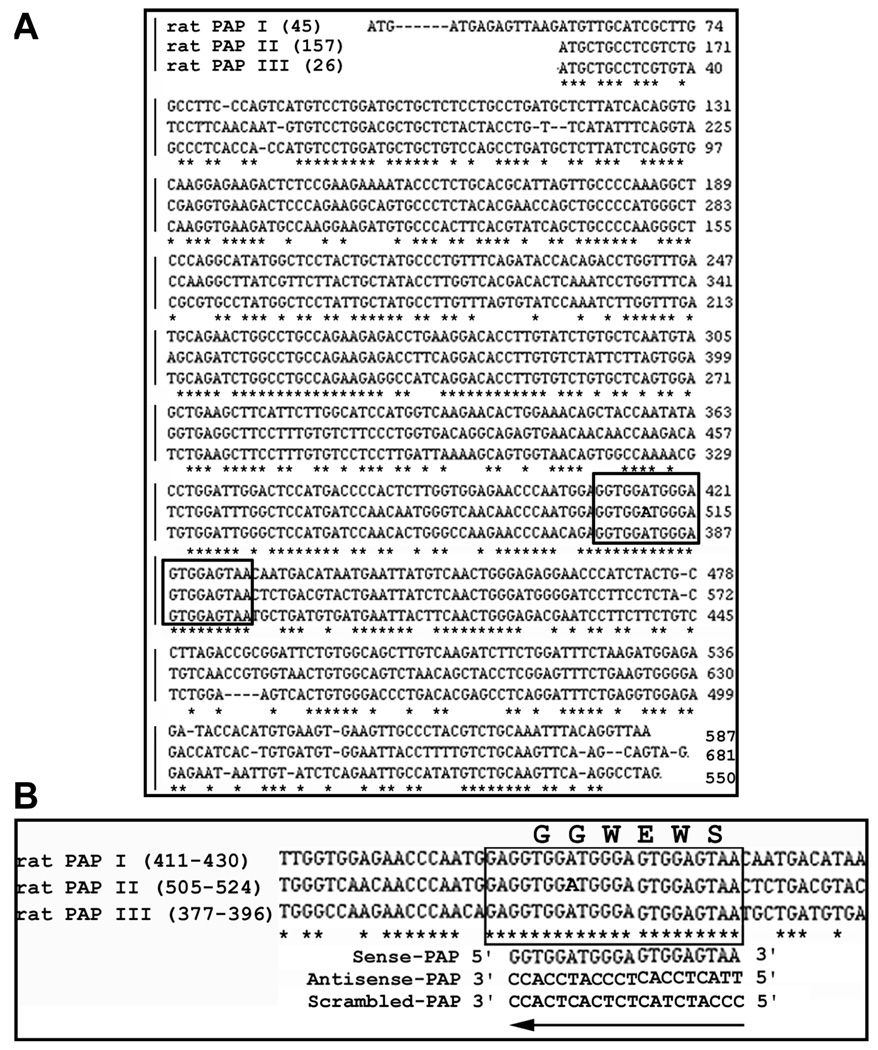
The mRNA (cDNA) sequences of all three isoforms of rat PAP (PAP I,6 PAP II,7 and PAP III8) were retrieved from the National Center for Biotechnology Information nucleotide database. The open reading frames of the three sequences were aligned using GeneTool 2.0 software (BioTools Incorporated, Edmonton, Alberta, Canada) and searched for potential binding sites. As shown in Figure 1, a 20-base region (5′ – ggt gga tgg gag tgg agt aa – 3′) coding for the GGWEWSN peptide was found to be homologous among all three PAP isoforms. This sequence corresponds to PAP I6 mRNA (NM_053289) nt no. 411–430, PAP II7 mRNA (L10229) nt no. 505–524, or PAP III8 mRNA (L20869) nt no. 377–396. An AS-PAP oligonucleotide was then designed to be complementary to this 20-base consensus region, and targeted to inhibit all three isoforms of PAP gene expression (Figure 1B). Purposely scrambled variants of the sequences were used as controls. Phosphorothioate oligodeoxyribonucleotides of both AS-PAP and SC-PAP were synthesized and purified by Molecular Research Laboratory (Herndon, VA) using protocols reported earlier.17 The sequences of oligonucleotides are as follows: AS-PAP: 5′ – tta ctc cac tcc cat cca cc – 3 ′, SC-PAP: 5′ – ccc atc tac tct cac tca cc – 3′. The sequence homologies were checked against reported DNA sequences available in the BLAST program in order to limit the chances of hybridization to other known targets.
Figure 1.
Design of the AS-PAP oligonucleotide targeting all three isoforms of rat PAP. (A.) Alignment result of the cDNA (open reading frame) sequence for rat PAP I, II, and III. A 20-base region homologous among all three isoforms of PAP was located (boxed). (B.) Enlarged view of the 20-base homologous nucleotide sequence and its deduced amino acid sequence. The antisense-PAP oligonucleotide was then designed to be complementary to this 20-base consensus region and target the inhibition of all three PAP isoforms. Purposely scrambled variants of the antisense-PAP sequences (scrambled-PAP) were designed to serve as controls. (Arrows) Amplification direction (5′ → 3′). *Nucleotides that are identical in all 3 species.
Pancreatitis induction and treatment with antisense or scrambled oligonucleotides
Pancreatitis was induced using retrograde infusion of 4% sodium taurocholate (NaT) (Sigma, St. Louis, MO) into the pancreatic duct as previously described.13 Briefly, under pentobarbital (Abbott Laboratories, North Chicago, IL) anesthesia (50 mg/kg given intraperitoneally), a midline incision was performed. The common bile duct was identified and cannulated in an antegrade direction with PE-10 tubing (Fisher Scientific, Pittsburgh, PA) such that the proximal end of the tube was beyond the ampulla of Vater in the duodenum. The bile duct was then ligated to prevent the flow of bile and 4% NaT in sterile saline was infused into the pancreatic duct at a rate of 1 ml/kg over 10 min.18
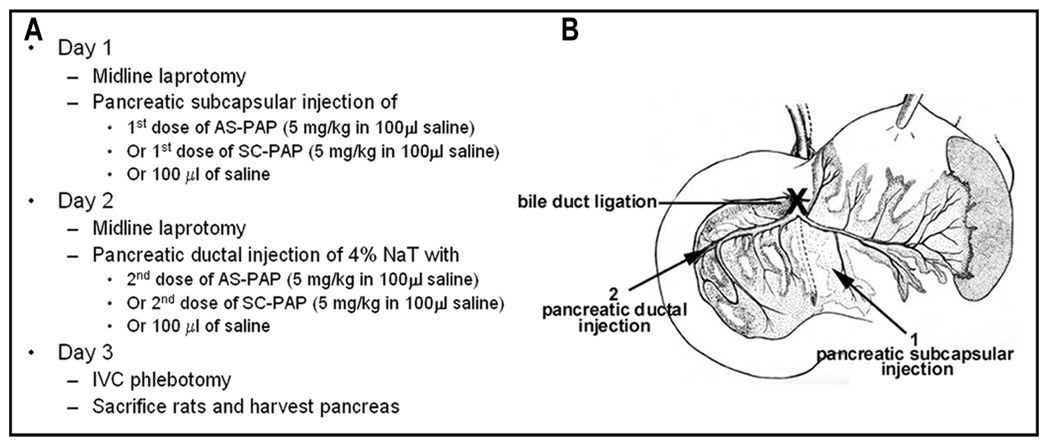
Phosphorothioate AS-PAP or the control SC-PAP was suspended in sterile saline and given twice at a dose of 5 mg/kg/day during the experiment. As shown in Figure 2, the first dose was given via intrapancreatic subcapsular injection (100 µl in saline) 24 h prior to the administration of NaT for induction of pancreatitis. The second dose was given at the time of pancreatitis induction via pancreatic duct infusion (100 µl in saline) together with NaT administration. Control rats were given a comparable injection of SC-PAP or saline. Twenty-four h after pancreatitis induction, rats were sacrificed. Pancreas and blood samples were harvested immediately after sacrifice. Eight rats were included in each of the three groups (saline, SC-PAP, AS-PAP).
