Abstract
Angiotensin II (ANG II) blockade delays progression of chronic kidney disease (CKD) by modifying intrarenal hemodynamics, but the effect on metabolic adaptations has not been examined. Using renal ablation/infarction (A/I) model of CKD in rats at one week, the effects of ANG II blockade by captopril (CAP) and losartan (LOS) on renal O2 consumption (QO2), renal nitric oxide (NO) activity and nitric oxide synthase (NOS) protein expression was examined. A/I kidneys exhibited proteinuria, reduced GFR, renal blood flow (RBF) and NOS-1 protein expression, while QO2 factored by sodium reabsorption (QO2/TNa) was markedly increased. CAP + LOS treatment increased GFR, RBF, and TNa, while QO2 remained unchanged, thus normalizing QO2/TNa. NOS-1 expression was normalized with CAP + LOS, as was proteinuria. Triple antihypertensive therapy administered to control for the blood pressure reduction, and lysine administration to increase GFR and RBF, did not normalize QO2/TNa, suggesting a specific effect of ANG II in elevating QO2/TNa. NOS blockade, to test functional NO activity on QO2 and QO2/TNa, increased QO2 in shams, but not in untreated A/I. The increase in QO2 was restored in CAP + LOS treated A/I. CAP + LOS treatment normalized the increased QO2/TNa and functional NO activity in A/I independent of the blood pressure and GFR effects, providing evidence for an additional mechanism underlying the benefits of ANG II inhibition therapy.
Keywords: NOS-1, Remnant Kidney, Glomerular filtration rate, Oxygen consumption, ANG II
Introduction
The kidney receives 20–25% of the cardiac output, however, due to its unique structure and physiology, it usually borders of hypoxia. This is in part due to a preglomerular oxygen diffusion shunt maintaining a low O2 tension (pO2) in the cortex, around 40–45 mmHg (1–3) and a relatively high rate of oxygen consumption per gram tissue. In the normal kidney, this high rate of oxygen consumption is primarily obligated to sodium reabsorption as the proximal tubule primarily functions in an aerobic environment. Therefore, kidney ischemia and hypoxia can be dictated not only by adequacy of blood flow and supply of oxygen, but also by changes in the rate of oxygen consumption. Varieties of pathophysiologic conditions are characterized by alterations in the rate of kidney oxygen consumption. Hypoxia, resulting from decreased oxygen supply or increased oxygen consumption, can affect the expression of a wide array of genes, including many fibrogenic factors, and has been postulated as a common pathway to progressive chronic kidney disease (CKD) (4). Therefore, the regulation and efficient utilization of O2 can be an important contributor to the pathogenesis of progression of CKD.
Recent studies have suggested that renal oxygen consumption is regulated by nitric oxide (NO) and angiotensin II (ANG II) in normal and diseased kidneys. Acute nitric oxide synthase (NOS) inhibition, specifically of NOS-1, or chronic ANG II infusion increases renal oxygen consumption in normal animals (5–7). There is evidence for increased oxygen consumption in experimental models of hypertension and in early diabetes. In the remnant kidney or 5/6thkidney ablation/infarction (A/I) model, a traditional model of CKD, increased renal oxygen consumption per nephron or per sodium ion transported has also been observed (8, 9), along with indirect evidence of intrarenal hypoxia (10). Moreover, evidence of intrarenal hypoxia is evident prior to structural changes affecting the supply of oxygen (11). Interestingly, the remnant kidney model shows reduced renal expression of neuronal NOS and other functional evidence of NO deficiency (12, 13), while high ANG II activity has been implicated, at least in the early stages (14). Treatment of CKD with renin-angiotensin system (RAS) blockers has been shown to delay the progression of CKD (15–19), suggesting increased ANG II activity as one of the important factors contributing to the progression of CKD. While the beneficial effects of RAS blockers on glomerular hemodynamics have been widely published, the effects on renal metabolism have not received enough attention.
In this study we tested the hypothesis that in A/I kidneys an imbalance in the activities of NO and ANG II contributes to increased oxygen consumption. We confirmed an increase in oxygen consumption factored for sodium reabsorption in the early stages of remnant kidneys, and found a deficiency in NOS-1 protein expression. We assessed the effects of ANG II inhibition on oxygen consumption, and found that combined ACE inhibition and angiotensin type 1 (AT-1) receptor blockade significantly decreased the elevated values for oxygen consumption factored by sodium reabsorption and markedly increased RBF and GFR, with restoration of NOS-1 protein expression, and demonstrated that these beneficial metabolic effects of ANG II blockade can be distinctly separated from the effect on systemic blood pressure and the increase in filtered load.
Results
Systolic blood pressure in conscious rats
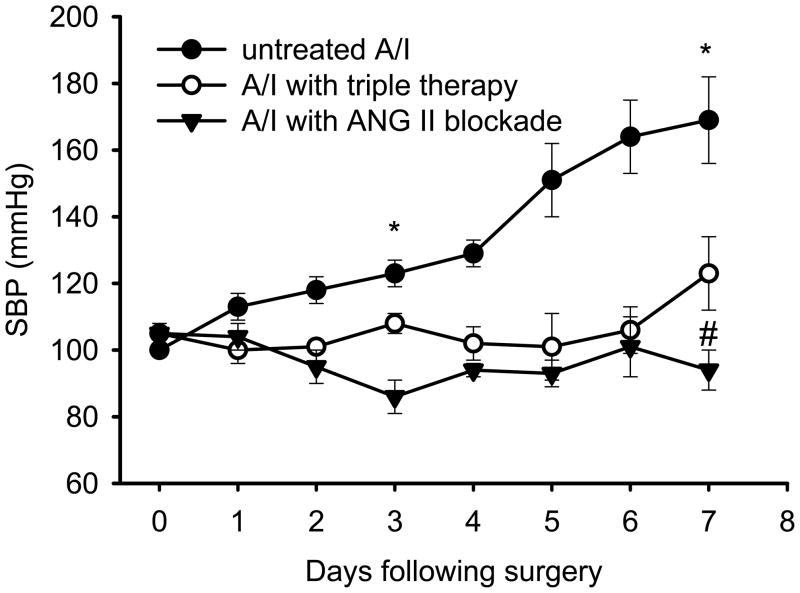
Systolic blood pressure (SBP) was measured in three groups of conscious rats by the tail-cuff method. As shown by Figure 1, SBP significantly increased as early as day 4 following the A/I, and continued increasing, even more rapidly, to day 7. This early blood pressure rise was effectively prevented by dual ANG II blockade. Triple therapy with hydralazine, reserpine, and hydrochlorothiazide had the same anti-hypertensive effect. Treatment of A/I rats with lysine had no impact on systolic blood pressure and values were similar to untreated remnant kidney rats (Table 1).
