Abstract
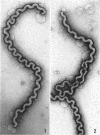
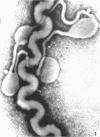
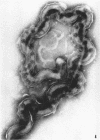
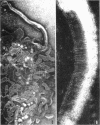
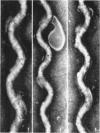
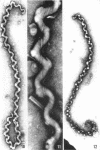
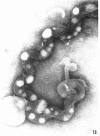
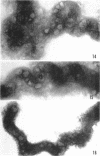
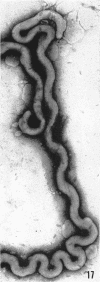
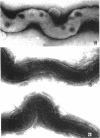
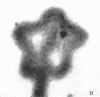
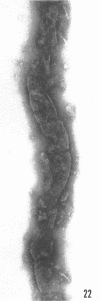
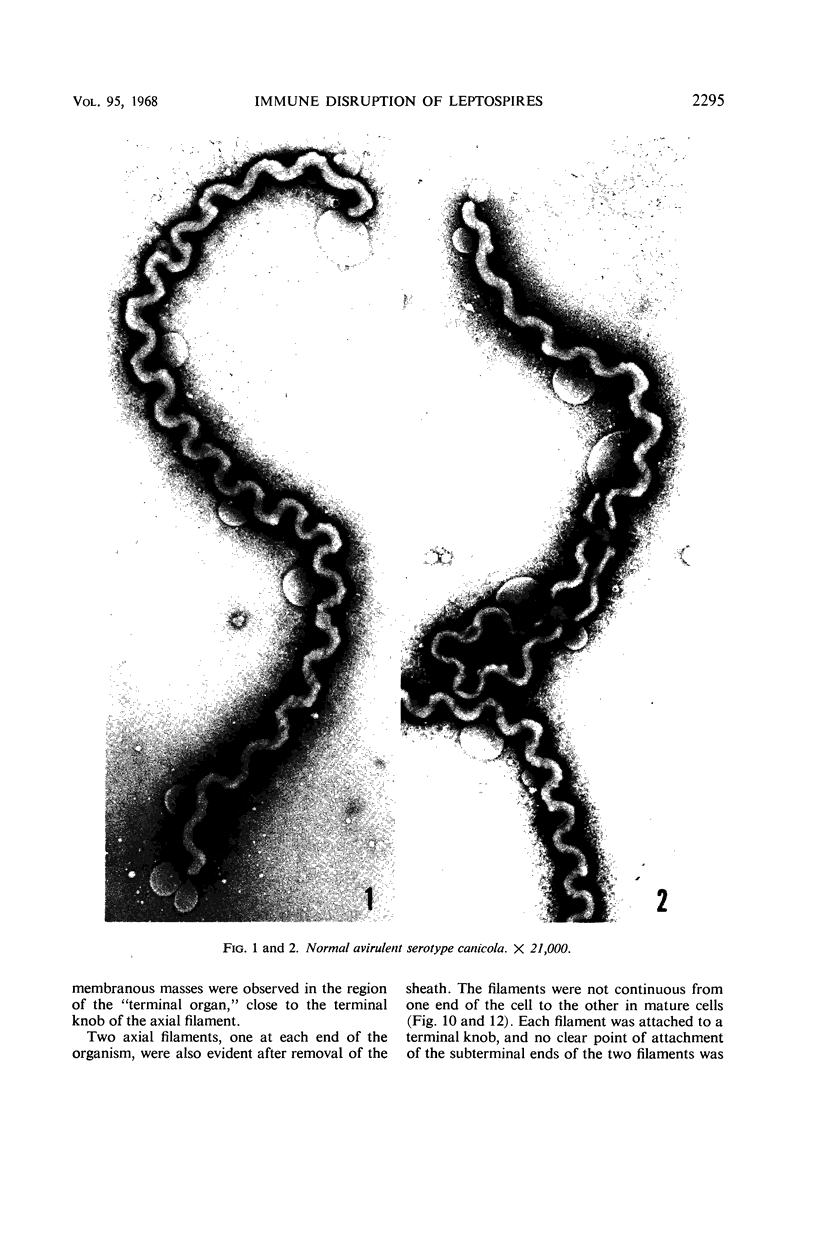
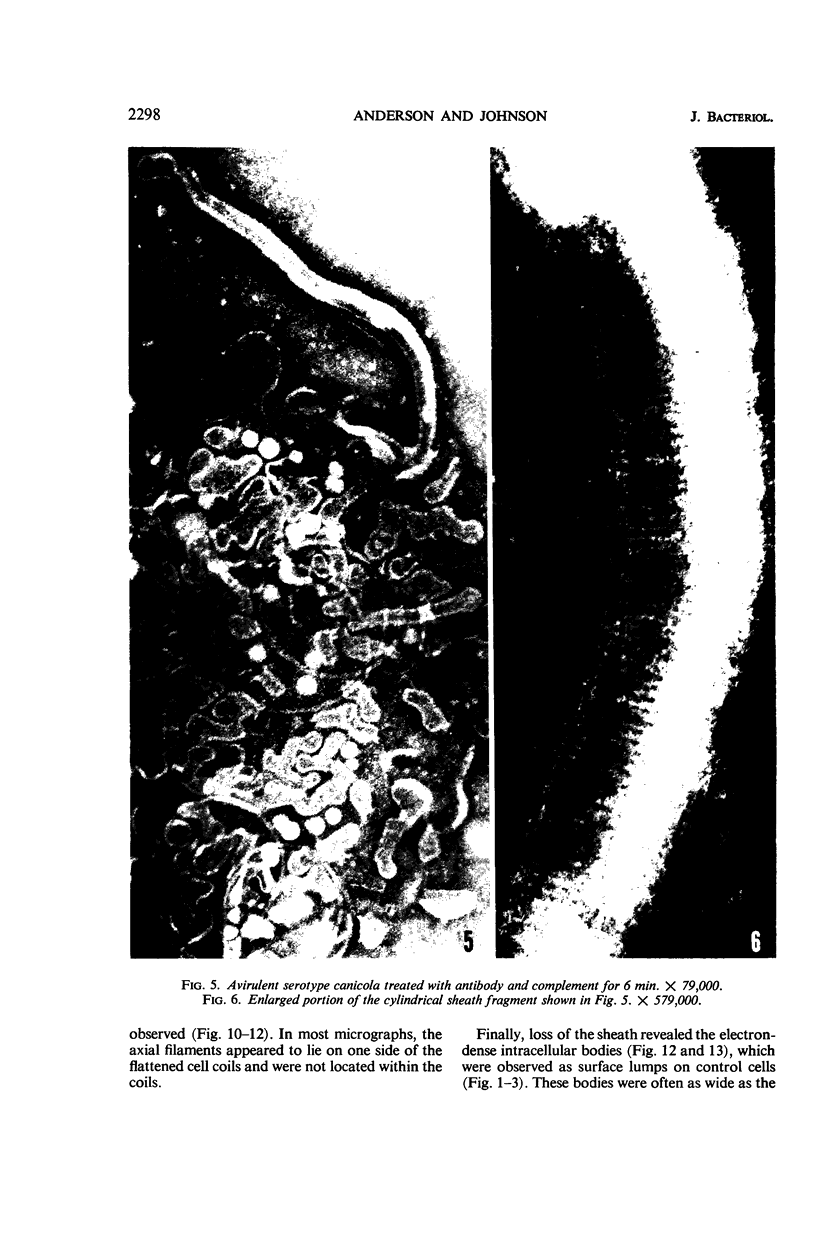
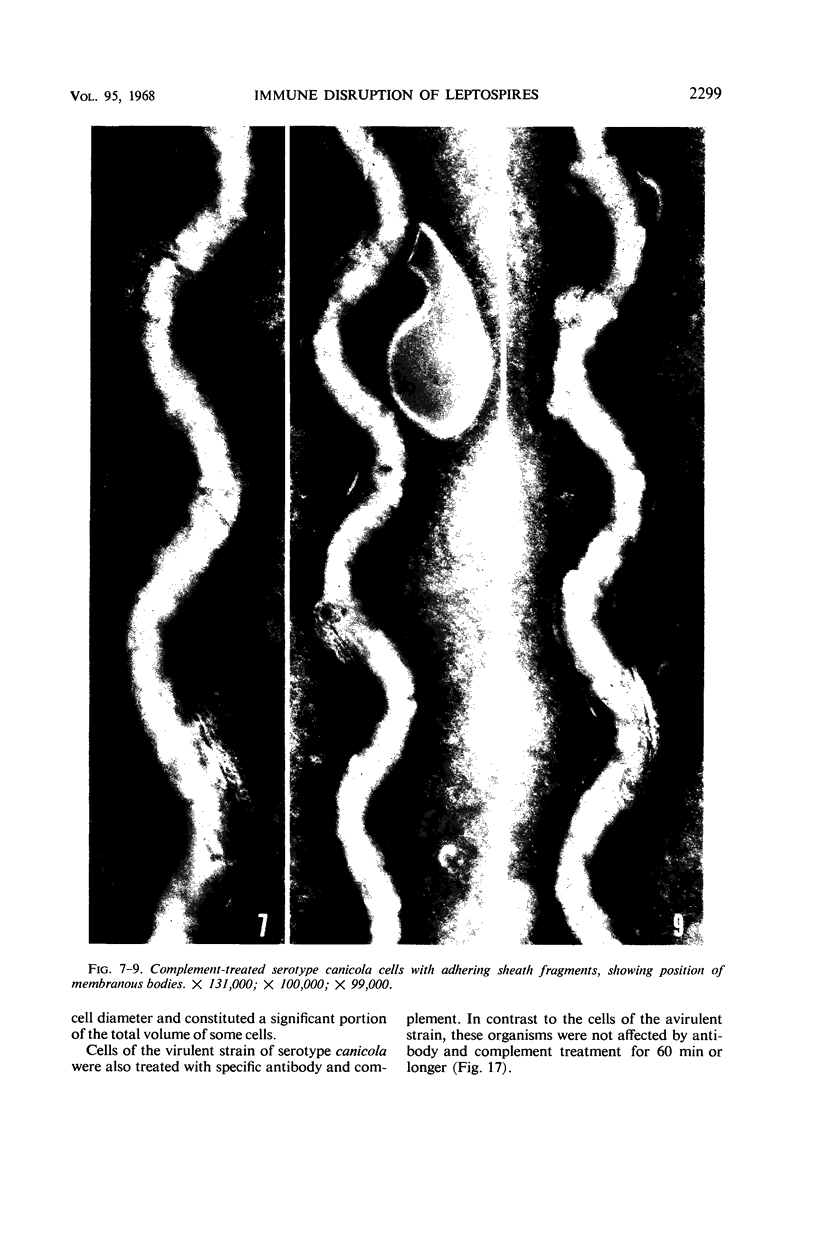