Figure 2.
Intrapancreatic administration of oligonucleotides in rats. (A.) Three-day surgery and treatment protocol for saline, SC-PAP, and AS-PAP infusion in rats. (B.) Schematic drawing of bile duct ligation, and pancreatic subcapsular and ductal injection.
Semiquantitative RT-PCR
Total RNA was isolated from rat pancreas using TRIzol (Gibco BRL, Grand Island, NY) as previously reported.19 Samples were then treated with ribonuclease (RNase)-free deoxyribonuclease (DNase) (Ambion, Austin, TX), and subjected to semiquantitative RT-PCR.19 One µg of DNase-pretreated total RNA was reverse-transcribed with random decamer in 20-µl reactions using a RETROscript Kit (Ambion). PCR amplification of each gene product was carried out in parallel 50-µl reactions using PCR Core Systems I (Promega, Madison, WI) with 1 mM of specific forward and reverse primers and 2 µl of RT product. β-actin was used as an internal control for sample loading. Amplifications were carried out as follows: first, denaturation at 94°C for 2 min; subsequently, 30 (for PAP) or 20 (β-actin) cycles of denaturation at 94°C for 10 s, annealing at 55°C for 2 min and extension at 74°C for 2 min; and finally, extension at 74°C for 10 min. The specific forward and reverse primers were designed based on published cDNA sequences of rat PAP I, II, and III (GenBank accession nos. NM_053289, L10229 and L20869). The sequences and sizes of the amplified cDNAs are listed in Table 1. After PCR, a 20-µl aliquot from each PCR amplification was loaded onto 1.5% agarose gels with 0.5 mg/ml of ethidium bromide and subjected to electrophoresis. Gels were photographed using Polaroid 667 film and digitized using an Epson 636 scanner. Band density analysis was performed using the public domain NIH Image program (available at http://rsb.info.nih.gov/nih-image/) to determine the quantity of PCR product. To account for differences in the amounts of starting RNA between samples, the density of each PAP I, II, or III band was normalized by division by the density of the β-actin band for the same sample.
Table 1.
Primers for semi-quantitative PCR.
| Gene (Accession no.) | Primer Sequences (Forward/Reverse) | Position (nt no.) | Size (bp) |
|---|---|---|---|
| PAP I | F 5′-GAAGACTCTCCGAAGAAAATACCC-3′ | 138–161 | 447 |
| (NM_053289) | R 5′-ACCTGTAAATTTGCAGACGTAGGG-3′ | 584-561 | |
| PAP II | F 5′-ATCCCAGATCACTGCAAGGC-3′ | 1–20 | 370 |
| (L10229) | R 5′-AAGGTCTCTTCTGGCAGGCC-3′ | 370-351 | |
| PAP III | F 5′-GAAGATGCCAAGGAAGATGTG-3′ | 104–124 | 447 |
| (L_20869) | R 5′-CTAGGCCTTGAACTTGCAGAC-3′ | 550-530 | |
| β-actin | F 5′-TGGTGGGTATGGGTCAGAAGG-3′ | 131–151 | 808 |
| R 5′-ATCCTGTCAGCGATGCCTGGG-3′ | 938-919 |
Real-time quantitative RT-PCR
One-step real-time quantitative RT-PCR for PAP I, II, and III was performed based on the fluorogenic 5′-nuclease assays previously described.20, 21 The assay, which confers very high specificity, was carried out using a GeneAmp 5700 sequence-detection system (PE Biosystems, Foster City, CA), with β-actin as an endogenous control to standardize the amount of sample RNA added to a reaction. Primers and probes were designed using Primer Express software (PE Biosystems). Sequences for all primers and probes used in these analyses are listed in Table 2. All primers and probes and other reagents for real-time quantitative PCR were purchased from Applied Biosystems. One hundred ng of total RNA was used to set up 25-µl real-time quantitative PCRs that consisted of 1 X TaqMan Universal PCR Master Mix, 500 nM forward and reverse primers, and 200 nM TaqMan probe. PCR amplification was carried out with the following temperature profile: 30 min at 48°C; 10 min at 95°C; and 40 cycles of 15 s at 95°C and 1 min at 60°C. Assays were performed in triplicate.
Table 2.
Taqman primers and probes for real-time quantitative PCR.
| Gene (Accession no.) | Primer/Probe Sequences (Forward/Reverse/Probe) | Position (nt no.) | Size (bp) |
|---|---|---|---|
| PAP I | F 5′-AAAATACCCTCTGCACGCATTAG-3′ | 153–175 | 67 |
| (NM_053289) | R 5′-GGGCATAGCAGTAGGAGCCATA-3′ | 219-198 | |
| P 5′-FAM-TGCCCCAAAGGCTCCCAGGC-TAMRA-3′ | 177–196 | ||
| PAP II | F 5′-CCAGAAGGCAGTGCCCTCTA-3′ | 240–259 | 67 |
| (L10229) | R 5′-GCAGTAAGAACGATAAGCCTTGGA-3′ | 306-283 | |
| P 5′-FAM-ACGAACCAGCTGCCCCATGG-TAMRA-3′ | 261–280 | ||
| PAP III | F 5′-TGTGCCCACTTCACGTATCAG-3′ | 121–140 | 64 |
| (L_20869) | R 5′-GGCATAGCAATAGGAGCCATAGG-3′ | 184-162 | |
| P 5′-FAM-TGCCCCAAGGGCTCGCG-TAMRA-3′ | 143–159 | ||
| β-actin | F 5’-TTCAACACCCCAGCCATGT-3’ | 379–397 | 68 |
| R 5’-GTGGTACGACCAGAGGCATAC A-3’ | 446-425 | ||
| P 5’-FAM-CGTAGCCATCCAGGCTGTGTTGTCC-MGBNFQ-3’ | 399–422 |
Data were analyzed with the relative standard curve method. Standard curves of the genes of interest and β-actin were prepared with three 1:2 dilutions (four points, eightfold range) of total RNA from one of the samples that was expected to have the highest amount of mRNA for the gene of interest. For each reaction tube, the amount of target or endogenous reference was determined from the standard curves. The mean amount of each sample was calculated from the triplicate data and was normalized by division by the mean quantity of β-actin RNA for the same sample. The mean and SD of each treated group were calculated from the normalized value for each rat in that group. The mean value of the saline group was arbitrarily set at 100%. Results from the ASPAP- and SC-PAP-treated groups were divided by the mean value of the saline group to generate the relative expression levels. Tests for significance were performed with Student’s t test, two-tailed, to assess differences between data from saline-, SC-PAP-, or AS-PAP-treated groups. Means are presented ± 1 SD. Significance level was set at P < .05.