Figure 1. Changes of systolic blood pressure (SBP) in awake A/I rats measured by tail-cuff method.
Data are reported as mean ± SE. Untreated A/I rats developed hypertension within one week. Both treatments significantly prevented the blood pressure from rising. * P < 0.05; ** P < 0.01 compared with baseline (day 0); # P < 0.01 compared with untreated A/I.
Table 1. Renal oxygen consumption, transport efficiency, renal function, blood pressure and urinary protein in the five groups.
Data are reported as mean ± SE. Abbreviations: A/I: renal ablation and infarction; T1: treatment with captopril and losartan; T2: treatment with hydralazine, reserpine and hydrochlorothiazide (triple therapy); T3: treatment with Lysine; QO2: renal oxygen consumption; TNa: transported sodium; QO2/TNa: sodium transport efficiency; GFR: glomerular filtration rate; RBF: renal blood flow; BP: systemic blood pressure. The urine was drawn directly from the bladder using a syringe right after anesthesia. The urinary protein concentration was detected using urinary 32 strips, where + stands for trace to 0.3 mg/ml, ++ 1 mg/ml and +++ 5 mg/ml.
| Group | QO2 (ml/min) | QO2/TNa (M/M) | GFR (ml/min) | RBF (ml/min) | BP (mmHg) | Urinary protein |
|---|---|---|---|---|---|---|
|
(I) Sham n=7 |
0.16±0.01 | 1.04±0.11 | 1.11±0.15 | 7.03±0.5 | 99±4 | 0 -- + |
|
(II) A/I n=20 |
0.14±0.01 | 1.70±0.15 | 0.69±0.07 | 4.25±0.34 | 116±3 | +++ |
| P vs. I | NS | <0.05 | <0.05 | <0.01 | <0.05 | |
|
(III) A/I+T1 n=19 |
0.15±0.01 | 1.23±0.09 | 0.91±0.06 | 6.80±0.29 | 85±3 | 0 -- + |
| P vs. II | NS | <0.05 | <0.05 | <0.05 | <0.01 | |
|
(IV) A/I +T2 n=6 |
0.14±0.01 | 1.54±0.12 | 0.76±0.09 | 4.7±0.40 | 85±7 | + -- ++ |
| P vs. II | NS | NS | NS | NS | <0.01 | |
|
(V) A/I + T3 n=6 |
0.20±0.03 | 1.61±0.14 | 0.97±0.04 | 6.67±0.48 | 128±10 | |
| P vs. II | <0.05 | NS | <0.01 | <0.01 | NS |
Renal oxygen consumption and kidney function
Table 1 shows the means ± SE for renal oxygen consumption (QO2), sodium transport efficiency (QO2/TNa), glomerular filtration rate (GFR), renal blood flow (RBF), mean arterial pressure (BP), and urinary protein in the five groups of rats: (I) sham operated, (II) untreated ablation/infarction, (III) A/I treated with dual ANG II inhibition by captopril and losartan, (IV) A/I with triple anti hypertensive therapy, and (V) A/I with lysine.