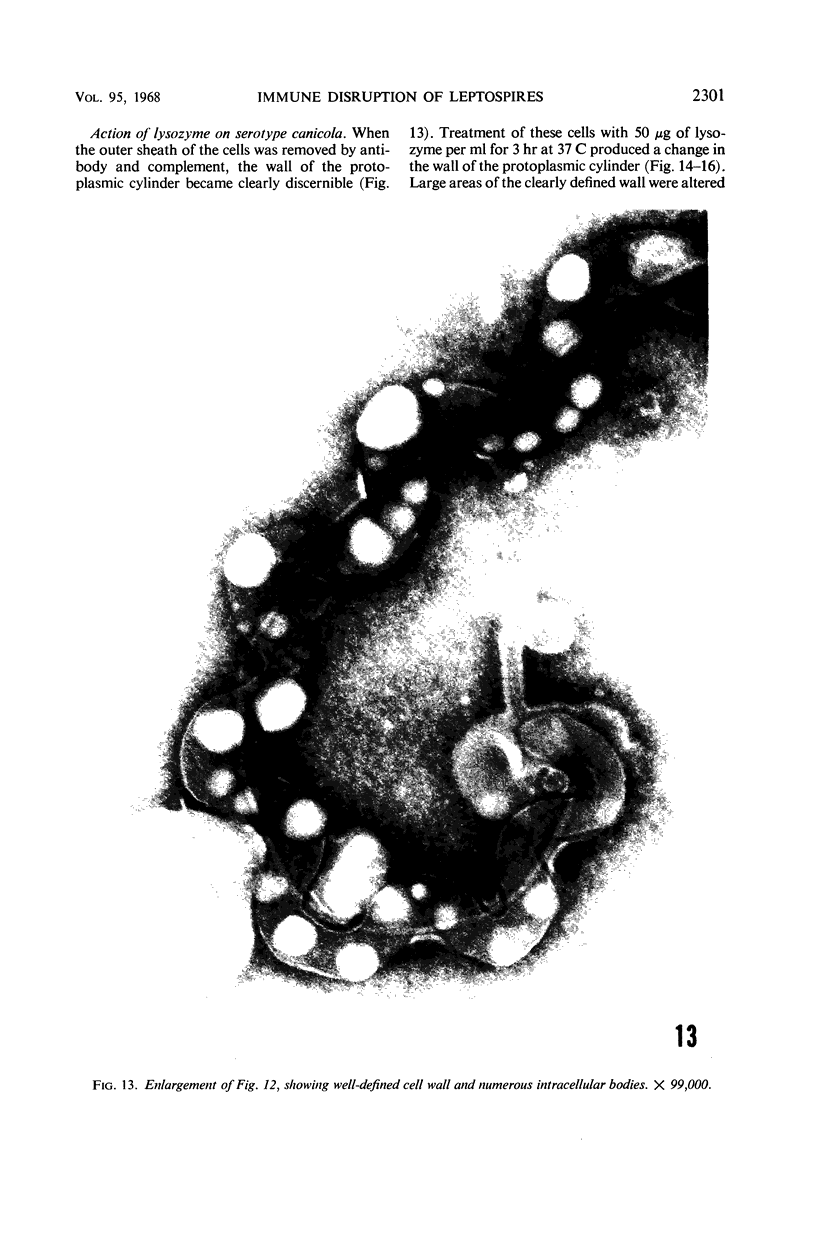
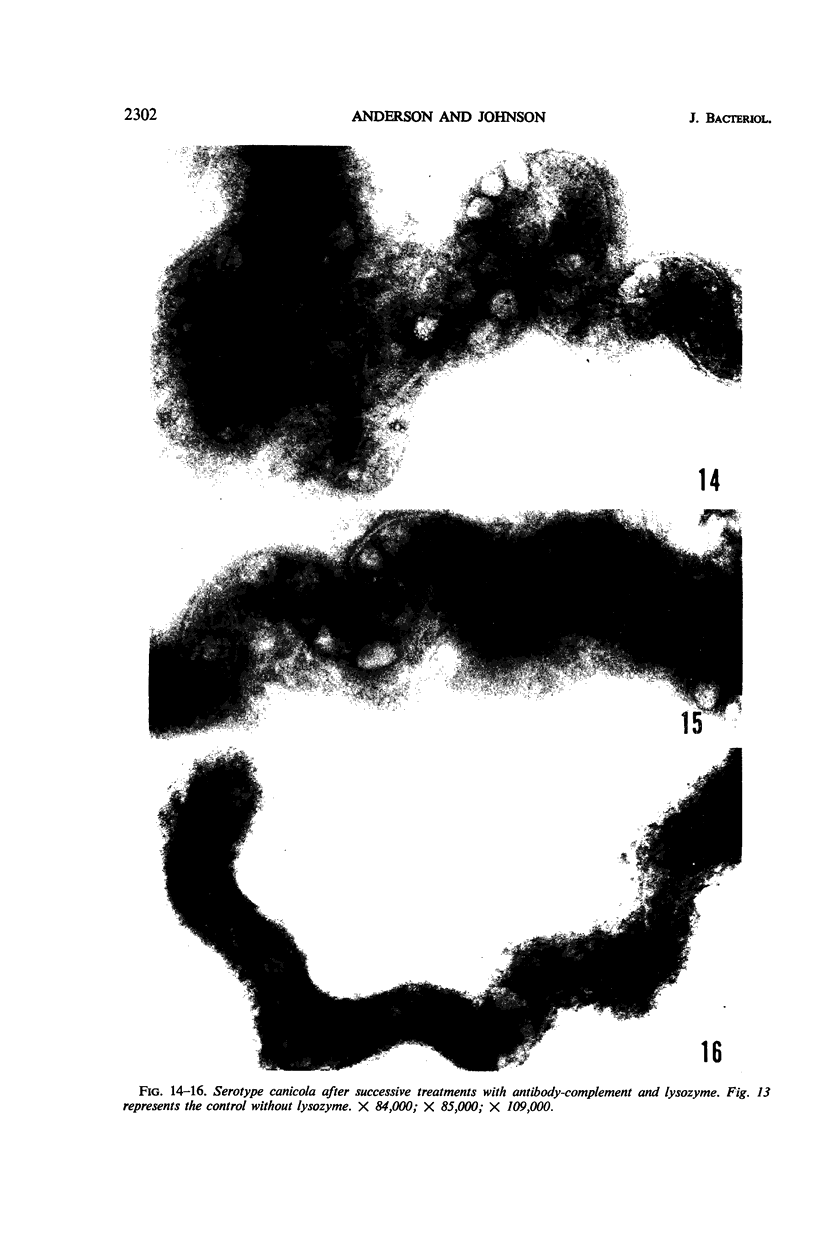
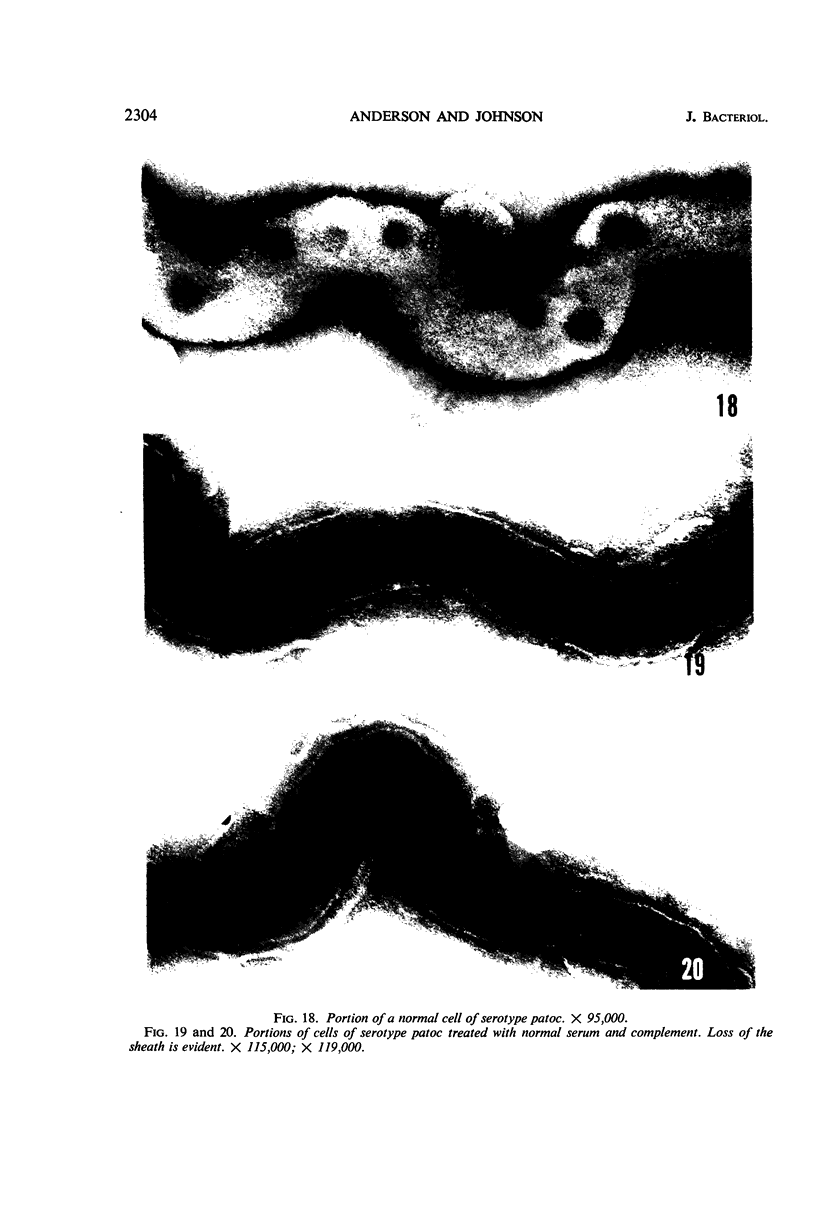
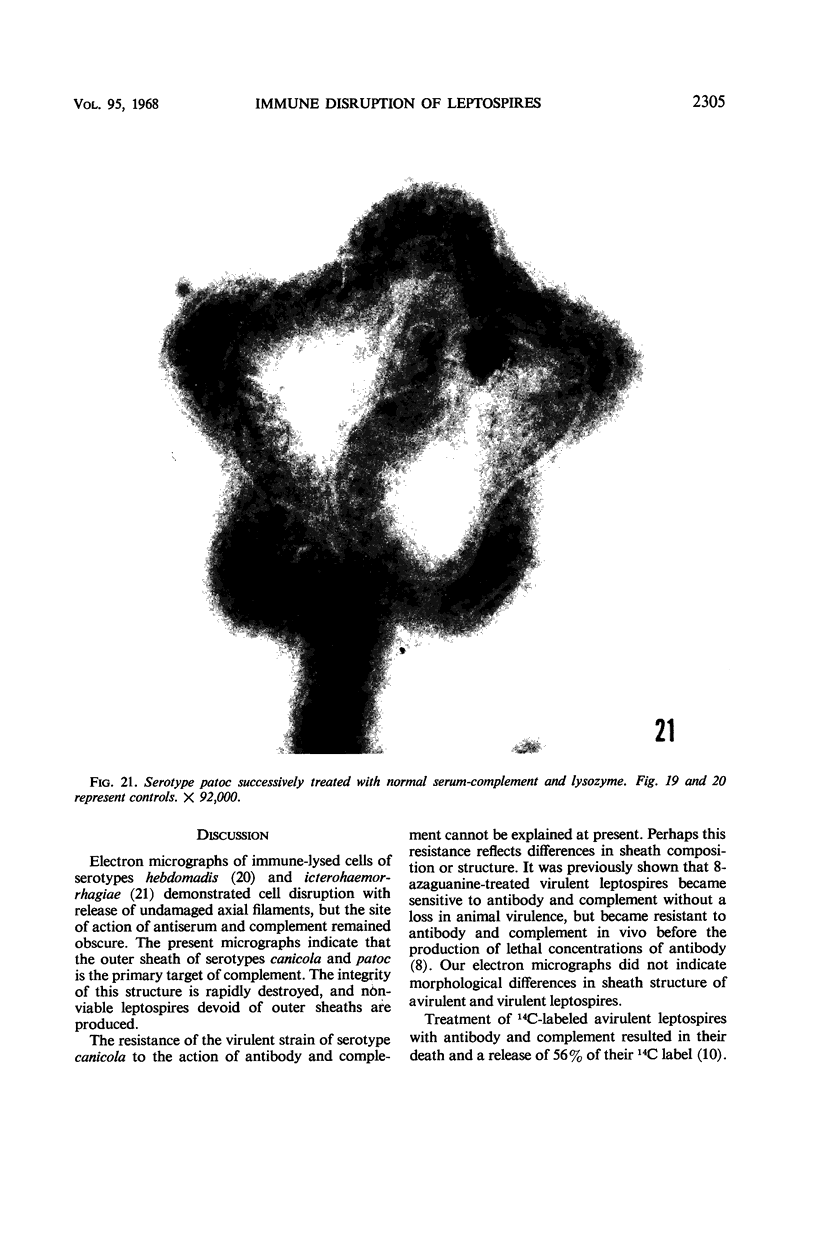
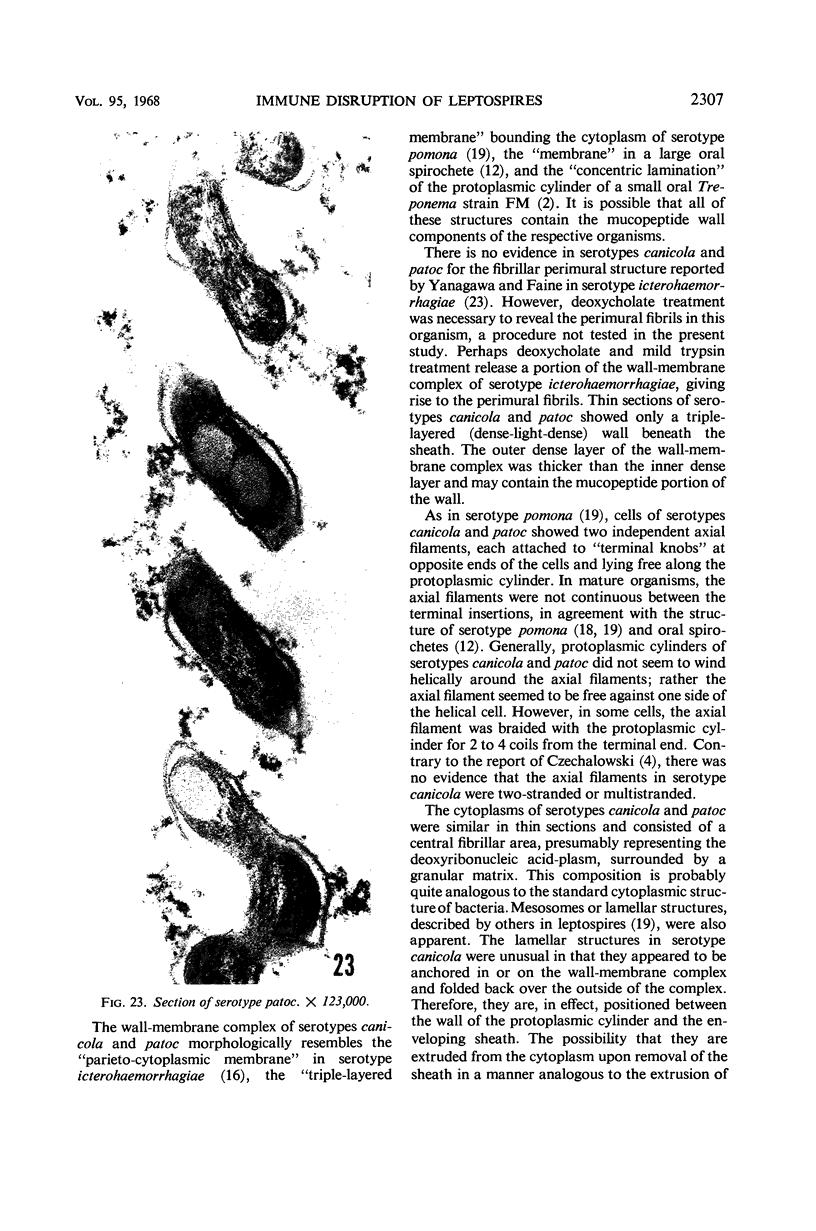
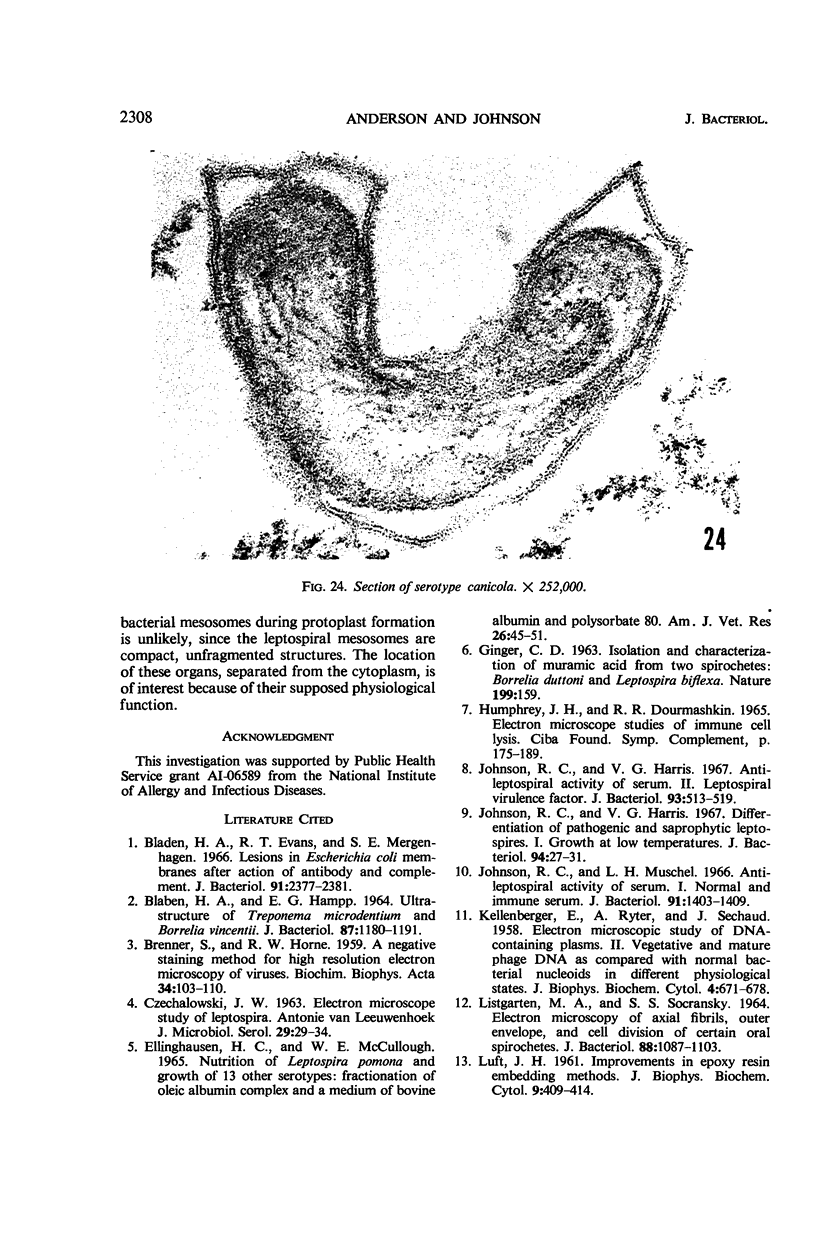
Sequential disruption of the sheath of avirulent leptospires of the serotype canicola with antibody and complement was monitored by electron microscopy. Loosening and separation of the sheath from the protoplasmic cylinder was observed as early as 2 min after exposure to complement. Virulent leptospires of this serotype were morphologically intact after 1 hr of exposure to antibody