Quantitation of serum amylase activity, C-reactive protein (CRP), and pancreatic edema, and evaluation of pancreatic morphology
Serum amylase activity was measured using 4,6-ethylidene (G7)-p-nitrophenyl (G1)-α1D-maltoheptaoside as the substrate.22 CRP levels were determined in the clinical laboratory using an immunoassay system and Beckman nephelometer (Beckman Coulter, Inc., Galway, Ireland).23 The extent of pancreas edema was quantitated by the ratio of pancreas wet weight over subjects’ total body weights.13 For morphologic analysis, 5-µm-thick paraffin sections of pancreas samples were stained with H&E. Ten randomly chosen microscopic fields were examined for each tissue sample, and inflammation as well as necrosis were scored as follows: none = 0; mild = 1; moderate = 2; and severe = 3.24
Cytokine gene expression profile by RNase protection assay
Peripheral blood mononuclear lymphocytes (PBMC) were isolated from rat whole blood using Ficoll-Paque (Pharmacia, Biotech AB, Uppsala, Sweden). Total RNA was extracted from PBMC after induction of pancreatitis and treatment with antisense PAP or scrambled PAP using TRIzol (Gibco) and quantified as previously described.25 RNase protection analysis was done using the RiboQuant Multi-Probe RNase protection assay system (PharMingen, San Diego, CA). In this assay, high-specific-activity RNA probes are synthesized from a mixture of DNA templates of distinct lengths, and that correspond to a sequence in a distinct messenger RNA (mRNA) species. Template set hCK-1 (PharMingen) was used; hCK-1 contains templates for IL-1α/β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, tumor necrosis factor-α/β (TNF-α/β) and interferon-γ (IFN-γ), and for the ubiquitously expressed genes for ribosomal protein L32 and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH). T7 polymerase-directed antisense RNA probe synthesis, hybridization to total RNA, RNase treatments, and gel resolution of protected probes were done as described by the manufacturer. Briefly, the probe was synthesized by labeling the template with [α-32P] UTP (New England Nuclear, Boston, MA) using T7 RNA polymerase. A total of 6 × 105 cpm of labeled probe in hybridization solution was added to 10 µg total RNA, and the samples were overlaid with mineral oil before brief heating to 90°C; hybridization continued for 16 h at 56°C. After hybridization, the samples were digested for 45 min at 30°C with a mixture of RNase A and RNase T1 (as recommended in the kit), treated with proteinase K, extracted with phenol/chloroform/isoamyl alcohol (50:50:0.5), precipitated with ethanol, and resolved on 5% denaturing polyacrylamide sequencing gels. Radioactive fragments were detected by autoradiography and also by PhosphorImager (Molecular Dynamics, Sunnyvale, CA), from which quantification of mRNA was done by a volume integration protocol using ImageQuant software (Molecular Dynamics). To control for differences in sample processing, hybridization signals in each sample were divided by the signal for L32. Fold increases in mRNA levels in AS-PAP-treated groups were determined by comparison with SC-PAP-treated controls.
Statistical analysis
The results reported represent mean ± SD or SEM values obtained from multiple determinations in 3 or more separate experiments. In any one experiment, there were 8 animals in each treatment group. Statistical analyses were carried out by 2-way or 3-way analysis of variance, with the independent variables being saline, SC-PAP, or AS-PAP treatment. The significance of changes was evaluated by Tukey’s post hoc test. A value of P < .05 was considered statistically significant.
Results
PAP mRNA levels are highly induced in acute pancreatitis
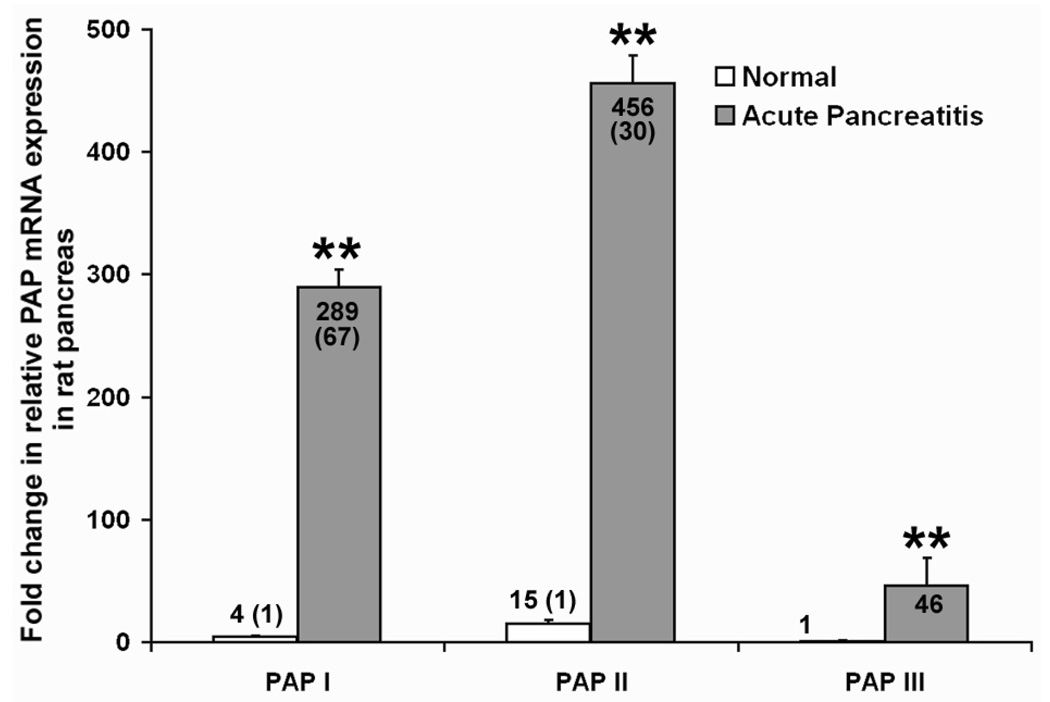
As shown in Figure 3, all three isoforms of PAP are normally expressed at very low levels in the healthy pancreas. After induction of pancreatitis by retrograde infusion of the pancreatic duct with of 4% NaT, pancreatic PAP mRNA levels dramatically increased within 24 h. Real-time quantitative PCR results (Figure 3) show that PAP II was the most abundant isoform, followed by PAP I, and then PAP III. Compared to normal PAP III (the lowest expressed), mRNA expression of PAP I, II, and III was 289.3 ± 14.4-fold, 456.1 ± 22.8-fold, and 46.3 ± 22.8-fold higher, respectively, in the pancreas 24 h after pancreatitis induction. PAP mRNA expression increased 67-, 30-, and 46-fold for PAP I, II, and III, respectively, after acute pancreatitis, when compared to its own normal expression (P < .05 for all comparisons).
Figure 3.
PAP mRNA levels in normal rats vs. rats with acute induced pancreatitis. Acute pancreatitis was induced by 4% NaT, and subjects were sacrificed 24 h later. RNA was prepared from pancreas samples from nonoperated or treated rats. PAP I, II, and III mRNA levels were determined by real-time RT-PCR analysis, using PAP isoform-specific TaqMan primers and probes. The amount of mRNA in each group was normalized to β-actin. Results are presented as fold change in PAP I, II, or III mRNA in normal (white bars) or acute pancreatitis (gray bars) conditions when compared to normal PAP III levels. A ratio of 1 represents the PAP III (the lowest expressed) mRNA level in non-operated rats. Fold change values of PAP I, II and III when compared its own normal expression are presented in brackets. The bar represents the mean ± SD (n = 8, ** P < .05 for PAP I, II and III when comparing the pancreatitis-induced group with the non-operated control group).
