Abstract
Background
The pancreatitis-associated protein (PAP) family of genes is induced in acute pancreatitis. We have previously demonstrated that antisense mediated gene knockdown of PAP in vivo decreased PAP gene expression and worsened pancreatitis. Here we investigated the effect of a more stable inhibition of PAP using siRNA gene knockdown in vitro and in an in vivo model of experimental pancreatitis.
Methods
In vitro, pancreatic acinar cell line, AR42J, was cultured with Dexamethasone and IL6 (Dex/IL6) to induce expression of PAP with subsequent transfection of siRNA into stimulated AR42J cells. In vivo, acute pancreatitis was induced in Sprague Dawley rats by retrograde infusion of 4% sodium taurocholate (NaT) into the pancreatic duct. PAP-specific siRNA was subsequently administrated, subcapsularly, after infusion of NaT. Controls included administration of scrambled siRNA (SC-RNA) or vehicle alone. After 24hr, pancreata were harvested and assessed for worsening pancreatitis by histopathology; serum was analyzed for PAP, amylase, lipase and cytokines protein levels. In both models endogenous PAP (PAPI, PAPII, PAPIII) gene expression was assessed at 24 hrs using real time RT-PCR.
Results
In vitro, PAP isoform (PAPI, PAPII, PAPIII) protein and mRNA levels were reduced (PAPI: 76%, PAPII: 8%, PAPIII: 24%) in cells treated with PAP siRNA when compared with control treatment. In vivo, induction of pancreatitis was confirmed by histopathology, serum amylase and lipase levels. PAP isoforms I and III expression were reduced (PAPI: 36%, PAPIII: 66%) in siRNA treated rats, compared with controls; there was no difference in PAP II isoform mRNA expression and serum protein levels. Serum amylase levels decreased after administration of siRNA compared with vehicle control (1583 ±312.U/L vs. 3013±317 U/L; p<0.05). In addition, serum lipase levels decreased after administration of PAP siRNA compared with vehicle control (162 ±42 U/L vs. 478±125 U/L; p<0.05). Serum levels of IL-1β, IL-4 and IL-6 increased (20%, 96.9%, 118%, respectively) while CRP and TNFα decreased (18.8%, and 13.7%, respectively) when compared with vehicle control. Administration of PAP siRNA correlated with worsening leukocytic infiltration and necrosis but not edema.
Conclusions
siRNA mediated gene knockdown of PAP appeared to worsen pancreatitis severity but demonstrated some different effects when compared to antisense gene knockdown in certain instances. This observed difference may be due to the inhibition profile discrepancy between two knockdown methods and/or different mechanisms of action for siRNA compared with antisense technology.
Keywords: pancreatitis associated proteins, PAP, pancreatitis, sodium taurocholate, AR42J, SiRNA, antisense, gene knockdown, cytokines, severity, in vivo
INTRODUCTION
Acute pancreatitis is an acute inflammatory process of the pancreas with variable involvement of other regional tissues or remote organ systems. During acute pancreatitis, the gene expression of pancreatitis associated protein (PAP), a member of the regeneration (Reg) family of pancreatic proteins, is highly upregulated in the pancreas (1). Currently, three PAP genes have been characterized in rat (2) and human (3). There is a high degree of conservation among all three PAP isoforms’ protein sequences (3). However, their expression patterns and tissue distribution are different; PAP II mRNA is specifically expressed in the pancreas, whereas PAP I and III mRNA are normally expressed in the small intestine (4). The maximal expression of PAP II can be detected in pancreas and in serum within 24 hours of induction of acute pancreatitis (5) It has been shown that PAP I can aggregate bacteria in suspension and may function as an endogenous anti-bacterial agent and be protective against infectious complications of pancreatitis (6). In vitro studies showed that PAP expression is also upregulated by free radicals or cytokines, and such upregulation confers cellular resistance to apoptosis (6). Recent studies have shown that PAP III can serve as a macrophage chemoattractant during inflammation and is involved in peripheral nerve regeneration (7).
The function of PAP in vivo during acute pancreatitis remains unknown. A limited number of studies have been done with regard to the in vivo function of PAP, and the current results have been controversial. Previous studies in our laboratory showed that Reg/PAP isolated from cow is mitogenic for pancreatic-derived cells, thus implying that PAP may be part of the pancreatic regeneration process post-pancreatic injury (8). Some studies have confirmed a protective anti-inflammatory effect of PAP on leukocyte-induced lung injury (9), whereas others have shown that PAP released from pancreas could mediate lung inflammation through induction of tumor necrosis factor (TNF-α) expression with subsequent increases in circulating TNF-α (10).
In vitro studies have shown that when the rat acinar cell line, AR 42J, is stimulated with dexamethasone and IL-6 (Dex/IL6), all three isoforms of PAP can be upregulated (11) thus providing a cellular basis of PAP investigation. In addition, we have demonstrated in a rat model of experimental acute pancreatitis induced by bile salt sodium taurocholate (NaT), all three PAP family genes have also been shown to be over-expressed during the acute phase of pancreatitis providing a in vivo model of necrotizing pancreatitis (12). In previous studies, we have applied anti-sense (AS) gene inhibition technology to investigate PAP’s function in pancreatic acinar (AR42J) cells and rat acute pancreatitis model (12). We found that AS mediated gene knockdown decreased PAP gene expression in Dex-IL6 induced AR42J cells. Furthermore we demonstrated that AS knockdown of PAP in NaT induced acute pancreatitis worsened pancreatitis severity by increased leukocytic infiltration, edema and necrosis in the pancreatic parenchyma and increased inflammatory cytokines (12). Additionally, antibody neutralization of PAP with anti-PAP antibodies in vivo demonstrated a similar worsening in the severity of pancreatitis (13).
To further elucidate the function of PAP, siRNA gene knockdown was applied in the present study. Small interference RNA (SiRNA) knockdown technology represents a new powerful approach to studying protein function by a mechanism distinct from antisense technology and has been shown to be effective in vitro and in vivo (14).
Materials and Methods
Cell culture and reagents
The AR42J rat pancreatic acinar cell line (ATCC Inc. Manassas, VA) were used after 15 passages. The cell were routinely cultivated in a 5% CO2, 95% air atmosphere in Dulbecco’s modified Eagle’s medium containing 10% (v/v) fetal calf serum (Gibco Inc. Carlsbad, Calif.), 4mM L-glutamine, 50 units/ml penicillin, and 50ug/ml streptomycin. Cells were incubated at 80 to 90% confluence with SiRNA (100 nM) along in cultures with or without IL-6 (100 Units/ml) (R & D sstems, Minneapolis, MN) and dexamethasone (100 nM)(Sigma, St Louis, MO). After different length of treatment, cells were lysed for RNA extraction using the Trizol reagent as recommended by the supplier (Invitrogen Inc. Carlsbad, Calif.).