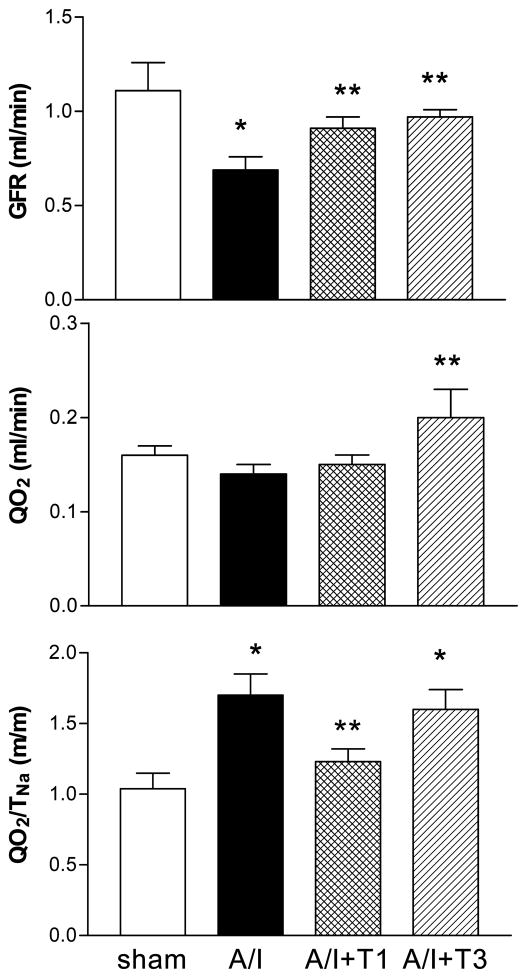
A/I rats had a lower RBF (4.25 ± 0.34 vs. 7.04 ± 0.51, ml/min, p < 0.01) and a lower GFR (0.69 ± 0.07 vs. 1.14 ± 0.13, ml/min, p < 0.01), compared to shams, as expected with the marked reduction in nephron number. The GFR was increased to normal values in the A/I rats treated with ANG II inhibition, but not normalized by triple anti-hypertensive therapy, suggesting a significant ANG II association with renal hemodynamics in this early A/I model. In the normal kidney, the major renal oxygen consumption is used for sodium reabsorption (TNa) and the ratio of QO2/TNa is relatively stable, which is around 1, as shown with the sham group in Table 1. In spite of the reduction in renal mass by about two-thirds, the A/I kidney had a total QO2 similar to that of the sham kidney (0.14 ± 0.01 vs. 0.16 ± 0.01, ml/min, P >0.05), suggesting that the A/I kidney had an increased oxygen consumption per nephron. This observation was further supported by a substantially higher value of QO2/TNa (1.70 ± 0.15, vs. 1.00 ± 0.12, ml/mole, P < 0.05). The elevation in QO2/TNa was reduced by ANG II inhibition (1.70 ± 0.15, vs. 1.23 ± 0.09, P < 0.05), but not affected by the triple therapy (NS), (1.54 ± 0.12) suggesting that ANG II has a significant impact on renal oxidative metabolism separate from effects on systemic blood pressure in this early A/I model. As shown in Table 1 and Figure 2, both ANG II inhibition and lysine feeding significantly increased GFR. However, the GFR increase by lysine feeding was accompanied by a significant increase in QO2 whereas the GFR increase by ANG II inhibition was characterized by a constant QO2. Therefore QO2/TNa after lysine was not different from untreated A/I rats and values remained significantly higher that A/I + T1 rats. These findings suggest that the increase in QO2 associated with an increase in GFR was offset by a prevention of QO2 elevation caused by non-transport cellular processes in A/I kidneys treated with ANG II blockade, implying that there was an increased non Na-reabsorptive work requiring oxygen in untreated A/I kidneys.
Figure 2. Glomerular filtration rate, renal oxygen consumption and sodium transport efficiency in rats on day 7 following A/I (renal ablation/infarction) surgery.
T1: treatment with dual ANG II inhibition (captopril 20mg/kg/day and losartan 20mg/kg/day); T3:treatment with L-lysine (0.5g × 2/rat/day). All the drugs were given by daily gavage for 8 consecutive days. * P < 0.05 compared with sham; ** P < 0.05 compared with A/I. A/I rats treated with lysine (T3) had an increased glomerular filtration rate (GFR) and an increased total renal oxygen consumption (QO2) with the sodium transport efficiency (QO2/TNa) remaining high. In contrast, A/I rats treated with ANG II inhibition (T1) had an increased GFR and unchanged QO2 with a normalized QO2/TNa.
Effects of NOS blockade on renal oxygen consumption and renal function in A/I kidneys
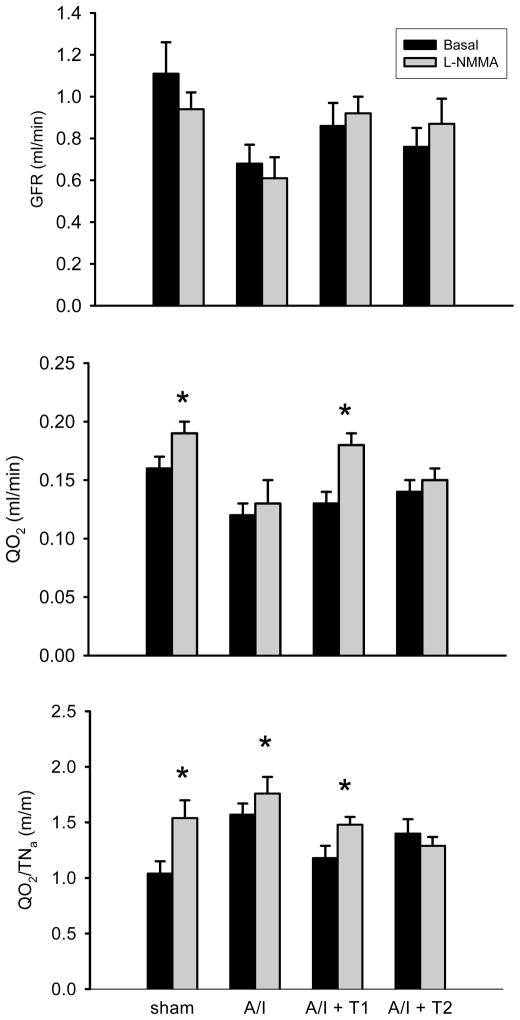
In this study of A/I kidney, which exhibits increased oxygen consumption, we further tested if inhibition of NO generation can induce additional increases in renal oxygen consumption in early stages of A/I kidney. L-NMMA was used for acute NO inhibition in four groups of rats: (I) sham operated (Sham), (II) untreated ablation/infarction (A/I), (III) A/I treated with dual ANG II inhibition by captopril and losartan (A/I + T1) and (IV) A/I with triple therapy by hydralazine, reserpine, and hydrochlorothiazide (A/I + T2). As shown in Table 2 and Figure 3, with respect to GFR, acute NOS blockade with L-NMMA did not produce significant changes in any of the four groups. However, acute NOS blockade exerted different effects on QO2 in the four groups. NOS blockade produced a significant increase in QO2 in the sham group (0.16 ± 0.01 vs. 0.19 ± 0.01, P < 0.05) and no change in QO2 in the untreated A/I group (0.12 ± 0.01 vs. 0.13 ± 0.02, P > 0.05), but there was a small and significant increase in QO2/TNa in the untreated A/I kidney. NOS blockade produced a significant increase in both QO2/TNa and QO2 in the A/I group treated with ANG II inhibition (0.13 ± 0.01 vs. 0.18 ± 0.01, P< 0.05) but produced no change in QO2/TNa and QO2 in the A/I group treated with triple therapy (0.14 ± 0.01 vs. 0.15 ± 0.01, P> 0.05), suggesting that the NO deficiency in this condition is related to ANG II and not just increased blood pressure.
