Abstract
Naturally occurring smallpox has been eradicated, yet it remains as one of the highest priority pathogens due to its potential as a biological weapon. The majority of the US population would be vulnerable in a smallpox outbreak. SIGA Technologies, Inc. has responded to the call of the US government to develop and supply to the Strategic National Stockpile a smallpox antiviral to be deployed in the event of a smallpox outbreak. ST-246® (tecovirimat) was initially identified via a high-throughput screen in 2002, and in the ensuing years, our drug-development activities have spanned in vitro analysis, preclinical safety, pharmacokinetics and efficacy testing (all according to the ‘animal rule’). Additionally, SIGA has conducted Phase I and II clinical trials to evaluate the safety, tolerability and pharmacokinetics of ST-246, bringing us to our current late stage of clinical development. This article reviews the need for a smallpox therapeutic and our experience in developing ST-246, and provides perspective on the role of a smallpox antiviral during a smallpox public health emergency.
Keywords: animal rule, antiviral, CBRN medical countermeasures, FDA approval, monkeypox, smallpox, strategic national stockpile, zoonotic orthopoxvirus
Smallpox & its eradication
In a time when infectious disease was rampant and deadly epidemics were commonplace, the British historian Lord Thomas B Macaulay (1800–1859) described smallpox as “the greatest of all the ministers of death.” Smallpox is estimated to have killed 300–500 million people in the 20th century alone, crippling or disfiguring many more [101]. During the 17th and 18th centuries, the death rate was a sixth of the birth rate and a third of all blindness in Europe was due to smallpox. As late as the 1950s, an estimated 50 million cases a year occurred – a number that dropped to 10–15 million per year by 1967 due to increasing implementation of prophylactic vaccination [101]. In 1967, the WHO started a worldwide campaign to eradicate smallpox, a feat accomplished in 10 years due to massive vaccination efforts. There has not been a naturally occurring case of smallpox since 1977, and in 1980, the World Health Assembly declared the world free of smallpox.
Variola virus, an orthopoxvirus, causes smallpox. The infectious dose is unknown but it is thought that between ten and 100 virions are able to cause infection in naive individuals [1], although it may be as low as one infectious particle [2]. Following exposure, there is an incubation period of 7–19 days during which no disease is evident and the infected individual is not contagious. After the incubation period, there is a prodrome phase characterized by high fever and excruciating headaches, backaches, joint and abdominal pain. The virus spreads through the blood and lymph, seeding capillary endothelial cells and initiating replication in the skin and oropharyngeal mucous membranes within 1–4 days following onset of the prodrome phase. After the prodrome, pocks begin to form as the result of virus replication on the extremities and face. Pocks develop in characteristic phases and (if the individual does not succumb to disease) are resolved within approximately 21 days after their initial appearance. During the pock or rash phase of the disease, smallpox is contagious. The disease is spread most often by respiratory droplets during face-to-face contact but may also be spread by fine particle aerosols capable of traveling significant distances, and by contaminated objects such as clothing and bedding. The pocks eventually scab over and heal. The scabs contain significant amounts of virus although the virus in scabs is not highly infectious. Nevertheless, the scabs allow for the virus, to persist in the environment for extended periods of time following an outbreak and may account for new outbreaks in seemingly disease-free environments. Up to 30% of unvaccinated and 1% of vaccinated individuals will die if they contract ‘typical’ smallpox. Occasionally the disease manifests as ‘flat’ or ‘hemorrhagic’ smallpox and these forms of disease are universally fatal. Currently there is no treatment for smallpox other than supportive care, although vaccination in the early incubation period is thought to reduce the incidence of disease and improve chances of survival. Survivors are scarred for life, many are blinded and a number have slowly resolving neurological complications such as encephalitis [3].
Currently, the smallpox virus no longer exists in nature and, officially, the only remaining stocks exist in two locations: the CDC in Atlanta, GA, USA, and the State Research Center of Virology and Biotechnology in Koltsovo, Russia. Much of the former Soviet Union's supply is unaccounted for and a Washington Post investigative report suggests that clandestine stores (undeclared to the WHO) of smallpox may be in the hands of at least four countries (Iraq, North Korea, Russia and France) [4]. In February 2003, the US military reinstated the smallpox vaccination program in response to the renewed threat of smallpox as bioweapon [5].