Efficient inhibition of three isoforms of PAP by antisense treatment
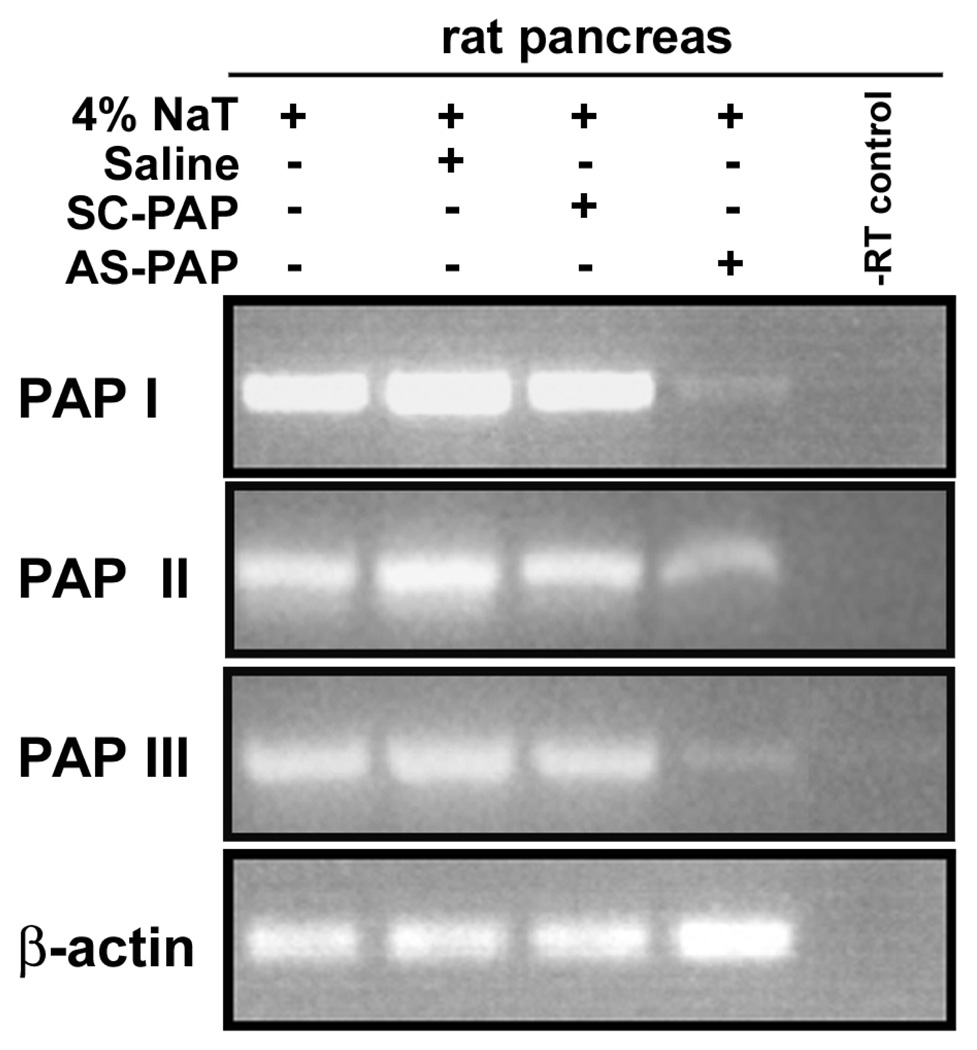
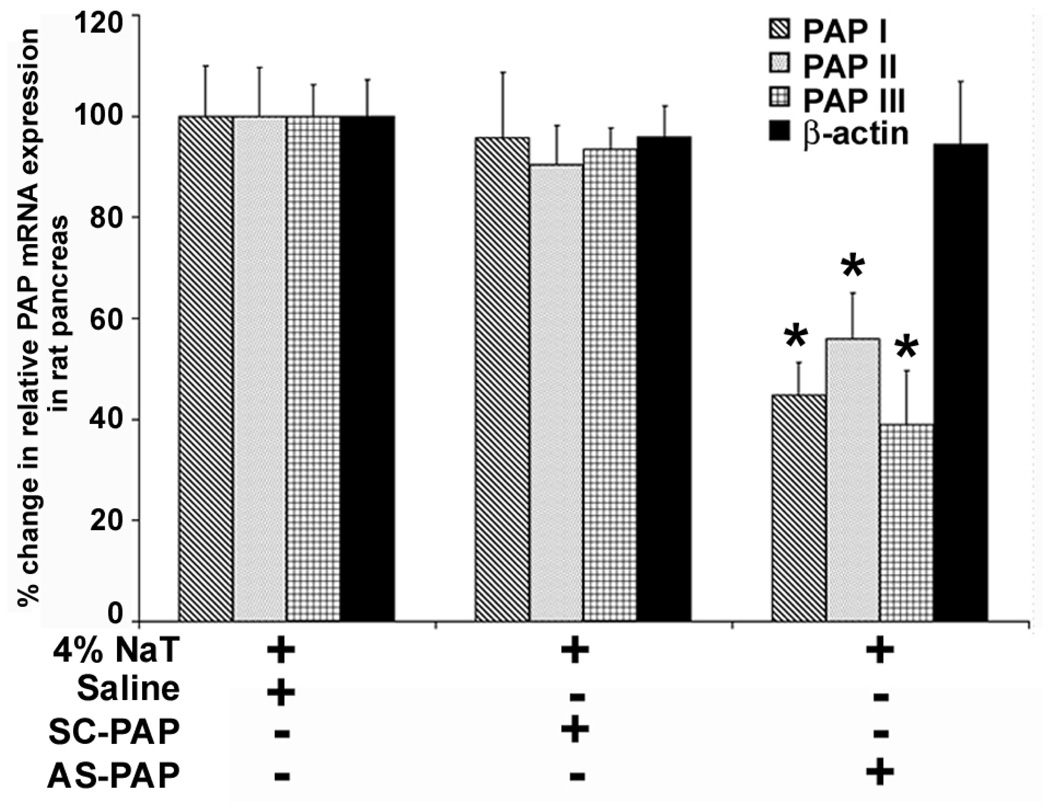
Due to the unavailability of an antibody against rat PAP I, II, or III, semiquantitative RT-PCR and real-time quantitative RT-PCR were performed to demonstrate the inhibitory effects of AS-PAP oligonucleotide on the expression of targeted genes. As shown in Figure 4, gel electrophoresis of the RT-PCR products with PAP I-, II-, and III-, and β-actin-specific primers revealed a single band of 447, 370, 447, and 808 bp, respectively. These bands corresponded in size to the RT-PCR products expected from rat PAP I, II, and III mRNA, respectively. Sequence analysis of these RT-PCR products confirmed their identities. As shown in Figure 4, administration of the AS-PAP oligonucleotide, but not SC-PAP oligonucleotide before induction of pancreatitis significantly and comparably inhibited expression of all three PAP isoforms. Real-time quantitative RTPCR (Figure 5) further quantified the inhibitory effect of AS-PAP, and revealed that mRNA levels of PAP I, II, and III were decreased by 55.2 ± 6.4%, 44.0 ± 8.9%, and 38.9 ± 10.7%, respectively, after AS-PAP treatment, compared to saline-treated control animals, which expression (highest among all three groups) was arbitrarily set to 100% (P < .05 for all). As shown by both semiquantitative RT-PCR and real-time quantitative PCR, treatment with SC-PAP oligonucleotide, in contrast to AS-PAP, did not attenuate pancreatitis-induced PAP levels (95.7 ± 13.0%, 90.4 ± 7.7%, and 93.4 ± 4.3% for PAP I, II, and III compared to saline-treated controls). Also, administration of neither AS-PAP nor SC-PAP oligonucleotide altered levels of β-actin, thereby confirming the specificity of the antisense oligonucleotide for PAP genes. Overall, semi-quantitative PCR showed results similar to those from real-time quantitative RT-PCR, confirming a significant and specific inhibitory effect of the AS-PAP oligonucleotide.
Figure 4.
Semiquantitative RT-PCR analysis of the effects of the administration of AS-PAP or SC-PAP on pancreatic PAP expression in rats with acute pancreatitis. Rats were pre-treated with saline, SC-PAP, or AS-PAP (5 mg/kg/day) for 2 days and then acute pancreatitis was induced by 4% NaT. After 24 h, rats were sacrificed and RNA was prepared from pancreas samples from all rats. PAP I, II, III, and β-actin mRNA levels were determined by semi-quantitative RT-PCR analysis using PAP-isoform-specific primers. Representative RT-PCR products for PAP I, II, III and β-actin from rats with pancreatitis and treated with saline, SC-PAP, or AS-PAP are shown. The experiment shown is representative of at least three experiments.