Pancreatitis induction and treatment with siRNA
a) Animals
Female Sprague Dawley rats were obtained from Harlan Sprague Dawley (Indianapolis, Ind.), and weighing 175–200 g at onset of the studies, served as subjects. Animals were fed standard laboratory rats chow, given water add libitum, and randomly assigned to control or experimental groups. All animal studies have been approved by the Division of Animal and Laboratory Resources, SUNY Downstate Medical Center.
b) Pancreatitis Induction
Acute pancreatitis was induced with 4% sodium taurocholate (NaT) as previously described (12). Breifly, under pentobarbital (Abbott Laboratories, North Chicago, Ill., USA) anesthesia (50 mg /kg given intraperitoneally), a midline incision was performed. The common bile duct was identified and cannulated in an antegrade direction with PE-10 tubing (Fisher Scientific, Pittsburgh, PA., USA) such that the proximal end of the tube was beyond the ampulla of Vater in the duodenum. The bile duct was then ligated to prevent the flow of bile and 4% NaT in sterile saline was consistently infused into the pancreatic duct at a rate of 1 mL/ kg over 10 min.
c) siRNA administration
PAP specific (SiRNA-PAP) or scrambled control siRNA (SC-PAP) (Dharmacon, Lafayette, CO) was suspended in lipofectamine 2000 and administered via intrapancreatic subcapsular injection (800nm, 300uL total volume) 1 h after the administration of NaT for induction of pancreatitis. SiRNA-PAP consisted of a mixture of three siRNA oligonucleotide constructs which demonstrated maximal gene knockdown of all three PAP isoforms in vitro (15). Twenty-four h after pancreatitis induction, the rats were killed. Pancreas and blood samples were harvested immediately afterwards; pancreata were snap frozen in liquid nitrogen and sera were frozen at −80C. The integrity of RNA obtained from pancreas was ascertained by visualization of 28S, 18S and 5S ribosomal RNA banding by electropheresis (data not shown). Six rats were included in each of the three groups (saline, SC-PAP, SiRNA-PAP).
siRNA oligonucleotides
The mRNA (cDNA) sequences of all three isoforms of rat PAP were retrieved from the National Center for Biotechnology Information nucleotide database (http://www.ncbi.nlm.nih.gov/gquery/gquery.fcgi). The 21-nt siRNA duplex was provided in the 2′-deprotected and desalted form of 2′-O-ACE-RNA (Dharmacon). The nontargeting RNA Cy3-labeled siGLO RISC-free siRNA (Dharmacon) was used to visualize transfection efficiency. Three target siRNA sequences were designed based on the rat PAP I (GenBank Accession No. NM_053289), PAP II (L10229) and PAP III (L_20869) mRNA sequences following the Rational siRNA Design algorithm. PAPI-5’GUG GAG UAA CAA UGA CAU AdTdT3’, PAP II-5’UGC CUC GUC UGU CCU UCA AdTdT3’, PAP III-5’CUG GGA GAC GAA UCC UUC UdTdT3’. Cells were transfected with siRNA using the Silencer Transfection kit (Ambion Austin, TX). Briefly, cells were grown in 35 mm dishes (BD Bioscience, Franklin Lakes, NJ) and overlaid with the transfection mixture containing siRNA (100 nmol/L) and siPORT Lipid (Ambion) in Opti-MEM I Reduced Serum Medium (Invitrogen, Carlsbad, CA). After 4-hour incubation, cells were stimulated with IL6/Dex plus medium without FBS for another 24 hours. Scrambled siRNA (SC-RNA) served as a negative control. These PAP specific siRNA oligonucleotides, which were able to knockdown all three PAP isoforms in vitro (15) were combined as a mixture for in vivo intrapancreatic gene knockdown studies.
Real-time quantitative RT-PCR
One-step real-time quantitative RT-PCR for PAP I, II, and III was performed as previously described (12), using a GeneAmp 5700 sequence-detection system (PE Biosystems, Foster City, Calif.), with β-actin as an endogenous control to standardize the amount of sample RNA added to a reaction. Primers and probes were designed using Primer Express software (PE Biosystems); the specific forward and reverse primers were designed based on published cDNA sequences of rat PAP I, II, and III (GenBank accession nos.NM_053289, L10229 and L20869, respectively)(12). All primers and probes and other reagents for real-time quantitative PCR were purchased from Applied Biosystems. One hundred nanograms of total RNA was used to set up 25-µL real-time quantitative PCRs that consisted of 1 X TaqMan Universal PCR Master Mix, 500 nM forward and reverse primers, and 200 nM TaqMan probe. PCR amplification was carried out with the following temperature profile: 30 min at 48 °C; 10 min at 95 °C; and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Assays were performed in triplicate. Data were analyzed with the relative standard curve method. Standard curves of the genes of interest and β-actin were prepared with three 1:2 dilutions (four points, eight-fold range) of total RNA from one of the samples that was expected to have the highest amount of mRNA for the gene of interest. For each reaction tube, the amount of target or endogenous reference was determined from the standard curves. The mean amount of each sample was calculated from the triplicate data and was normalized by division by the mean quantity of β-actin RNA for the same sample. The mean and standard deviation of each treated group were calculated from the normalized value for each rat in that group. The mean value of the saline group was arbitrarily set at 100%. Results from the siRNA-PAP- and SC-PAP-treated groups were divided by the mean value of the saline group to generate the relative expression levels. Tests for significance were performed with Student’s t test, two-tailed, to assess differences between data from saline-, SC-PAP-, or siRNA-PAP-treated groups. Means are presented ± 1SD. Significance level was set at P < 0.05.
Blood
Blood was collected into red-top tubes which did not contain any anticoagulant (Fisher, Piscataway, NJ) and allowed to clot for 30 minutes at room temperature. Tubes were rimmed and centrifuged at 1,000 rpm for 10 minutes. Sera were collected and stored at −20° C until analyzed.
Western Analysis
siRNA treated and control AR42J cells were homogenized and lysate was run on 15% acrylamide gel (10ug total protein/lane) and subsequently blotted with appropriately diluted anti-PAP antibody (1:400). (13). Anti-PAP antibody is species specific and has minimal overlap with other Reg isoforms (16). PAP protein size ranges from 13–18Kd and is dependent on PAP cleavage, post translational modification and glycosylation (17,18).