Table 2. The effects of nitric oxide synthase blockade with L-NMMA on renal oxygen consumption, glomerular filtration rate, ratio of oxygen consumption and sodium transport and blood pressure in four groups.
Data are reported as mean ± SE.
| Group | QO2 (ml/min) | GFR (ml/min) | QO 2/TNa (m/m) | BP (mmHg) | ||||
|---|---|---|---|---|---|---|---|---|
| basal | L-NMMA | basal | L-NMMA | basal | L-NMMA | basal | L-NMMA | |
|
Sham n=7 |
0.16±0.01 | 0.19±0.01* | 1.11±0.15 | 0.94±0.08 | 1.04±0.11 | 1.54±0.16* | 102±3 | 127±4** |
|
A/I n=10 |
0.12±0.01 | 0.13±0.02 | 0.68±0.09 | 0.61±0.10 | 1.57±0.10 | 1.76±0.15* | 115±12 | 151±11** |
|
A/I + T1 n=9 |
0.13±0.01 | 0.18±0.01* | 0.86±0.11 | 0.92±0.08 | 1.18±0.11 | 1.48±0.07* | 80±3 | 103±3** |
|
A/I + T2 n=8 |
0.14±0.01 | 0.15±0.01 | 0.76±0.09 | 0.87±0.12 | 1.40±0.13 | 1.29±0.08 | 85±7 | 113±11** |
stands for P < 0.05 vs. basal;
P < 0.01 vs. basal. Abbreviations: A/I -- renal ablation and infarction; T1 – treatment with captopril and losartan; T2 -- treatment with hydralazine, reserpine and hydrochlorothiazide (triple therapy); QO2 -- renal oxygen consumption; GFR -- glomerular filtration rate; BP -- systemic blood pressure; L-NMMA -- NG-monomethyl-L-arginine, a non-selective nitric oxide synthase inhibitor.
Figure 3. Changes of renal oxygen consumption in response to acute nitric oxide synthase (NOS) inhibition in four groups.
Data are reported as mean ± SE values on day 7 following A/I surgery. A/I -- untreated ablation/infarction; T1 -- dual ANG II inhibition with captopril and losartan; T2 -- triple therapy with hadralazine, reserpine and hydrochlorothiazide. * P < 0.05 compared with basal line. Acute superimposed NOS inhibition by L-NMMA did not alter GFR in any group. The NOS inhibition caused a significant increase in QO2 in the sham group, but this response (increase in QO2) was not seen in untreated A/I rats. This response to NOS inhibition was recovered by ANG II inhibition (T1) but was not recovered by another anti-hypertensive treatment (T2).
Changes of NOS protein abundance in A/I kidneys
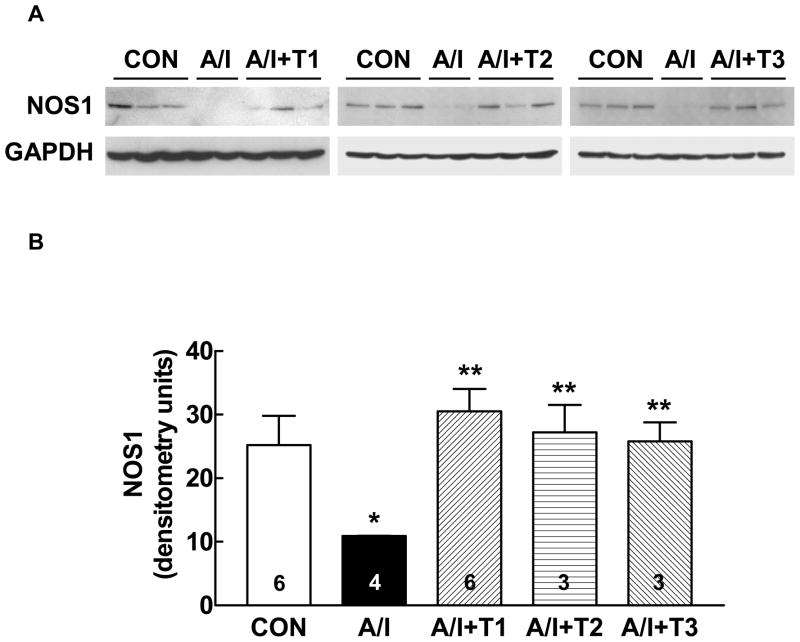
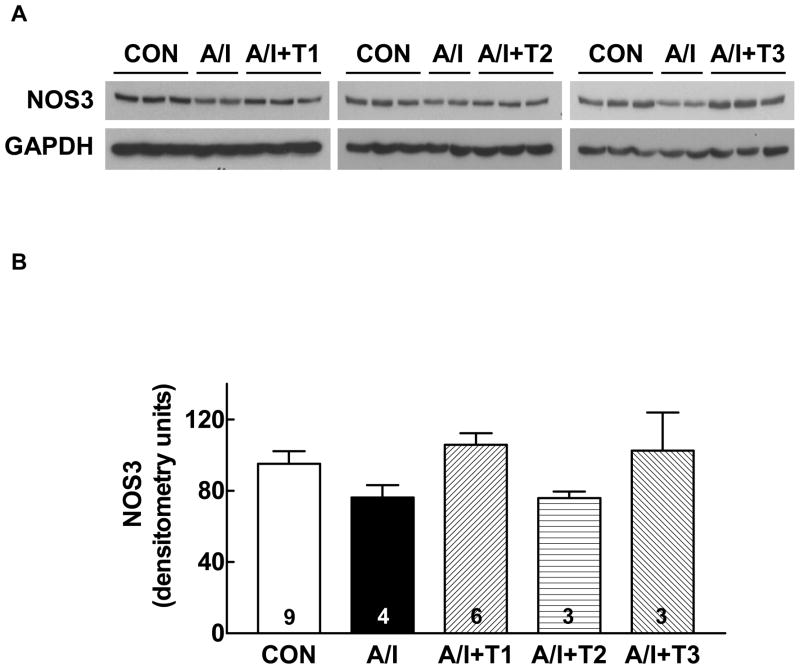
We determined NOS-1 protein abundance using the Western blot analysis in untreated A/I vs. treated A/I, with the right kidney as normal control. We chose NOS-1 as it has been demonstrated by Szabo and Baylis that renal NOS-1 protein expression is a marker of renal injury (20); and mitochondrial NOS is believed to be of NOS-1 type (21, 22). As shown in Figure 4, the NOS-1 protein abundance in the renal cortex was significantly reduced in A/I group, but was prevented by dual ANG II blockade. However, NOS-1 protein expression was also restored in the other A/I groups treated with triple therapy and lysine. We, then, evaluated NOS-3 protein expression to help explain the reduction in functional NO activity in A/I kidneys and restoration of this activity by Ang II blockade. NOS-3 was not different in sham and all A/I kidney groups (Fig. 5). While NOS-2 expression was different in the groups, it did not correlate with functional NO activity either (unpublished observations).
Figure 4. Western blot analysis of NOS-1 protein expressions in renal cortex in the five groups of rats.
A: Representative Western blots showing the NOS-1 protein expression levels in renal cortex from normal (CON), untreated renal ablation/infarction (A/I), A/I with dual ANG II blockade (A/I + T1), A/I treated with hydralazine, peserpine and hydrochlorothiazide (A/I + T2) and A/I treated wit lysine (A/I + T3).