Figure 5.
Real-time RT-PCR analysis of the effects of the administration of AS-PAP or SC-PAP on pancreatic PAP expression in rats with acute pancreatitis. The experimental details are the same as in Figure 4. PAP I, II, and III mRNA levels were determined by real-time quantitative RT-PCR analysis using PAP-isoform-specific TaqMan primers and probes. The amount of mRNA in each group was normalized to β-actin. Results are presented as percent change in PAP I, II, or III mRNA in saline-, SC-PAP-, or AS-PAP-treated rats with acute pancreatitis. The expression of PAP in saline-treated rats was arbitrarily set at 100%. Percent change was calculated based on the ratio of PAP I, II, and III expression in SC-PAP or AS-PAP treated rats to saline-treated rats. The bar represents the mean ± SD (n = 8, * P < .05 for PAP I, II, and III when comparing the AS-PAP-treated group with the saline-treated control group).
Significant worsening of pancreatitis after targeted disruption of PAP gene expression
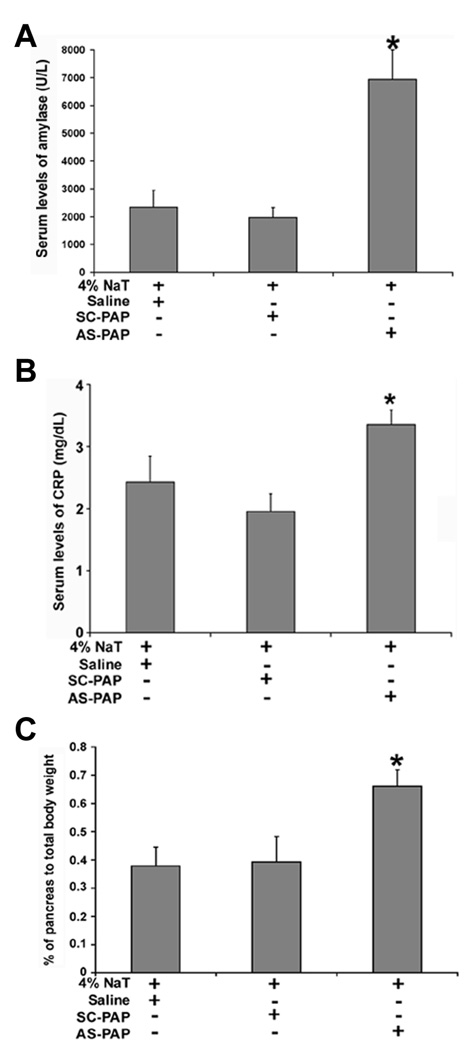
Hyperamylasemia, pancreatic edema, elevated serum CRP levels, and acinar-cell inflammation are the hallmark parameters associated with the severity of pancreatitis. Quantitation of serum amylase activity, CRP, and pancreatic edema, as well as evaluation of pancreatic morphology was performed to evaluate severity of pancreatitis. We observed adverse changes in each of these parameters in our present study after retrograde infusion of the pancreatic duct with 4% NaT and AS-PAP oligonucleotides. As shown in Figure 6A, in the AS-PAP-oligonucleotide-treated group, serum amylase activity significantly increased (by 3.5-fold, from 1970 ± 931 U/L to 6938 ± 1067 U/L) compared to the SC-PAP-treated group (P < .05). Similarly, as shown in Figure 6B, serum CRP levels were significantly elevated (by 1.7-fold, from 2.0 ± 0.3 mg/dL to 3.4 ± 0.2 mg/dL) in AS-PAP-treated animals compared to the SC-PAP-treated group (P < .05). Finally, as shown in Figure 6C, pancreas wet weight, which reflects pancreatic edema, was significantly elevated (by 1.7-fold, from 0.39 ± 0.09% to 0.66 ± 0.06%) in AS-PAP-treated animals compared to the saline-treated control group (P < .05). No significant change was noted between the saline-treated and the SC-PAPoligonucleotide-treated groups.
Figure 6.
Effect of administration of AS-PAP or SC-PAP on (A) serum amylase; (B) Serum CRP; and (C) pancreatic wet weight in rats with acute pancreatitis. The experimental details are as described in Figure 3. The bar represents the mean ± SD (n = 8, * P < .05 for all when comparing the AS-PAP-treated group with the SC-PAP-treated control group).
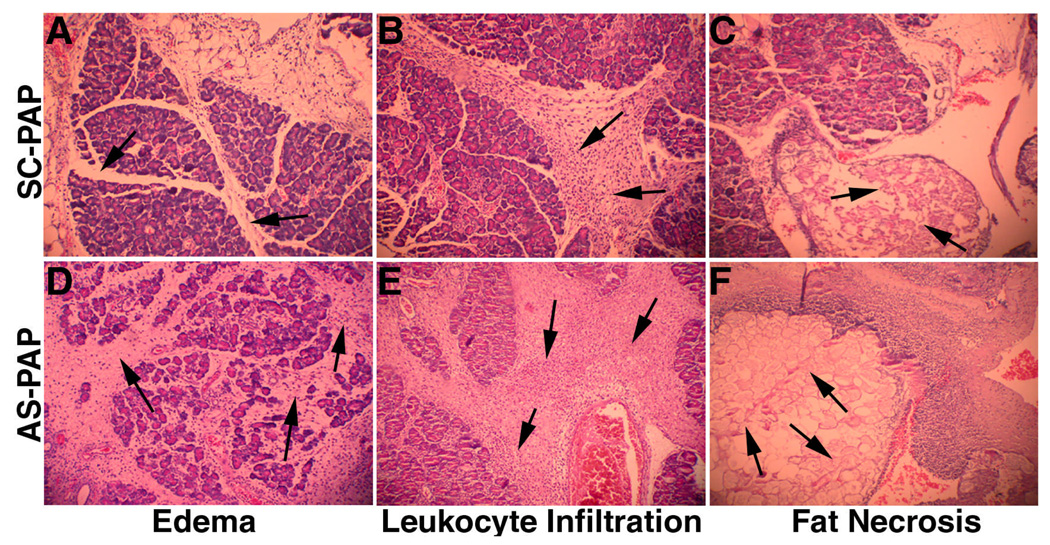
The effects of AS-PAP treatment on the morphology of pancreatic tissue infused with NaT in vivo are shown in Figure 7 and Table 3. Significantly increase in pancreatic inflammation indicated by edema, leukocyte infiltration, and fat necrosis was observed. “Blinded” histology grading results show that inflammation score increased from 1.0 ± 0.1 of SC-PAP-treated group to 1.8 ± 0.4 of AS-PAP-treated group. Area percentage of pancreatic necrosis also increased from approximately 45% of SC-PAP treated group to 67% of AS-PAP-treated group. (P < .05 for both).
Figure 7.
The effects of the administration of AS-PAP or SC-PAP on the morphology of the pancreas in rats with acute pancreatitis. Representative light micrographs of the H&E–stained pancreas sections from SC-PAP-treated (A through C) and AS-PAP-treated (D through F) groups are presented. Pancreatic inflammation was indicated by edema (A and D), leukocyte infiltration (B and E), and fat necrosis (C and F). Arrows indicate morphologic changes.
Table 3.