Quantitation of serum PAP levels
Serum PAP levels were determined by ELISA as previously described (16). Briefly, 96 well plates (Bethyl Labs, Montgomery AL) were coated with goat anti-rat, REG III (PAP2) antibody (R&D Biosystems, Minneapolis, MN) 100ng/ul to 0.01 mg/ml in Coating Buffer (Bethyl) and incubated for 60 minutes at RT or overnight at 4C. Plates were washed in Wash Buffer (Bethyl) and were blocked with Postcoat Blocking Solution according to manufacturer’s recommendations (Bethyl) for 30 minutes. Plates were washed 5x in Wash Buffer at which point rat serum samples were diluted 1:10 or 1:100 in sample diluent (Bethyl) added to plates and incubated at room temperature for 60 minutes. Plates were washed and biotinylated anti-rat REG III/(PAP) antibody (R&D Systems) (1:200) in sample diluent was added to wells (100 ul/well) and incubate the plate for 1 hour at RT. Plates were washed and streptavidin-HRP (Bethyl) (1:200) in sample diluent was added to each well (100ul/well) and incubated for 60 minutes at RT. Plates were washed and plates were developed by adding TMB substrate solution (Bethyl) 100ul/well and incubated for 10–15 minutes. Reaction was stopped by adding 100 ul of 2M H2SO4 to each well and plates were read using an automated microplate reader (Model ELx800; Bio-Tek Instruments, Winooski, VT), with a 450-nm measurement filter. Optical densities were compared with recombinant PAP2 protein fragment standards (13), diluted with 1/3rd normal rat serum, and converted to ug/ml protein. Assays were run in duplicate and data represent mean serum PAP2 levels ± SD.
Quantitation of serum amylase and lipase activity, C-reactive protein (CRP), cytokines and pancreatic edema, and evaluation of pancreatic morphology
Serum amylase activity was measured using 4,6-ethylidene (G7)-p-nitrophenyl (G1)-α1D-maltoheptaoside as the suabstrate (12) and is reported as units per liter (U/L). Serum lipase was measured by the Clinical Laboratories, SUNY Downstate Medical Center and is reported as U/L. CRP levels were determined in the Clinical Laboratory using a conventional immunoassay system and Beckman nephelometer (Beckman Coulter, Inc., Galway, Ireland). The method employed in the Beckman Coulter CRP Test measures the rate of increase in light scattered from particles suspended in solution as a result of complexes formed during an antigen–antibody reaction. Serum levels of IL-1β, IL-4, IL-6 and TNFα were measured using Quantikine Rat Immunoassay (R & D Systems, Minneapolis, MN) and are reported as picograms per milliliter (pg/ml). The extent of pancreas edema was quantitated by the ratio of pancreas wet weight over subject’s total body weights. For morphologic analysis, 5-µm-thick paraffin sections of pancreas samples were stained with H&E. Ten randomly chosen microscopic fields were examined for each tissue sample, and inflammation was scored as follows: none = 0; mild = 1; moderate = 2; and severe = 3.
Statistical analysis
The results reported represent mean ± SD values obtained from multiple determinations in three or more separate experiments. In any one experiment, there were ten animals in each group. Statistical analyses were carried out by 2 way or 3 ways analysis of variance, with the independent variables being saline, SCRNA or SiRNA treatment. The significance of changes was evaluated by Tukey’s post hoc test. A value of P< 0.05 was considered statistically significant.
RESULTS
In vitro SiRNA gene inhibition of PAP
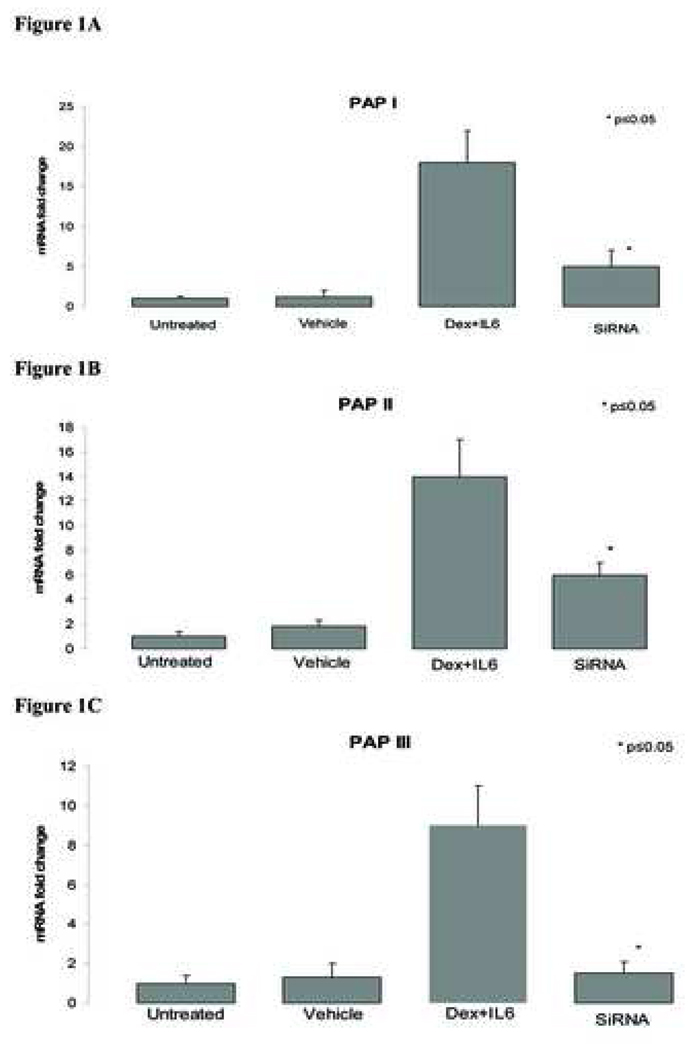
When AR 42 J cells were stimulated with Dex/IL6, expression of all PAP isoforms were increased when compared with vehicle control cells (Figure 1A,B,C). In contrast, when Dex/IL6 stimulated AR42J cells were treated with a mixture of PAP specific SiRNA, PAP gene expression significantly decreased when compared with vehicle treated controls; PAP 1 decreased 68%, PAP 2 decreased 57%, PAP 3 decreased 83% (Fig 1A,B,C). Furthermore western blot analysis of cellular lystates revealed decreased protein levels of PAP when treated with siRNA when compared with vehicle control (Fig 1D).
Figure 1. SiRNA gene inhibition of Dex/IL6 treated AR42J cells.