B: Summary graph representing the relative abundance of NOS-1 protein levels in the renal cortex analyzed using densitometry. There was a significant reduction of NOS-1 protein expression in one week A/I cortex,, and this reduction was prevented not only by dual ANG II blockade (T1) but by both T2 and T3.
* P < 0.01 (A/I vs. CON) ** P < 0.01 (A/I + T n vs. A/I).
Figure 5. Western blot analysis of NOS-3 protein expressions in renal cortex in the five groups of rats.
A: Representative Western blots showing the NOS-1 protein expression levels in renal cortex from normal (CON), untreated renal ablation/infarction (A/I), A/I with dual ANG II blockade (A/I + T1), A/I treated with hydralazine, peserpine and hydrochlorothiazide (A/I + T2) and A/I treated wit lysine (A/I + T3).
B: Summary graph representing the relative abundance of NOS-1 protein levels in the renal cortex analyzed using densitometry. There is no differences in renal cortical NOS3 expression among five groups.
Discussion
This study confirms that the A/I kidney exhibits increased oxygen consumption when factored by estimated remaining nephrons or factored by total Na reabsorption (QO2/TNa) as observed by others (8, 9). ANG II inhibition normalized this increase in QO2/TNa, suggesting a functional high ANG II state in the early stages of remnant kidney that regulates oxygen utilization. We have further supplied evidence that the effects of ANG II blockade on these parameters are independent of their hemodynamic effects.
It has been traditional to factor QO2 by TNa because studies have suggested that in the normal kidney approximately 80% of oxygen consumption is obligated to the process of Na reabsorption, therefore generally linked to the absolute values for GFR. Since both QO2 and QO2/TNa are increased in the untreated remnant kidney we should summarize the elements that could contribute to this. Oxygen requirements could increase because of either acquired inefficiencies in the process of Na reabsorption or shifts in the site of Na reabsorption to more distal tubular sites that exhibit higher oxygen and ATP costs per ion transported. Secondly, QO2/TNa and QO2 could be increased due to effects on mitochondrial efficiency. An example would be the effects of NO to reduce mitochondrial oxygen consumption via effects on cytochrome c oxidase and at other sites influencing oxidative metabolism. Finally, there could be increases in “work” other than Na reabsorption that requires oxygen consumption either for ATP requirements or increases in chemical utilization of oxygen.
In this study, ANG II blockade not only improved the QO2/TNa but may have exerted other effects on the oxidative status of the kidney. ANG II blockade normalized blood pressure and also produced a major increase in RBF and GFR. Therefore, we generated another group in which blood pressure was normalized using triple therapy, a treatment that likely did not reduce kidney ANG II activity. The QO2/TNa in this group remained increased above values observed in CAP + LOS treated A/I kidneys, similar to values in the untreated A/I remnant kidney. We addressed the second issue by increasing GFR to a degree similar to that observed after combined ANG II blockade by daily administration of lysine. Increasing the GFR and RBF did not normalize QO2/TNa and duplicate the effects of combined ANG II blockade. Therefore, these two hemodynamic effects of ANG II blockade in the remnant kidney rats did not contribute to the correction of QO2/TNa achieved by CAP + LOS treatment.