Effect of oligonucleotide treatment on NaT-induced pancreatic inflammation and necrosis.
| Groups | Inflammation | % of Necrosis |
|---|---|---|
| Saline + NaT | 1.1 ± 0.1 | 14.3% |
| SC- PAP + NaT | 1.0 ± 0.1 | 44.9% |
| AS-PAP + NaT | 1.8 ± 0.4* | 66.7%* |
Experimental details are same as described for Figure 2. Values are mean ± SEM obtained from at least 3 independent experiments.
P < .05 when compare
PAP suppress IL-1 cytokine expression in PBMC during acute pancreatitis
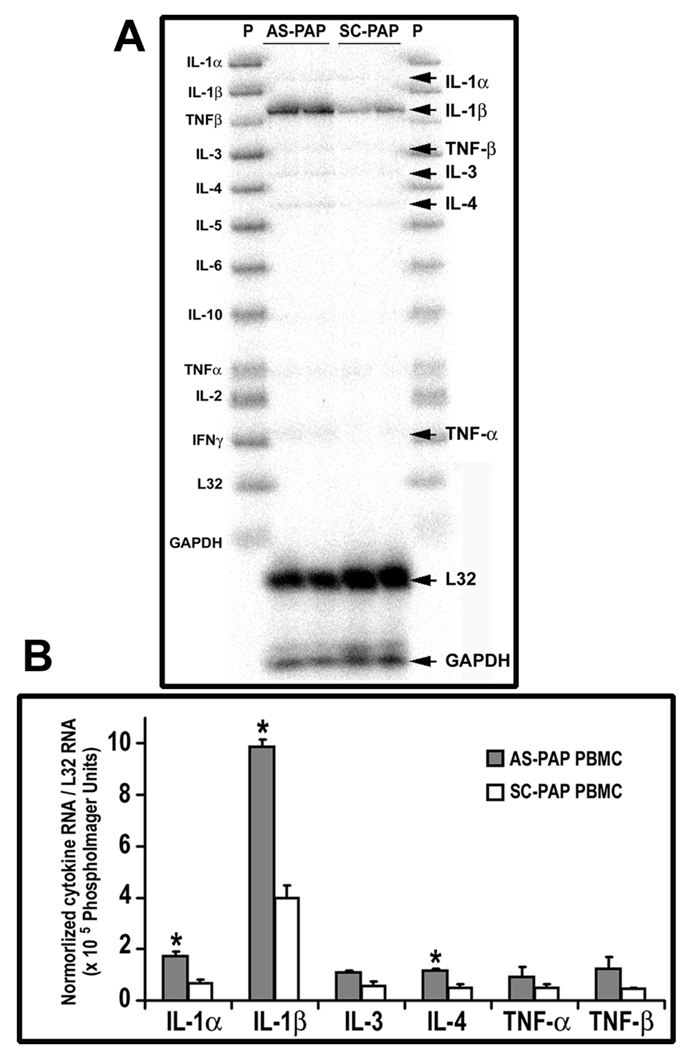
To further study the protective function of PAP in acute pancreatitis, the role of PAP in cytokine expression in PBMC was studied next. mRNA expression of IL-1α/β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, TNF-α/β, and IFN-γ was measured in PBMC isolated from AS-PAP- or SC-PAP-treated animals using an RNase protection assay. As shown in Figure 8, among the many cytokines tested, the acute phase cytokine IL-1β was predominantly expressed in PBMC 24 h after pancreatitis induction by NaT. Administration of the ASPAP oligonucleotide upregulated both IL-1α, IL-1β, and IL-4 gene expression in PBMC by 2.6-, 2.5, and 2.3-fold respectively when compared to control SC-PAP oligonucleotide treatment (P < .05 for both). Expression of IL-3, TNF-α, and TNF-β were also observed to be higher in AS-PAP-treated than SC-PAP-treated group, however the changes are not statistically significant. Our results thus suggest that the protective effects of PAP may be at least partially through the downregulation of proinflammatory cytokine gene expression in peripheral blood lymphocytes.
Figure 8.
The effects of the administration of AS-PAP or SC-PAP on the cytokine gene expression profile in PBMC from rats with acute pancreatitis. (A) RNase protection analysis of RNA isolated from PBMC is shown. Total RNA was extracted from PBMC from AS-PAP-treated or SC-PAP-treated rats with acute pancreatitis. Each lane contained 10 µg of total RNA hybridized to an antisense RNA probe cocktail that contained the templates for genes, protected fragments of which were separated on a 5% DNA sequencing gel and are indicated by arrows. (B) This graph represents the quantification results from 3 independent experiments. The IL-1β signal was normalized to that from L32 (mRNA for the ribosomal protein subunit); Each subsequent bar represents the mean ± SD in PhosphorImager units for the indicated group. *P < .05 when comparing the AS-PAP treated group with the SC-PAP-treated group.
Discussion
In the current study, using antisense-mediated in vivo gene knockdown technology, PAP gene expression was inhibited by locally administering a specific anti-PAP oligonucleotide , which targeted all three isoforms of PAP. Inhibiting PAP expression significantly worsened experimentally induced pancreatitis. Furthermore, our results suggest that PAP has an anti-inflammatory effect in acute inflammation response. We also demonstrated an inverse correlation between PAP expression and parameters associated with local pancreatic inflammation such as pancreatic edema, neutrophilic infiltration, as well as system inflammatory parameters such as serum CRP levels, and cytokine gene expression of IL-1α/β in PBMC. We show, for the first time, that PAP induction during pancreatitis mediates significant protection against subsequent injury, and that this protective function may be anti-inflammatory in nature.
Even though it has been more than 10 years since PAP was found to be the most highly induced protein in the pancreas during pancreatitis, final proof of its role in pancreatitis by a direct genetic approach using knockout technology has not been done. One of the problems with using a PAP knockout system is that multiple isoforms of PAP are expressed in both mice and rats. It is likely that the multiple isoforms of the reg gene play redundant roles in their biological functions. A distinguished phenotype in a single PAP-deficient animal may be due to functional compensation by the other PAP gene family members during the disease. In order to define a definitive function of PAP in acute pancreatitis, we pursued an alternative reverse genetic approach by inhibiting expression of all three isoforms of PAP in vivo using a single antisense oligonucleotide.
A 20-base-pair consensus nucleotide sequence preserved among all three isoforms of PAP was identified based on sequence comparisons from the open reading frame of PAP mRNA sequences (Figure 1). It has been demonstrated that a 15- to 30-base-pair oligonucleotide is required for complete degradation of both injected and endogenous mRNAs.26 Although PAP I, II, and III share more than 60% amino acid identity, this 20-base-pair sequence is the only sequence homologous among PAP I, II, and III that is long enough to be used as a target for antisense technology. As assessed by both semi-quantitative and real-time quantitative RT-PCR, all three isoforms of PAP were simultaneously suppressed after administration of the AS-PAP. We conclude that we have identified a susceptible sequence that can be used as a knockdown target by antisense oligonucleotides for PAP genes. It is our hypothesis that this highly homologous sequence among all three isoforms may harbor important genetic information and may encode a bioactive fragment of PAP. Further study is needed to test the biological function of the peptide GGWEWS, which was targeted by the ASPAP.