and complement. Similarly, treatment of leptospires of the serotype patoc with normal serum and complement severely damaged the sheath structure. Removal of the sheath of both serotypes permitted lysozyme to act on the wall of the protoplasmic cylinder. Thus, morphological evidence for the location of the mucopeptide-containing structure of these leptospires was obtained. Viable leptospires with intact sheaths were resistant to lysozyme alone. Sections and negatively stained preparations of sheaths of serotypes canicola and patoc revealed three dense layers with two intermediate light zones and an overall thickness of about 110 A. A periodicity of 40 A was observed in sheath fragments produced by complement. The 70 A wallmembrane complex of leptospires of both serotypes consisted of two dense layers with an intermediate light zone. Structures apparent after removal of the outer sheath included membranous bodies or mesosomes, axial filaments attached to terminal knobs at opposite ends of the cell, and electron-dense intracellular bodies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Evans R. T., Mergenhagen S. E. Lesions in Escherichia coli membranes after action of antibody and complement. J Bacteriol. 1966 Jun;91(6):2377–2381. doi: 10.1128/jb.91.6.2377-2381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Hampp E. G. Ultrastructure of Treponema microdentium and Borrelia vincentii. J Bacteriol. 1964 May;87(5):1180–1191. doi: 10.1128/jb.87.5.1180-1191.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZEKALOWSKI J. W. Electron microscope study of Leptospira. Antonie Van Leeuwenhoek. 1963;29:29–34. doi: 10.1007/BF02046036. [DOI] [PubMed] [Google Scholar]
- ELLINGHAUSEN H. C., Jr, MCCULLOUGH W. G. NUTRITION OF LEPTOSPIRA POMONA AND GROWTH OF 13 OTHER SEROTYPES: FRACTIONATION OF OLEIC ALBUMIN COMPLEX AND A MEDIUM OF BOVINE ALBUMIN AND POLYSORBATE 80. Am J Vet Res. 1965 Jan;26:45–51. [PubMed] [Google Scholar]