Cells were transfected with siRNA 4 hours prior to treatment with Dex/IL6. After 24 hours of stimulation, cells were harvested and total mRNA was isolated and assessed for PAP expression (real time PCR). A - PAP 1; B - PAP 2; C - PAP 3; D - western blot assay of stimulated AR42J cells treated with individual and mixture of PAP specific siRNA. A-C -Data are presented as mean fold change mRNA expression ±SD; controls consisted of untreated and vehicle treatment alone. D - siRNA treated and control AR42J cells were homogenized and lysate was run on 15% acrylamide gel (10ug total protein/lane) and subsequently blotted with appropriately diluted anti-PAP antibody (1:400). (13). PAP protein size ranges from 13-18Kd and is dependent on PAP modifications (17,18). The protein level was most inhibited in the mixture treatment group. Tubulin served as protein control. * significance p≤0.05
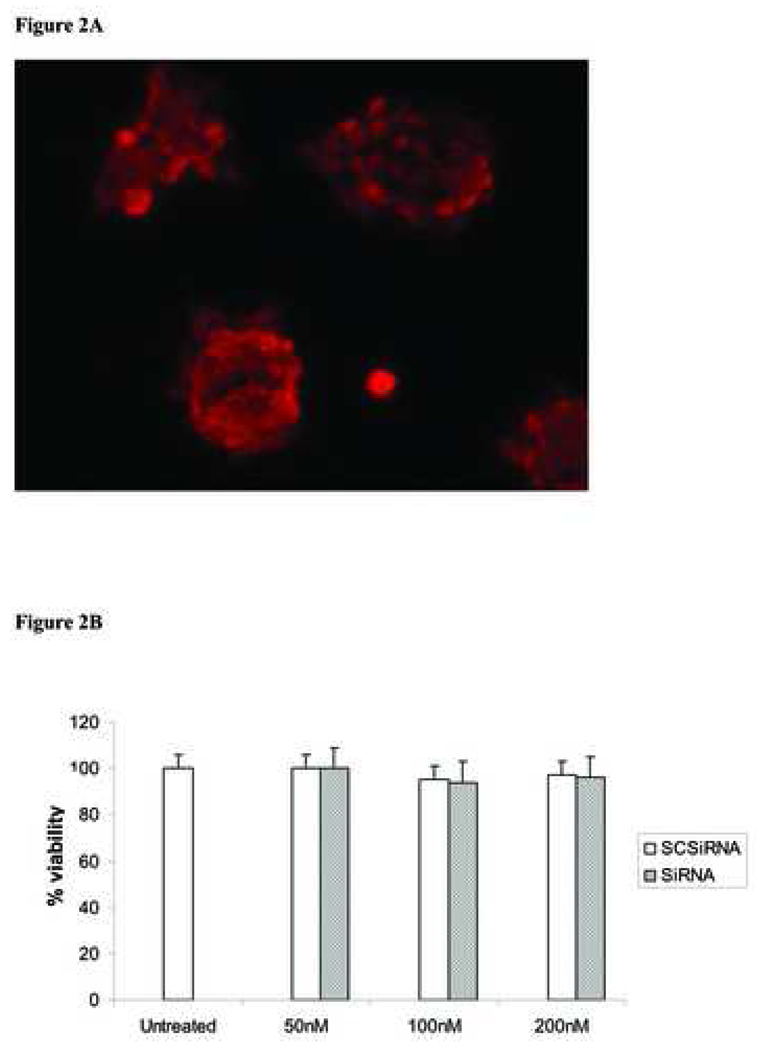
RNAi transfection efficiency was assessed on stimulated AR42J cells which were treated with cy3-conjugated scrambled SiRNA (Fig 2A) and cell viability was not affected by scrambled SiRNA treatment (Fig 2B).
Figure 2. SiRNA delivery and cell viability assay.
A - Fluorescent image of AR42J cells transfected with cy3-conjugated scrambled SiRNA. AR42J cell were transfected with 100 nM of scrambled siRNA with silencer transfection kit for four hours. Image magnification x400. B - AR42J cells were plated in 6 wells plate and transfected with increasing concentration of scrambled control siRNA (SCSiRNA) or mixture of PAP specific siRNA (siRNA) for 4 hrs. Complete media was added after transfection, cells were assessed for viability with tripan blue stain after 24 hrs. Data represent mean ±SD of triplicate experiments.
In vivo SiRNA treatment
SiRNA inhibition of PAP gene expression
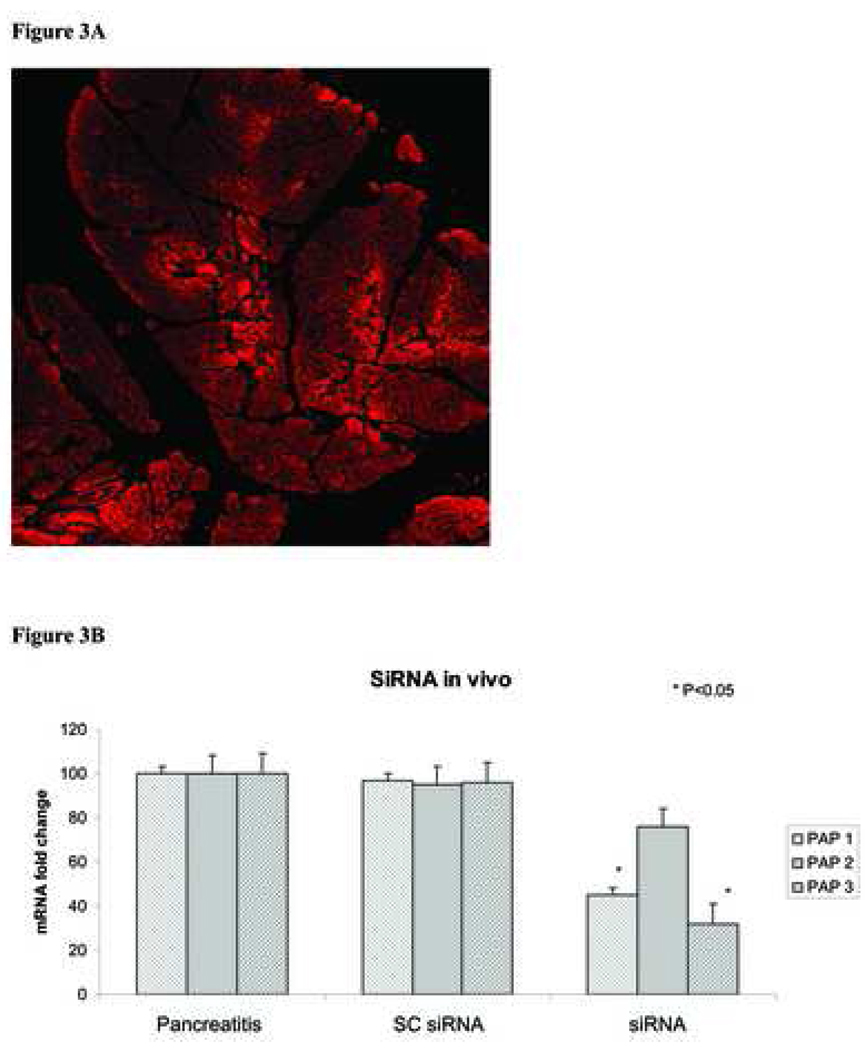
As in our previous studies, animals injected with NaT through the pancreatic duct demonstrated evidence of pancreatits including increased leukocytic infiltration, edema, and necrosis (12). Furthermore, acute pancreatitis was further confirmed by increased levels of serum amylase, lipase and CRP when compared with saline administered control animals (data not shown). As demonstrated in our previous studies (12), pancreata obtained from animals induced with NaT pancreatits had increased expression of all PAP isoforms (Figure 3). In contrast, pancreata obtained from PAP SiRNA treated animals had significantly inhibited expression of PAP I and III isoforms (PAP I decreased 55%, PAP III decreased 22%); there was no difference in expression of PAP II (Figure 3). Similarly, there was no difference in serum protein levels of PAP in PAP SiRNA treated animals when compared with SC-RNA treated controls. (212±19 vs. 230±20, ug/ml, respectively, data not shown)
Figure 3. Effect of siRNA on PAP expression in vivo.