The major principle of antisense oligodeoxyribonucleotide (ASODN) application is that ASODN-stimulated RNase H can digest the mRNA-ASODN hybrid.26–28 However, ASODN may also act through other mechanisms that lead to translational arrest. For example, ASODN-mRNA hybrid formation may cause a steric or conformational obstacle to ribosome reading and so prevents protein translation. ASODN may also bind to specific sites of genomic DNA and mRNA to prevent transcription, mRNA splicing, and/or mRNA transport out of the nucleus into the cytoplasm. All of these mechanisms lead to a reduction in mRNA and protein expression. There are currently no commercially available antibodies against rat PAP proteins. Therefore, semiquantitative and real-time quantitative RT-PCR were used to test the effectiveness of the AS-PAP oligonucleotide (Figure 4 and Figure 5). Both methods confirmed the effectiveness of the AS-PAP used in the present study. The change in target gene expression of PAP, as assessed by real-time quantitative RT-PCR, appeared to be similar among the three isoforms, with an average of approximately a 54% reduction when compared to saline-treated animals. As expected, the inhibition of a specific protein in response to antisense treatment in vivo was not complete due to the low uptake efficiency. A similar reduction in heat shock protein 70 using antisense treatment was observed, and significant physiological changes have been detected in pancreas tissue.29
A combination of pancreatic subcapsular and ductal injection was used to deliver ASPAP or SC-PAP locally into the pancreas (Figure 2). Direct intrapancreatic injection yields higher recovery of oligonucleotide in local pancreas tissue compared to intravenous or intraperitoneal injection, in which most of the oligonucleotide is diffused in the blood circulation and consumed by liver and lung.30 It is possible, though, that intrapancreatic injection causes pancreatic injury itself; however, the great difference observed between AS-PAP-treated and SC-PAP-treated animals indicate that any injury caused by intrapancreatic injection was minimal. Due to the rapid degradation of the oligodeoxyribonucleotides in their natural forms in blood and cells by exo- and endo-nucleases, we used phosphorothioated oligos to prolong their activity. One potential problem caused by phosphorothioation is that Phosphorothioate oligodeoxynucleotide, especially those with four repeated guanine (G) residues, tend to bind to proteins nonspecifically.31 Therefore, the G quartet sequences were avoided in our AS- and SCPAP oligonucleotides. In the present study, only AS-PAP produced significant biological changes and a reduction in specific protein/mRNA when compared to saline-treated animals, whereas SC-PAP was ineffective, indicating that the observed inflammatory changes were caused by antisense inhibition of target gene expression, not by the nonspecific action of Phosphorothioate oligodeoxynucleotide. Phosphorothioate oligonucleotides have been shown to be effective and distributed in most tissues when administered either intravenously or intraperitoneally in mice and rats in doses ranging from 5 to 30 mg/kg body weight.30, 32, 33 In a separate group of two animals using 10 mg/kg/day for 2 days, we have observed similar inhibition of PAP expression, indicating that local pancreatic indication of 5 mg/kg/day for 2 days have reached maximum inhibitory effect. Therefore we chose to use 5 mg/kg for 2 days for all animals in this study. Our results show this regimen to be effective in inhibiting PAP mRNA expression in the pancreas.
To evaluate the role of pancreatitis-induced upregulation of PAP expression in limiting the severity of pancreatitis, AS-PAP was administered before pancreatitis induction with NaT. NaT-induced pancreatitis is an experimental model of necrotizing acute pancreatitis.34 Pretreatment with AS-PAP attenuated the rise of PAP and exacerbated the parameters associated with severity pancreatitis.35 The mechanism of PAP’s protective effect in pancreatitis is not yet clearly determined. It is unlikely that the effect is exerted through interference with intra-acinar cell trypsinogen activation, since PAP is without enzymatic activity, nor does it inhibit any enzyme present in pancreatic juice.36
Elevated edema, leukocyte infiltration and fat necrosis in pancreas (Figure 6 and Figure 7) indicated local reduction of PAP in pancreas tissue resulted in an elevated local inflammatory response. Furthermore, the present study’s findings regarding elevated CRP in AS-PAP-treated animals suggest a systemic antiinflammatory role for PAP. CRP is an acute phase reactant, its measure directly reflects the level of inflammation.37 We next investigated whether the local reduction of PAP had a parallel effect in the periphery as well. For this reason we studied PBMC for evidence of periphery inflammatory response (Figure 8). PBMC shuttle between tissue and circulation and the cells could have been activated locally during acute phase of inflammation. The persistence of increased IL-1 expression at 24 hours in circulating PBMC may indicate this activation to be secondary to the suppression of PAP. It is known that CRP production is induced by the liver under the influence of IL-1 and IL-6.37 It is possible that in the absence of PAP, IL-1 is upregulated, which in turn induced CRP expression. Both elevated IL-1 and CRP expression in AS-PAP-treated animal confirmed a systemic antiinflammatory role of PAP. We are currently studying the time course of cytokine activation in pancreas tissue and peripheral in parallel. Further study on in vivo function of PAP in the activation of the complement system, phagocytic cell function, and cell mediated cytotoxicity is needed.
Interestingly, in addition to IL-1, IL-4, a cytokine mainly involved in antibody-mediated immune response and inhibits the production of proinflammatory cytokines (IL-1 and TNF-α), was also found to be higher in PBMC isolated from AS-PAP-treated animals. It is conceivable though, pancreatic inflammation, like other inflammatory process, is a balance event between proinflammatory and anti-inflammatory actions. When PAP is suppressed, both IL-1 and IL-4 expressions are elevated, but IL-1 level is much higher than that of IL-4, indicating a dominant anti-inflammatory effect of PAP. A recent study shows that PAP I induces lung inflammation in rats via activation of TNF-α expression in hepatocytes.38 It is possible that PAP I may induce a proinflammatory response, while PAP II and III induce an anti-inflammatory response or the reported proinflammatory reaction is lung tissue specific. However, PAP I used in the study was purified from rat pancreatic juice using a polyclonal anti PAP I antibody. Further studies using isoform-specific pure recombinant PAP is needed for clarification.
In conclusion, the present study demonstrates a protective function of PAP in acute pancreatitis, and one mechanism of this effect may be via the downregulation of acute phase cytokine gene expression in the peripheral immune system. Our results underscore the role of PAP as an autocrine protector of the pancreas from injury.
Acknowledgments
Grant Support:
Supported by grant DK054511 (to M.E.Z.) from the National Institutes of Health.