The mechanisms whereby ANG II may increase kidney QO2 have not been fully explained. There may be multiple pathways involved, including stimulation of Na reabsorption. However, this transport effect of ANG II does not necessarily increase the QO2/TNa ratio or diminish the efficiency of Na transport. Normal kidney also expresses NADPH oxidases (23), and prolonged infusion of ANG II upregulates NADPH, accompanied by an increase in renal QO2 and a decrease in renal tissue oxygenation (7). Increased NADPH oxidase expression has been reported in rat kidneys 6 weeks after 5/6th nephrectomy (24). ANG II also activates the enzyme systems; lipooxygenases, cytochrome P-450 and cyclooxygenases, producing a variety of bioactive molecules (25). However, it is unlikely that ANG II stimulated oxidase activity would be sufficient to explain the large increases in QO2 and QO2/TNa in the untreated remnant kidney and the nearly complete amelioration by ANG II blockade.
Extensive in vitro studies have demonstrated that NO is an important regulator of oxygen utilization in mitochondria. More than 85% of the oxygen utilized by the cell is consumed by the mitochondrial electron transport system for ATP synthesis. Most investigations have demonstrated that NO and its derivatives inhibit mitochondrial respiration in two different ways: (1) Within the physiological range, NO exerts an acute and reversible inhibition of cytochrome c oxidase, in competition with O2, thereby decreasing respiration (26–28), implying a “braking” effect on oxidative metabolism; and (2) at higher concentrations, NO and its derivatives irreversibly inhibit multiple enzymes of the mitochondria, including aconitase, complex I, II, III and cytochrome b (29–33). Existence of mitochondrial NOS (mtNOS) was recently described in rat liver (34, 35), brain (36) heart and kidney (37), and has been identified as the alpha isoform of NOS-1 (21, 38). The study by Laycock et al demonstrated in vivo that NO inhibition caused an increase in renal oxygen consumption in normal conscious dog (6). We have further clarified in a previous study that the specific NOS-1 isoform inhibition leads to an increase in oxygen consumption in normal rat kidney in vivo and in isolated proximal tubules (5), the latter also demonstrated by Koivisto and Persson (39).
Various animal models of CKD are characterized by a deficiency in NO generation and NOS-1 expression (13) (12). Therefore we examined the potential role of NO as an intermediary that might explain the observed metabolic effects of combined ANG II blockade. We have confirmed the functional NO deficiency in the remnant kidney in the current study, by demonstrating that in contrast to normal kidneys, the untreated A/I kidney showed no change in oxygen consumption in response to acute inhibition of NOS. However combined ANG II blockade restored the normal response of kidneys to NOS blockade. In order to control for the normalization of blood pressure, we examined the effects of non-selective NOS blockade in rats whose blood pressure had been normalized by triple therapy. These rats exhibited no increases in oxygen consumption, similar to the untreated A/I kidneys. Protein expression of NOS-1 was also reduced in untreated A/I, but was restored in all A/I treatment groups. These results suggest that the suppression of NOS-1 protein expression in the untreated A/I kidney may derive from systemic and renal hemodynamic consequences and/or changes in temporal adaptation of the tubuloglomerular feedback system rather than the consequence of increased ANG II activity. This makes alteration in expression of NOS-1 an unlikely single explanation for changes in NO activity or the cause of the increase in oxygen consumption. This lack of correlation between functional activity and protein expression of NO has also been shown in the past to in conditions such as variations in NaCl intake (40, 41). Most importantly, the normalization of the increase in QO2/TNa by ANG II blockade and not other therapies in remnant kidneys did not correlate with protein expression of NOS isoforms. The combination of ACE inhibitors and AT-1 receptor blockers used in this study could have restored functional NO activity by several potential mechanisms involving AT-1, AT-2 receptors and elimination of NOS inhibitors that are known to be elevated in this model (42–44). We also observed that blockade of ANG II activity in the A/I kidney increased RBF and GFR to a greater extent than expected in a normal kidney, implying a greater ANG II impact in the A/I kidney. Blockade of ANG II activity likely produced changes in tubuloglomerular feedback activity potentially contributing to this greater effect on GFR and RBF.
In the remnant kidney, hypoxia has been demonstrated by indirect measurements in early (10) and late stages, and has been proposed as a final common pathway to progression of CKD (4). However, the structural changes leading to a decrease in blood supply implicated in the late stages cannot be invoked to explain the hypoxia observed in the early stages. An increase in demand or utilization of oxygen could also contribute to hypoxia in the early stages. We have verified previous observations in the remnant kidney of increased oxygen consumption per remaining kidney mass or factored by Na reabsorption in early stages (8, 9), and this finding was corrected with losartan and captopril treatment. ANG II blockade also appeared to eliminate functional NO deficiency. Early modification of hormonal status in the remnant kidney model of CKD is critical to the regulation of oxygen consumption and may prevent early hypoxia, thus suggesting another important mechanism by which ANG II blockers provide a salutary effect in progressive kidney diseases.
Materials and Methods
Animal experiments described herein were conducted in accordance with the NIH Guide for the Care and Use of Animals in Research. Studies were conducted on male Wistar rats, weighing initially between 220 and 250g when purchased from Harlan (Indianapolis, IN, USA).
Renal ablation and infarction surgery
The rats were anesthetized with sodium pentobarbital (50mg/kg, i.p.), and placed on temperature controlled surgical table. Using sterile precautions, the right kidney was exposed via a small right flank incision. After separating the adrenal gland, the right renal pedicle was ligated and the kidney removed. A left flank incision was then performed and the left kidney was retracted out of the peritoneal space to expose the renal artery. Two branches of the left renal artery were ligated with 3-0 silk sutures. The left kidney was then gently retracted back into the body and the incision was closed. Sham-operated rats underwent anesthesia and manipulation of the renal pedicles on both sides, without removal of any renal mass. The rats were kept warm until ambulatory.