A – Fluorscence image from pancreas obtained from rats injected (subcapsular) with cy3 conjugated scrambled siRNA. Pancreas tissue was harvested 24 hrs after the injection. Image maginifcation X200.B - Rats were injected with control siRNA (SCsiRNA) or mixture of PAP specific siRNA (siRNA) after ductal injection of 4%NaT. Pancreatic tissue was harvested 24hrs later, total mRNA was isolated and PAP isoforms were determined (real time PCR) and compared with rats induced with NaT pancreatitis. Data represent mean fold change PAP mRNA expression ± SD.
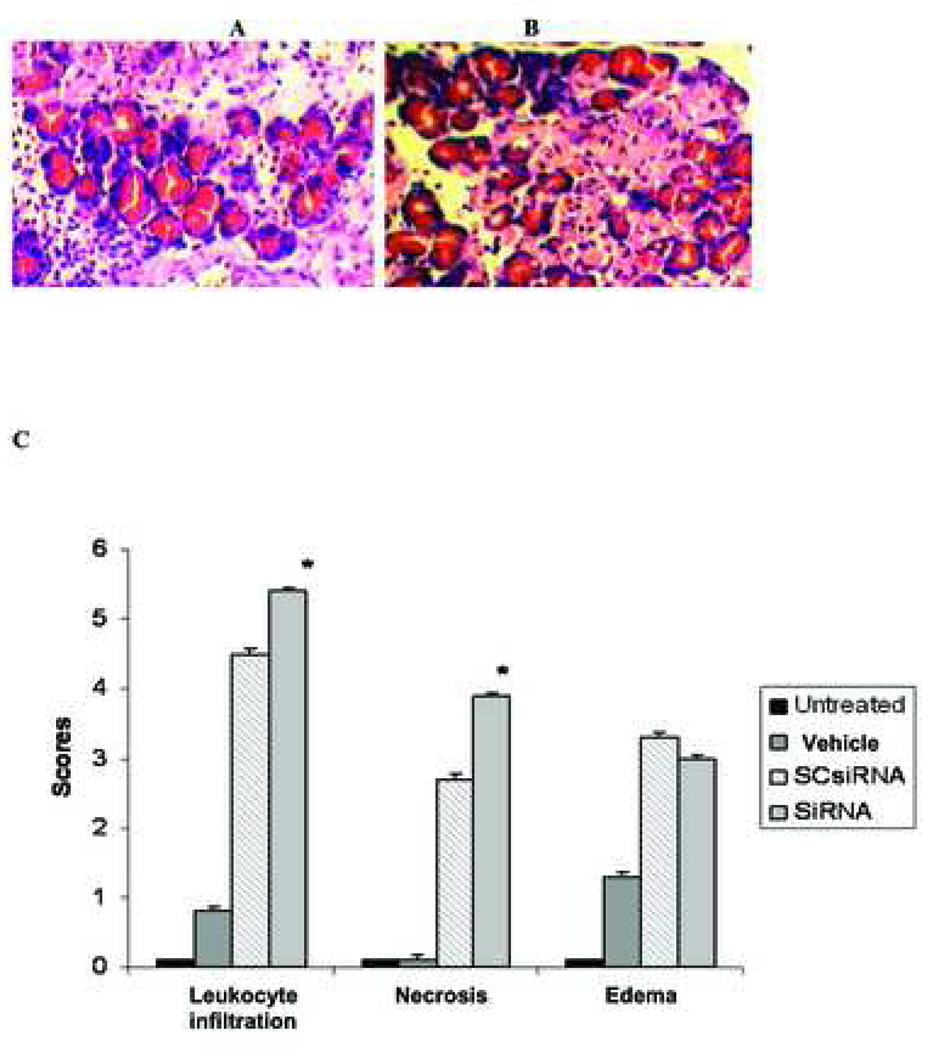
Effect of PAP SiRNA on Pancreatitis severity
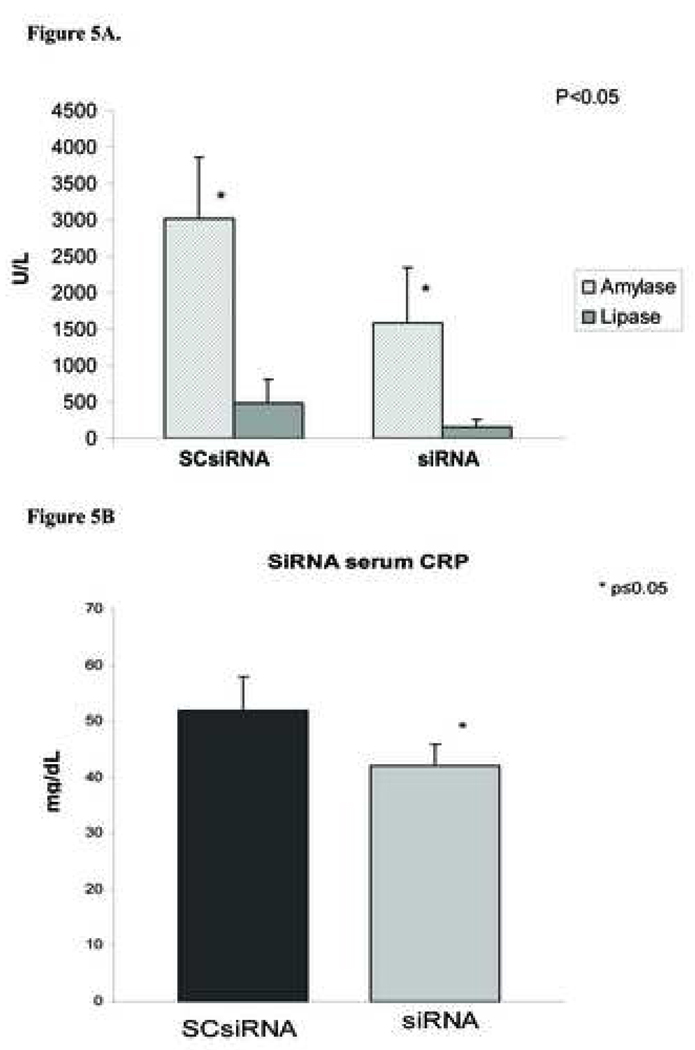
When mixtures of PAP specific siRNA was injected subcapsularly into the pancreas of NaT induced pancreatitis rats, pancreatitis severity was worsened as evidenced by increased leukocytic infiltration, and necrosis (Figure 4); however no differences in edema was observed when compared with scrambled siRNA treated tissue. Interestingly, serum levels of amylase, lipase and CRP were decreased with RNAi treatment (47.5%, 66.1%, 18.8%, respectively) (Figure 5) when compared with scrambled siRNA controls.