Abbreviations
- AS
antisense oligodeoxyribonucleotides
- IL
interleukin
- NaT
sodium taurocholate
- PAP
pancreatitis-associated protein
- PBMC
peripheral blood mononuclear cells
- SC
scrambled oligodeoxyribonucleotide
References
- 1.Iovanna JL, Keim V, Michel R, Dagorn JC. Pancreatic gene expression is altered during acute experimental pancreatitis in the rat. Am J Physiol. 1991;261:G485–G489. doi: 10.1152/ajpgi.1991.261.3.G485. [DOI] [PubMed] [Google Scholar]
- 2.Iovanna JL, Lechene de la Porte P, Dagorn JC. Expression of genes associated with dedifferentiation and cell proliferation during pancreatic regeneration following acute pancreatitis. Pancreas. 1992;7:712–718. doi: 10.1097/00006676-199211000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Keim V, Iovanna JL, Rohr G, Usadel KH, Dagorn JC. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology. 1991;100:775–782. doi: 10.1016/0016-5085(91)80025-5. [DOI] [PubMed] [Google Scholar]
- 4.Stephanova E, Tissir F, Dusetti N, Iovanna J, Szpirer J, Szpirer C. The rat genes encoding the pancreatitis-associated proteins I, II and III (Pap1, Pap2, Pap3), and the lithostathin/pancreatic stone protein/regeneration protein (Reg) colocalize at 4q33-->q34. Cytogenet Cell Genet. 1996;72:83–85. doi: 10.1159/000134168. [DOI] [PubMed] [Google Scholar]
- 5.Orelle B, Keim V, Masciotra L, Dagorn JC, Iovanna JL. Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J Clin Invest. 1992;90:2284–2291. doi: 10.1172/JCI116115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem. 1991;266:24664–24669. [PubMed] [Google Scholar]
- 7.Frigerio JM, Dusetti NJ, Keim V, Dagorn JC, Iovanna JL. Identification of a second rat pancreatitis-associated protein. Messenger RNA cloning, gene structure, and expression during acute pancreatitis. Biochemistry. 1993;32:9236–9241. doi: 10.1021/bi00086a032. [DOI] [PubMed] [Google Scholar]
- 8.Frigerio JM, Dusetti NJ, Garrido P, Dagorn JC, Iovanna JL. The pancreatitis associated protein III (PAP III), a new member of the PAP gene family. Biochim Biophys Acta. 1993;1216:329–331. doi: 10.1016/0167-4781(93)90167-c. [DOI] [PubMed] [Google Scholar]
- 9.Dusetti NJ, Frigerio JM, Keim V, Dagorn JC, Iovanna JL. Structural organization of the gene encoding the rat pancreatitis-associated protein. Analysis of its evolutionary history reveals an ancient divergence from the other carbohydrate-recognition domain-containing genes. J Biol Chem. 1993;268:14470–14475. [PubMed] [Google Scholar]
- 10.Dusetti NJ, Frigerio JM, Szpirer C, Dagorn JC, Iovanna JL. Cloning, expression and chromosomal localization of the rat pancreatitis-associated protein III gene. Biochem J. 1995;307:9–16. doi: 10.1042/bj3070009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iovanna JL, Keim V, Bosshard A, Orelle B, Frigerio JM, Dusetti N, Dagorn JC. PAP, a pancreatic secretory protein induced during acute pancreatitis, is expressed in rat intestine. Am J Physiol. 1993;265:G611–G618. doi: 10.1152/ajpgi.1993.265.4.G611. [DOI] [PubMed] [Google Scholar]
- 12.Keim V, Rohr G, Stockert HG, Haberich FJ. An additional secretory protein in the rat pancreas. Digestion. 1984;29:242–249. doi: 10.1159/000199041. [DOI] [PubMed] [Google Scholar]
- 13.Zenilman ME, Tuchman D, Zheng Q, Levine J, Delany H. Comparison of reg I and reg III levels during acute pancreatitis in the rat. Ann Surg. 2000;232:646–652. doi: 10.1097/00000658-200011000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz EM, Dusetti NJ, Vasseur S, Malka D, Bodeker H, Dagorn JC, Iovanna JL. The pancreatitis-associated protein is induced by free radicals in AR4-2J cells and confers cell resistance to apoptosis. Gastroenterology. 1998;114:808–816. doi: 10.1016/s0016-5085(98)70595-5. [DOI] [PubMed] [Google Scholar]
- 15.Zenilman ME, Magnuson TH, Swinson K, Egan J, Perfetti R, Shuldiner AR. Pancreatic thread protein is mitogenic to pancreatic-derived cells in culture. Gastroenterology. 1996;110:1208–1214. doi: 10.1053/gast.1996.v110.pm8613011. [DOI] [PubMed] [Google Scholar]
- 16.Heller A, Fiedler F, Schmeck J, Luck V, Iovanna JL, Koch T. Pancreatitis-associated protein protects the lung from leukocyte- induced injury. Anesthesiology. 1999;91:1408–1414. doi: 10.1097/00000542-199911000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Qing X, Keith IM. Targeted blocking of gene expression for CGRP receptors elevates pulmonary artery pressure in hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2003;7:7. doi: 10.1152/ajplung.00356.2002. [DOI] [PubMed] [Google Scholar]
- 18.Lankisch PG, Goke B, Folsch UR, Winckler K, Otto J, Creutzfeldt W. Influence of secretin on the course of acute experimental pancreatitis in rats. Digestion. 1983;26:187–191. doi: 10.1159/000198888. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Patel SA, Mueller CD, Zenilman ME. Pancreatic elastase is proven to be a mannose-binding protein-implications for systemic response in pancreatitis. Surgery. 2003;133 doi: 10.1067/msy.2003.175. 000-00. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Siddiqui MA, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2002;31:31. doi: 10.1182/blood-2002-02-0629. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 22.Kruse-Jarres JD, Kaiser C, Hafkenscheid JC, Hohenwallner W, Stein W, Bohner J, Klein G, Poppe W, Rauscher E. Evaluation of a new alpha-amylase assay using 4.6-ethylidene-(G7)-1-4-nitrophenyl-(G1)-alpha-D-maltoheptaoside as substrate. J Clin Chem Clin Biochem. 1989;27:103–113. [PubMed] [Google Scholar]
- 23.Imamura T, Tanaka S, Yoshida H, Kitamura K, Ikegami A, Takahashi A, Niikawa J, Mitamura K. Significance of measurement of high-sensitivity C-reactive protein in acute pancreatitis. J Gastroenterol. 2002;37:935–938. doi: 10.1007/s005350200157. [DOI] [PubMed] [Google Scholar]
- 24.Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akman HO, Zhang H, Siddiqui MA, Solomon W, Smith EL, Batuman OA. Response to hypoxia involves transforming growth factor-beta2 and Smad proteins in human endothelial cells. Blood. 2001;98:3324–3331. doi: 10.1182/blood.v98.12.3324. [DOI] [PubMed] [Google Scholar]
- 26.Dash P, Lotan I, Knapp M, Kandel ER, Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minshull J, Hunt T. The use of single-stranded DNA and RNase H to promote quantitative ‘hybrid arrest of translation’ of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986;14:6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walder RY, Walder JA. Role of RNase H in hybrid-arrested translation by antisense oligonucleotides. Proc Natl Acad Sci U S A. 1988;85:5011–5015. doi: 10.1073/pnas.85.14.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhagat L, Singh VP, Song AM, van Acker GJ, Agrawal S, Steer ML, Saluja AK. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal S, Temsamani J, Tang JY. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc Natl Acad Sci U S A. 1991;88:7595–7599. doi: 10.1073/pnas.88.17.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein CA. Phosphorothioate antisense oligodeoxynucleotides: questions of specificity. Trends Biotechnol. 1996;14:147–149. doi: 10.1016/0167-7799(96)20006-X. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Q, Zhou R, Temsamani J, Zhang Z, Roskey A, Agrawal S. Cellular distribution of phosphorothioate oligonucleotide following intravenous administration in mice. Antisense Nucleic Acid Drug Dev. 1998;8:451–458. doi: 10.1089/oli.1.1998.8.451. [DOI] [PubMed] [Google Scholar]
- 33.Akhtar S, Agrawal S. In vivo studies with antisense oligonucleotides. Trends Pharmacol Sci. 1997;18:12–18. doi: 10.1016/s0165-6147(96)01002-4. [DOI] [PubMed] [Google Scholar]
- 34.Aho HJ, Nevalainen TJ. Experimental pancreatitis in the rat. Ultrastructure of sodium taurocholate-induced pancreatic lesions. Scand J Gastroenterol. 1980;15:417–424. doi: 10.3109/00365528009181494. [DOI] [PubMed] [Google Scholar]
- 35.Steer M. Pancreatitis severity: who calls the shots? Gastroenterology. 2002;122:1168–1172. doi: 10.1053/gast.2002.32761. [DOI] [PubMed] [Google Scholar]
- 36.Keim V, Iovanna JL, Dagorn JC. The acute phase reaction of the exocrine pancreas. Gene expression and synthesis of pancreatitis-associated proteins. Digestion. 1994;55:65–72. doi: 10.1159/000201127. [DOI] [PubMed] [Google Scholar]
- 37.Kushner I. C-reactive protein and the acute-phase response. Hosp Pract (Off Ed) 1990;25:13(16):21–28. [PubMed] [Google Scholar]
- 38.Folch-Puy E, Garcia-Movtero A, Iovanna JL, Dagorn JC, Prats N, Vaccaro MI, Closa D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFalpha expression in hepatocytes. J Pathol. 2003;199:398–408. doi: 10.1002/path.1307. [DOI] [PubMed] [Google Scholar]