BP measurement in awake rats
Systolic BP (SBP) of rats was measured by the tail-cuff method. Nonanesthetized rats were placed in a plastic restraining holder mounted on a thermostatically controlled warm plate that was maintained at 37°C during measurement. The tail protruded from the holder. A metal tubular cuff, 7/16 inches, was placed around the tail of the rat. Cuff inflation/deflation rates (25 mm Hg/sec) and maximum cuff pressure (225 mmHg) were controlled by a programmed electro-sphygmomanometer PE-300 (Narco Bio Systems, Houston, TX). The reported values are the mean of four to six recordings performed at the same time of day by the same investigator on 8 consecutive days starting at one day before the A/I surgery. All the rats underwent restraining holder training (15 minutes/per day) for 3 to 6 days.
Study groups and drug administration
Five groups of rats were used for the study: SHAM -sham operated rats, A/I -renal ablation/infarction, A/I +T1-A/I treated with captopril (20mg/kg/day) and losartan (20mg/kg/day), A/I + T2 –A/I treated with hydralazine (20mg/kg/day), reserpine (4.5mg/kg/day) and hydrochlorothiazide (10mg/kg/day), and A/I +T3 –A/I treated with Lysine (3g/kg/day). All drugs were given by daily gavage. The treatment started one day before the surgery and was continued for eight consecutive days.
Whole kidney function and O2 consumption measurement
Clearance and O2 consumption experiments were performed as described in details earlier (5). Briefly, 1 week after A/I, the rats were anesthetized with Inactin (100mg/kg, i.p). After cannulation of trachea, left jugular vein, left femoral artery and urinary bladder was catheterized. The left kidney blood flow (RBF, ml/min) was monitored with a perivascular ultrasonic transit time flow probe (Transonics T420, Ithaca, NY, USA) connected to a computer for continuous recording. Proximal left renal vein was used for sampling of venous blood. GFR (ml/min) was 51 measured by clearance of 3H-inulin administered in Ringer’s NaCl-NaHCO3\ at 1.5ml/h (10μCi/ml.
Blood samples were taken from the femoral artery and renal vein for measurements of total arterial blood hemoglobin (tHb), (O2Hb), (pO2), (pCO2), pH, [Na+], [K+], [HCO3−] with a color spectrophotometer, 682 CO-Oximeter (Instrumentation Laboratory, Lexington, MA). O2 content (O2ct) was calculated by the formula:
The total left kidney O2 consumption (QO2, ml/min) was calculated from A-V difference in O2 content multiplied by RBF. TNa is equal to the total amount of sodium filtered (FNa) minus the amount of sodium excreted in the urine (UNaV). After the surgical preparation, the animals were allowed 60 minutes for stabilization with the flow probe in place and blood pressure and renal blood flow being recorded, after which measurements were taken. GN -Monomethyl-L-arginine (L-NMMA, 2 μmole/kg/min), a non selective nitric oxide synthase inhibitor was administered intravenous infusion for 60 minutes in a group of rats.
Western blotting analysis
Renal medulla and cortex were homogenized (4°C) in buffer (25 mM Tris-HCl, pH 7.4, 0.5 mM EDTA, 0.5 mM EGTA, 10 mMβ-mercaptoethanol, 50 mM β-glycerophosphate, 10 mM NaF, 1 mM Na3VO4) in the presence of protease inhibitor cocktail (Roche, IN). The tissue homogenates were extracted with 1% Triton X-100 for 10 min at 4°C and then spun at 5,000 56 rpm for 3 min. After protein concentration measurement by Bradford method, the supernatant were denatured at 95°C for 3 min in Laemmli buffer. Samples were subjected to SDS-PAGE in 10% polyacrylamide gels. After electrophoresis, proteins were electrotransferred to a polyvinylidene difluoride membrane (Bio-Rad), which was incubated in blocking buffer (5% milk, 6 20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20) at 4 °C 1 hr and then incubated with anti-NOS-1 antibody (1:200, sc-5302, Santa Cruz Biotechnology), anti-NOS2 (1:200, sc-650, Santa Cruz Biotechnology) and anti-NOS-3 antibody (1:250, Cat # 610296, BD Transduction Laboratories) overnight. Binding of the primary antibody was detected by an enhanced chemiluminescence method (ECL Plus; GE Healthcare Biosciences) using horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse IgG 1:5, 000 sc-2031, goat anti-rabbit IgG, 1:5,000 sc-2030, Santa Cruz Biotechnology). The blot membrane was then stripped by incubating in 1.2 M Glycine (pH 2) for 2 hr and used for additional protein expression detection. Quantification of protein expression was performed using Gel-Pro® Analyzer (Media Cybernetics, Silver Spring, Maryland).
Statistical analysis
Results were presented as mean ± SE. Differences between groups were analyzed by 1-way analysis of variance (ANOVA) and differences before and after drug administration were analyzed by 2-way ANOVA with repeated measures, using statistic software SPSS. Statistical significance is taken at p<0.05.
Acknowledgments
This study was supported by funds provided by the National Institutes of Health (NIH DK28602 and DK56248) and resources supplied by the Research Service of the Department of Veterans Affairs. Dr. Tang was supported in part by a Grant-In-Aid from the American Heart Association Western States Affiliate. Dr. Singh was supported by a grant from the National Kidney Foundation of Southern California.
Footnotes
There are no conflicts of interests by any of the authors involved.