Figure 4. Effect of siRNA on pancreatitis severity.
Pancreas tissue from NaT induced pancreatitic rats were injected with A - PAP specific siRNA and B - control (scrambled) siRNA. Pancreas was harvested 24 hours post RNAi administration and assessed for severity (leukocytic infiltration, edema and necrosis). C – histopathological scoring of leukocytic infiltration, edema and necrosis. Data represent mean scoring (± SD), * significance p≤0.05.
Figure 5. Effect of siRNA on serum amylase, lipase and C reaticve protein levels.
Serum from pancreatitic rats treated with either scrambled siRNA (SCsiRNA) or PAP specific siRNA (siRNA) were collected 24hr post NaT induced pancreatitis. A - serum amylase and lipase levels. B – serum CRP levels. Data represented mean serum levels of respective proteins ± SD. * significance p≤0.05.
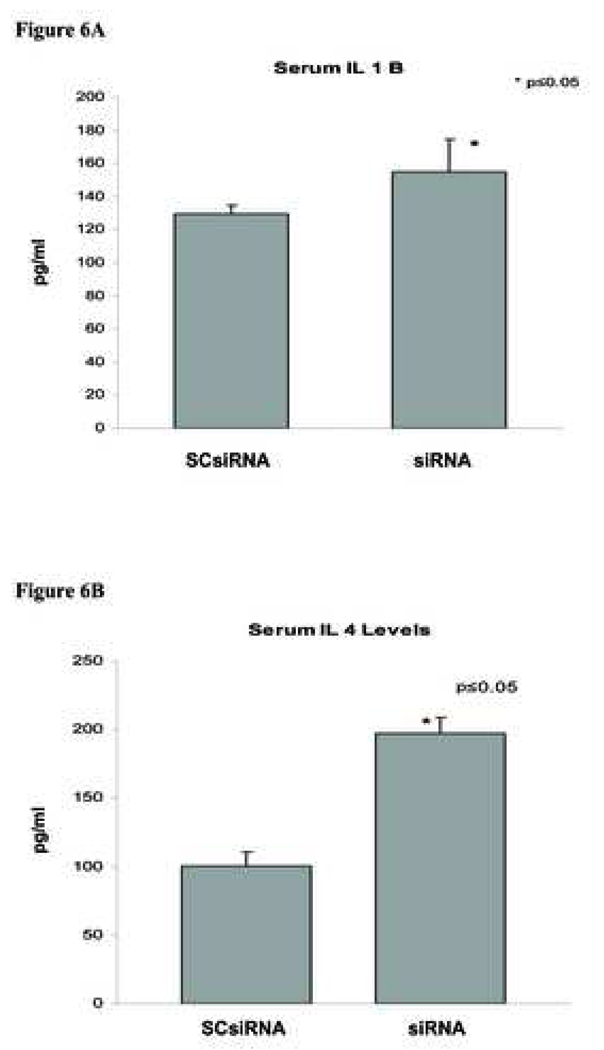
Effect of PAP SiRNA on proinflammatory cytokines
We have previously demonstrated that serum obtained from animals which were induced with NaT pancreatitis contained high levels of proinflammatory cytokine IL1β, IL-4, IL-6 and TNFα (12)(Figure 6). Interestingly, when these animals were treated with PAP SiRNA serum levels of IL-1β increased 20% (Figure 6A), IL-4 increased 96.9% (Figure 6B), and IL-6 increased 118% (Figure 6C) compared with control groups. In contrast serum levels of TNFα decreased 13.7% (Figure 6D) with PAP SiRNA treatment.
Figure 6. Effect of siRNA on inflammatory cytokines.
Serum obtained from pancreatitc rats treated with scrambled siRNA (SCsiRNA) or PAP specific siRNA (siRNA) was collected 24hrs after induction of acute pancreatitis. Serum cytokine levels were measured by ELISA as described in materials and methods. A - IL-1β, B - IL-4, C - IL-6, D – TNFα. Data represented mean serum levels of respective cytokines ± SD. * significance p≤0.05.
DISCUSSION
The expression of PAP is low in healthy pancreas and is highly up regulated during acute pancreatitis. The maximal expression of PAP can be detected in pancreas and in serum within 24 hours of induction of acute pancrestitis (5). The AR42J cell line can be induced to express PAP with stimulation of Dex/IL6 (11). In present study, we demonstrated that a mixture of PAP specific SiRNA was able to decrease PAP expression in rat acute pancreatitis.
RNAi technology represents a unique gene knockdown technology in its ability to study protein expression and function. Indeed, recent studies have demonstrated the ability of protein specific RNAi to decrease expression of pancreatic protein in vivo (14). However, the ability to design appropriate oligonucleotides is not trivial. Since the PAP protein system is highly homologous we initially screened a number of sequences derived from each PAP gene in their ability to decrease each PAP isoform. We selected potential target sequence by rational SiRNA design algorithm and all SiRNA targets were BLAST search to make sure that they were isoform-specific and did not cross react with other PAP isoforms or other known genes. Furthermore RNAi can be utilized in plasmid (circular) and linear based forms for targeted applications. In our preliminary studies (15), complementary DNA with 9 nts loop structure sequences were cloned into pSilencer 1.0-U6 Vectors and positive colonies were transiently transfected into stimulated AR 42J cells, using the lipofectamine method, to screen for their inhibition efficacy.