- GINGER C. D. ISOLATION AND CHARACTERIZATION OF MURAMIC ACID FROM TWO SPIROCHAETES: BORRELIA DUTTONI AND LEPTOSPIRA BIFLEXA. Nature. 1963 Jul 13;199:159–159. doi: 10.1038/199159a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Antileptospiral activity of serum. II. Leptospiral virulence factor. J Bacteriol. 1967 Feb;93(2):513–519. doi: 10.1128/jb.93.2.513-519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Muschel L. H. Antileptospiral activity of serum. I. Normal and immune serum. J Bacteriol. 1966 Apr;91(4):1403–1409. doi: 10.1128/jb.91.4.1403-1409.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Jackson J. E. The reactivity of serum against protoplasts and spheroplasts. J Immunol. 1966 Jul;97(1):46–51. [PubMed] [Google Scholar]
- Pillot J., Ryter A. Structure des spirochètes. 1. Etude des generes Treponema, Borrelia et Leptospira au microscope electronique. Ann Inst Pasteur (Paris) 1965 Jun;108(6):791–804. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE A. E., ELLINGHAUSEN H. C. ELECTRON MICROSCOPY OF LEPTOSPIRES. I. ANATOMICAL FEATURES OF LEPTOSPIRA POMONA. J Bacteriol. 1965 Jan;89:223–233. doi: 10.1128/jb.89.1.223-233.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEYA K., MORI R., TODA T. Studies on the structure of Leptospira as revealed by the electron microscope. Jpn J Microbiol. 1957 Apr;1(2):99–104. doi: 10.1111/j.1348-0421.1957.tb00014.x. [DOI] [PubMed] [Google Scholar]
- VARPHOLOMEEVA A. A., STANISLAVSKY E. S. Recherches sur la morphologie des Leptospires à l'aide du microscope électronique. Ann Inst Pasteur (Paris) 1958 Mar;94(3):361–366. [PubMed] [Google Scholar]
- Yanagawa R., Faine S. Morphological and serological analysis of leptospiral structure. Nature. 1966 Aug 20;211(5051):823–826. doi: 10.1038/211823a0. [DOI] [PubMed] [Google Scholar]