References
- 1.Leichtweiss HP, Lubbers DW, Weiss C, Baumgartl H, et al. The oxygen supply of the rat kidney: measurements of int4arenal pO2. Pflugers Arch. 1969;309:328–349. doi: 10.1007/BF00587756. [DOI] [PubMed] [Google Scholar]
- 2.Schurek HJ, Jost U, Baumgartl H, Bertram H, et al. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am J Physiol. 1990;259:F910–915. doi: 10.1152/ajprenal.1990.259.6.F910. [DOI] [PubMed] [Google Scholar]
- 3.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney Int. 2001;59:230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 4.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 5.Deng A, Miracle CM, Suarez JM, Lortie M, et al. Oxygen consumption in the kidney: effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int. 2005;68:723–730. doi: 10.1111/j.1523-1755.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 6.Laycock SK, Vogel T, Forfia PR, Tuzman J, et al. Role of nitric oxide in the control of renal oxygen consumption and the regulation of chemical work in the kidney. Circ Res. 1998;82:1263–1271. doi: 10.1161/01.res.82.12.1263. [DOI] [PubMed] [Google Scholar]
- 7.Welch WJ, Blau J, Xie H, Chabrashvili T, et al. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–28. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 8.Harris DC, Chan L, Schrier RW. Remnant kidney hypermetabolism and progression of chronic renal failure. Am J Physiol. 1988;254:F267–276. doi: 10.1152/ajprenal.1988.254.2.F267. [DOI] [PubMed] [Google Scholar]
- 9.Nath KA, Croatt AJ, Hostetter TH. Oxygen consumption and oxidant stress in surviving nephrons. Am J Physiol. 1990;258:F1354–1362. doi: 10.1152/ajprenal.1990.258.5.F1354. [DOI] [PubMed] [Google Scholar]
- 10.Manotham K, Tanaka T, Matsumoto M, Ohse T, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 11.Norman JT, Fine LG. Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol. 2006;33:989–996. doi: 10.1111/j.1440-1681.2006.04476.x. [DOI] [PubMed] [Google Scholar]
- 12.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1–9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- 13.Roczniak A, Fryer JN, Levine DZ, Burns KD. Downregulation of neuronal nitric oxide synthase in the rat remnant kidney. J Am Soc Nephrol. 1999;10:704–713. doi: 10.1681/ASN.V104704. [DOI] [PubMed] [Google Scholar]
- 14.Mackie FE, Campbell DJ, Meyer TW. Intrarenal angiotensin and bradykinin peptide levels in the remnant kidney model of renal insufficiency. Kidney Int. 2001;59:1458–1465. doi: 10.1046/j.1523-1755.2001.0590041458.x. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujihara CK, Velho M, Malheiros DM, Zatz R. An extremely high dose of losartan affords superior renoprotection in the remnant model. Kidney Int. 2005;67:1913–1924. doi: 10.1111/j.1523-1755.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 18.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 19.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 20.Szabo AJ, Wagner L, Erdely A, Lau K, et al. Renal neuronal nitric oxide synthase protein expression as a marker of renal injury. Kidney Int. 2003;64:1765–1771. doi: 10.1046/j.1523-1755.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 21.Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem. 2002;277:38079–38086. doi: 10.1074/jbc.M205256200. [DOI] [PubMed] [Google Scholar]
- 22.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–195. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 24.Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, et al. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003;63:179–185. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 25.Needleman P, Turk J, Jakschik BA, Morrison AR, et al. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 26.Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem J. 1996;315 (Pt 1):295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 28.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, et al. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 29.Andersson U, Leighton B, Young ME, Blomstrand E, et al. Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem Biophys Res Commun. 1998;249:512–516. doi: 10.1006/bbrc.1998.9171. [DOI] [PubMed] [Google Scholar]
- 30.Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46–57. doi: 10.1016/s0005-2728(00)00238-3. [DOI] [PubMed] [Google Scholar]
- 31.Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33:1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- 32.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran A, Ceaser E, Darley-Usmar VM. Chronic exposure to nitric oxide alters the free iron pool in endothelial cells: role of mitochondrial respiratory complexes and heat shock proteins. Proc Natl Acad Sci U S A. 2004;101:384–389. doi: 10.1073/pnas.0304653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 35.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 36.Riobo NA, Melani M, Sanjuan N, Fiszman ML, et al. The modulation of mitochondrial nitric-oxide synthase activity in rat brain development. J Biol Chem. 2002;277:42447–42455. doi: 10.1074/jbc.M204580200. [DOI] [PubMed] [Google Scholar]
- 37.Boveris A, Valdez LB, Alvarez S, Zaobornyj T, et al. Kidney mitochondrial nitric oxide synthase. Antioxid Redox Signal. 2003;5:265–271. doi: 10.1089/152308603322110841. [DOI] [PubMed] [Google Scholar]
- 38.Kanai AJ, Pearce LL, Clemens PR, Birder LA, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koivisto A, Pittner J, Froelich M, Persson AE. Oxygen-dependent inhibition of respiration in isolated renal tubules by nitric oxide. Kidney Int. 1999;55:2368–2375. doi: 10.1046/j.1523-1755.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 40.Bosse HM, Bohm R, Resch S, Bachmann S. Parallel regulation of constitutive NO synthase and renin at JGA of rat kidney under various stimuli. Am J Physiol. 1995;269:F793–805. doi: 10.1152/ajprenal.1995.269.6.F793. [DOI] [PubMed] [Google Scholar]
- 41.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat. Role of endogenous nitric oxide. J Clin Invest. 1993;91:642–650. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen MF, Xie XM, Yang TL, Wang YJ, et al. Role of asymmetric dimethylarginine in inflammatory reactions by angiotensin II. J Vasc Res. 2007;44:391–402. doi: 10.1159/000103284. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, et al. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ Res. 2007;101:e2–10. doi: 10.1161/CIRCRESAHA.107.156901. [DOI] [PubMed] [Google Scholar]
- 44.Matsuguma K, Ueda S, Yamagishi S, Matsumoto Y, et al. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17:2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]