The target specific SiRNA sequences used in these studies were generated from positive screened oligonucleotides which were found to have the highest downregulatory capacity for each respective isoform; some of these also had a moderate downregulatory effect on other isoforms. Over 50 candidate constructs were screened in Dex/IL6 stimulated AR42J cells for subsequent in vivo experiments, which consisted of 2 sequences representing the 5’, middle and 3’ end of each PAP isoform (15). The fact that one construct, which is specific to one isoform, was able to downregulate expression of another PAP isoform suggested the interactive nature of all three PAP isoforms. Studies have shown that PAP I interacts with PAPII and PAP III as well as itself to form homo/heterodimers, suggesting that PAP proteins may provide overlapping function for other members of the Reg protein family (19,20).
Administration of PAP specific SiRNA in vivo decreased expression of PAP isoforms I and PAP III, however PAP II was not affected. This could be due to the fact that PAP II is the most abundant isoform in serum and tissue (5), and that the amount of administered SiRNA mixture was not enough to effectively knockdown PAP II. Studies by Bradley and colleagues (14) demonstrated siRNA mediated gene knockdown of the Ins2 mRNA when administered IV through the tail vein. In those studies animals received a 100ug of siRNA which facilitated a 33% reduction of the targeted gene mRNA expression. However, the effect of intrapancreatic administration and siRNA turnover were not studied. Our subcapsular administration of PAP specific siRNA decreased specific PAP isoform mRNA expression up to 55%. Increasing the dose of the SiRNA for this isoform may result in more effective decreased gene expression of PAP, or select PAP isoforms, but may also result in toxic or non specific effects. In agreement with PAP II mRNA expression, serum levels of PAP II did not differ between NaT induced pancreatitic rats with or without PAP specific SiRNA (16) which supports the notion that PAP mRNA was not appropriately saturated with RNAi at the amounts used.
The administration of PAP specific SiRNA in vivo correlated with worsening pancreatitis severity as evidenced by increased leukocytic infiltration and necrosis which has been shown with our earlier AS studies (12). The fact that there was no difference in edema scores could be also be due to the lack of total knockdown of PAP by RNAi as opposed to AS technology. Whether PAP is directly related to the pathophysiology of edema remains further investigation.
Analysis of serum, CRP, amylase and lipase were decreased with RNAi administration. This is in contrast to our previous finding that AS mediated gene knockdown of PAP correlated with increased serum levels of PAP (12). It could be that the ineffective knockdown of PAP II alone was sufficient to exert a putative protective effect on certain aspects of pancreatic physiology. To this end, previous studies in our laboratory have shown that NaT induced pancreatitis decreases expression of many pancreatic digestive products but increases expression of PAP (21). It could be that PAP, and possibly to a large extent PAP II may contribute to the downregulation of these digestive products thereby avoiding autodigestion of pancreas. It could also be that RNAi may have some non-specific effects where one species of specific RNAi can decrease expression of other, presumably unrelated genes (personal communication Applied Biosystems). Although both of these gene knockdown technologies represent very powerful tools to study gene function additional work may be needed to determine the possibility of non-specific binding.
The NaT model of acute pancreatitis is a well established model of inducing a necrotizing pancreatitis which in many cases mimics certain aspects of clinical disease (22). We have previously demonstrated that AS technology can be effectively applied to this in vivo system to downregulate PAP expression (12). SiRNA gene knockdown technology has also recently been shown to effectively knockdown gene expression in vivo though different delivery methods (14). Our studies administered SiRNA subcapsularly in an effort to maximize the local gene knockdown effect. The reduction of PAP after 24 hours demonstrates its efficacy in this context and appears to be an effective method of gene knockdown when compared with other methods.
Furthermore, serum cytokine levels of cytokines IL-1β, IL-4 and IL-6 were increased with administration of PAP specific SiRNA levels where as levels of TNFα was decreased. These data are consistent with our earlier data which showed that AS gene knockdown of PAP correlated with increased serum levels of IL-1α, IL-1β and IL4, (IL-6 was not tested) (12). In contrast serum levels of TNFα decreased in these studies with RNAi but were not affected with AS treatment. It could be that the remaining levels of PAP which were inefficiently downregulated with RNAi treatment in vivo were sufficient to promote a protective effect in decreasing serum levels of TNFα. Indeed, recent studies in our laboratory have demonstrated that macrophages cultured in the presence of recombinant PAPII generated increased RNA expression and serum levels of IL-1, IL-6 suggesting a direct relationship of PAP2 on the immune system (23). Other studies have also demonstrated an immunmodulatory role for PAP. Studies by Folch-Puy have demonstrated that the PAP I isoform inhibits NF kappa B activation and TNF alpha expression and is induced by itself and IL-10 (24,25). However, those studies were limited to the PAP I isoform. It is likely that the relationship of all three PAP isoforms as a collective in conjunction with putative binding proteins would modulate and elicit different inflammatory patterns in vivo. This is supported by the current observation that serum levels of TNFa were decreased with knockdown on PAP I and III whereas in our earlier studies, in which all three isoforms were knocked down, showed no difference in TNFa levels compared with controls (12).
PAP has been shown to have an anti-apoptotic and regenerative effects in vitro and in vivo (6,8,26). Studies by Simon et al (26) have demonstrated that HIP/PAP transgenic mice had increased liver regeneration after partial hepatectomy. In those studies HIP/PAP transgenic mice displayed evidence of liver regeneration after 48 hours. Regeneration at 24 hours was not significant compared with control animals; the role of HIP/PAP on apoptosis in vivo and the effects on pancreas were not determined. In our current studies it is unknown whether or not apoptosis was affected with siRNA gene knockdown in vivo. Further studies with respect to the role of PAP and their isoforms on organ specific apoptosis over time are necessary to better understand the physiological role of PAP in vivo.
In conclusion, gene knockdown technology represents a powerful tool to study PAP function. Differences in PAP isoform expression may account for the multiple reported functions of PAP.
REFERENCES
- 1.Orelle B, Keim V, Masciotra L, Dagorn JC, Iovanna JL. Human pancreatitis-associated protein. Messenger RNA cloning and expression in pancreatic diseases. J Clin Invest. 1992;90:2284–2291. doi: 10.1172/JCI116115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iovanna J, Orelle B, Keim V, Dagorn JC. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J Biol Chem. 1991;266:24664–24669. [PubMed] [Google Scholar]
- 3.Dusetti NJ, Frigerio JM, Keim V, Dagorn JC, Iovanna JL. Structural organization of the gene encoding the rat pancreatitis-associated protein. Analysis of its evolutionary history reveals an ancient divergence from the other carbohydrate-recognition domain containing genes. J Biol Chem. 1993;268:14470–14475. [PubMed] [Google Scholar]
- 4.Iovanna JL, Keim V, Bosshard A, Orelle B, Frigerio JM, Dusetti N, Dagorn JC. PAP, a pancreatic secretory protein induced during acute pancreatitis, is expressed in rat intestine. Am J Physiol. 1993;265:G611–G618. doi: 10.1152/ajpgi.1993.265.4.G611. [DOI] [PubMed] [Google Scholar]
- 5.Zenilman ME, Tuchman D, Zheng Q, Levine J, Delany H. Comparison of reg I and reg III levels during acute pancreatitis in the rat. Ann Surg. 2000;232:646–652. doi: 10.1097/00000658-200011000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz EM, Dusetti NJ, Vasseur S, Malka D, Bodeker H, Dagorn JC, Iovanna JL. The pancreatitis-associated protein is induced by free radicals in AR42J cells and confers cell resistance to apoptosis. Gastroenterology. 1998;114:808–816. doi: 10.1016/s0016-5085(98)70595-5. [DOI] [PubMed] [Google Scholar]
- 7.Namikawa K, Okamoto T, Suzuki A, Konishi H, Kiyama H. Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J Neurosci. 2006;26:7460–7467. doi: 10.1523/JNEUROSCI.0023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zenilman ME, Zheng Q, et al. Pancreatic reg I and a conserved bioactive fragment are mitogenic through the MAPK p38 pathway. J Am Coll Surg. 2000;191:S29. doi: 10.1016/S1072-7515(00)00456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heller A, Fiedler F, Schmeck J, Luck V, Iovanna JL, Koch T. Pancreatitis-associated protein protects the lung from leukocyte-induced injury. Anesthesiology. 1999;91:1408–1414. doi: 10.1097/00000542-199911000-00034. [DOI] [PubMed] [Google Scholar]
- 10.Folch-Puy E, Garcia-Movtero A, Iovanna JL, Dagorn JC, Prats N, Vaccaro MI, Closa D. The pancreatitis-associated protein induces lung inflammation in the rat through activation of TNFalpha expression in hepatocytes. J Pathol. 2003;199:398–408. doi: 10.1002/path.1307. [DOI] [PubMed] [Google Scholar]
- 11.Kandil E, Lin YY, Bluth MH, Zhang H, Levi G, Zenilman ME. Dexamethasone mediates protection against acute pancreatitis via upregulation of pancreatitis-associated proteins (PAP) World J Gastroenterol. 2006;12:6806–6811. doi: 10.3748/wjg.v12.i42.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Kandil E, Lin YY, Levi G, Zenilman ME. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats.″. Scand J Gastroenterol. 2004;9:870–881. doi: 10.1080/00365520410006477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viterbo D, Bluth M, Mueller C, Callender G, Lin YY, Murray S, Ocasio V, DiMaio T, Zenilman M. Anti-Reg I and III antibodies worsen pancreatitis in vivo. Gastroenterology. 2005;128:A791. [Google Scholar]
- 14.Bradley SP, Rastellini C, da Costa MA, Kowalik TF, Bloomenthal AB, Brown M, Cicalese L, Basadonna GP, Uknis ME. Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas. 2005;31:373–379. doi: 10.1097/01.mpa.0000179730.69081.64. [DOI] [PubMed] [Google Scholar]
- 15.Lin YY, Bluth MH, Viterbo D, Mueller CM, Zenilman ME. Linear siRNA mediated gene knockdown of pancreatitis-associated protein (PAP) in cellular pancreatitis. Pancreas. 2005;31:452. doi: 10.1097/MPA.0b013e31815f3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluth MH, Smith-Norowitz TA, Drew H, Viterbo D, Lin YY, Pierre J, Mueller CM, Stanek A, Zenilman ME. A sensitive assay for the determination of pancreatitis-associated protein in experimental acute pancreatitis. Am J Clin Pathol. 2006;126:635. [Google Scholar]
- 17.Graf R, Schiesser M, Scheele GA, Marquardt K, Frick TW, Ammann RW, Bimmler D. A family of 16-kDa pancreatic secretory stress proteins form highly organized fibrillar structures upon tryptic activation. J Biol Chem. 2001;276:21028–21038. doi: 10.1074/jbc.M010717200. [DOI] [PubMed] [Google Scholar]
- 18.De Reggi M, Gharib B, Protein X. Pancreatic Stone-, Pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Curr Protein Pept Sci. 2001;2:19–42. doi: 10.2174/1389203013381233. [DOI] [PubMed] [Google Scholar]
- 19.Bodeker H, Keim V, Fiedler F, Dagorn JC, Iovanna JL. PAP I interacts with itself, PAP II, PAP III, and lithostathine/regIalpha.″. Mol Cell Biol Res Commun. 1999;2:150–154. doi: 10.1006/mcbr.1999.0166. [DOI] [PubMed] [Google Scholar]
- 20.Honda H, Nakamura H, Otsuki M. The elongated PAP II/Reg III mRNA is upregulated in rat pancreas during acute experimental pancreatitis. Pancreas. 2002;25:192–197. doi: 10.1097/00006676-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Bluth MH, Viterbo D, Kevins M, Lin YY, Malhado L, Kandil E, Zenilman M. Gene expression profiling of a rat model for acute pancreatitis reveals subtle transcriptional changes in peripheral blood lymphocytes. Gastroenterology. 2003;124:A803. [Google Scholar]
- 22.Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1–14. doi: 10.1097/01.mpa.0000246658.38375.04. [DOI] [PubMed] [Google Scholar]
- 23.Viterbo D, Lin YY, Mueller CM, Zenilman ME, Bluth MH. Pancreatitis associated protein (PAP) modulates cytokine expression in macrophages. Gastroenterology. 2006;130:A384. [Google Scholar]
- 24.Folch-Puy E, Granell S, Dagorn JC, Iovanna JL, Closa D. Pancreatitis-associated protein I suppresses NF-kappa B activation through a JAK/STAT-mediated mechanism in epithelial cells. J Immunol. 2006;176:3774–3779. doi: 10.4049/jimmunol.176.6.3774. [DOI] [PubMed] [Google Scholar]
- 25.Vasseur S, Folch-Puy E, Hlouschek V, Garcia S, Fiedler F, Lerch MM, Dagorn JC, Closa D, Iovanna JL. p8 improves pancreatic response to acute pancreatitis by enhancing the expression of the anti-inflammatory protein pancreatitis-associated protein I. J Biol Chem. 2004;279:7199–7207. doi: 10.1074/jbc.M309152200. [DOI] [PubMed] [Google Scholar]
- 26.Simon MT, Pauloin A, Normand G, Lieu HT, Mouly H, Pivert G, Carnot F, Tralhao JG, Brechot C, Christa L. HIP/PAP stimulates liver regeneration after partial hepatectomy and combines mitogenic and anti-apoptotic functions through the PKA signaling pathway. FASEB J. 2003;17:1441–1450. doi: 10.1096/fj.02-1013com. [DOI] [PubMed] [Google Scholar]