Smallpox as a bioterror agent
There are numerous contributing factors that make smallpox an ideal biological weapon: first, smallpox is contagious. A very small dose is required for infectivity [6] and the ‘attack rate’ or the likelihood that a susceptible individual will contract disease if exposed to an infected individual ranges from 26% to as high as 90% [7]. Although the CDC estimates that an infected individual will spread the disease to three others, some in the scientific community consider this to be an overly optimistic prediction, and historical records suggest that one infected individual may spread the disease to ten or 20 others [6]. Second, the US population is highly susceptible to the virus and will remain so for the foreseeable future. Routine vaccination was stopped more than 30 years ago and the vaccine offers limited protection after 3–5 years [102]. In fact, 60% of smallpox cases in the 1960s had scars from prior vaccination [103]. Owing to concern over adverse events, the vaccine is contraindicated for the general public. In 2003, the Smallpox Vaccination Program was initiated with intent to vaccinate ‘first-responders’ in a smallpox emergency. Fear of potential vaccine-related adverse events, both real and exaggerated, resulted in poor implementation of the program [8], and only a small fraction of those originally intended to receive the vaccine were vaccinated. Third, publicly accessible scientific literature yields relatively simple and inexpensive methods for the growth, purification and genetic engineering of smallpox [9,10], as well as evidence that poxvirus virulence may be enhanced via insertion of cytokine genes such as IL-4, although this has only been demonstrated in mice [11].
The genome of smallpox is published, allowing for genetic manipulation [12]. Fourth, the widespread dispersal of the virus may be enhanced by ‘weaponization’ [13] or aerosolization [7]. Fifth, decontamination may be difficult. Scabs shed from healing smallpox lesions contain large amounts of virus and may effectively stabilize the virus in the environment. The virus may persist in the environment for as long as a year in dust and cloth [14]. Sixth, there is no US FDA-approved effective therapy for smallpox (although ST-246® may eventually negate this statement). Postexposure vaccination may prevent or limit the disease if administered within 4 days of exposure [102] but is unlikely to be effective thereafter [7]. Cidofovir has been shown to be effective at controlling poxviral disease in animals but is toxic, causing kidney damage [7]. Methisazone is only 30–40% effective against smallpox and is toxic, causing nausea and vomiting [7]. Finally, a smallpox outbreak would likely result in severe socioeconomic disruption.
The smallpox response plan
A single case of smallpox anywhere in the world would be considered a global health emergency. The deliberate or accidental release of the smallpox virus into today's largely unvaccinated and highly mobile population would wreak far-reaching havoc in medical, social and economic terms. In the event of a smallpox outbreak in the USA, the CDC would immediately implement the Smallpox Response Plan [15–19]. As there is currently no FDA-approved effective treatment for smallpox, the plan largely seeks to contain the outbreak. The sufficiency of this plan is based on 30–40-year-old observations made during the smallpox eradication campaign and is based on the best-case scenarios presented in Table 1, which compares the CDC model (as a best-case scenario) to a worst-case scenario based on information presented in the scientific literature. In a large urban setting, the policy is to isolate suspected cases, trace and vaccinate their contacts (‘race to trace’), quarantine any of those contacts who are feverish, but then vaccinate more widely if the outbreak is not contained by these measures. This is more commonly referred to as a ‘ring vaccination’ strategy, and is the method that was employed in the developing world during the smallpox eradication campaign. In that setting it was extremely effective for three distinct reasons:
Table 1. Comparison of best- and worst-case scenarios in a smallpox outbreak.
| Parameter | Best case† | Worst case‡ | Ref. |
|---|---|---|---|
| No. of index cases§ | 10–100 | 1000s (sporting venues, multiple simultaneous attacks, and so on) | |
| Spread from index cases | Up to three individuals (CDC model) | 10–20 individuals | [6] |
| Transmissibility | 26% | >96% | [7] |
| Potential number infected before final containment | 4200 | Millions | [18] |
| Time to containment | 1 year | Smallpox becomes endemic; years | [18] |
Based on CDC estimates.
Based on scenarios presented in the references from this table.
Scenario postulated by the authors for the purposes of modeling a terrorist release of smallpox.
-
▪
The number of index cases was generally very small (less than ten);
-
▪
Tracing the contacts of infected people was very accurate due to the communal nature, physical layout and isolation of villages;
-
▪
A large number of individuals had pre-existing immunity to smallpox due to prior vaccination or recovery from smallpox infection, thus reducing the likelihood of spread from person to person.
In basing the Smallpox Response Plan on the successful eradication of smallpox from the developing world, assumptions regarding a smallpox outbreak in a large urban area are made that may not be accurate. According to the Executive Summary of the Smallpox Response Plan (issued 20 March 2003), “A single case of smallpox is likely to represent a bioterrorism release…” and as such, the best-case scenario is probably overly optimistic [104]. It is likely that following a bioterrorist release of smallpox, the overwhelming burden of tracing contacts and maintaining a ring vaccination strategy would necessitate the implementation of mass vaccinations [16,18]. The risk: benefit ratio of the smallpox vaccine prevents its use in the general population in the absence of risk from smallpox, but in the event of a smallpox outbreak, the vaccine would be administered to everyone at risk of exposure regardless of previously existing contraindication. The risk of adverse events due to vaccination would be acceptable given the lethality of smallpox.
The vaccine used to eradicate smallpox is a live attenuated vaccinia virus, a close relative of variola. During the eradication campaign, in the USA, the most widely used version of the vaccine was an attenuated New York City Board of Health strain manufactured by Wyeth (NJ, USA) and licensed under the trade name Dryvax®. While the vaccine was proven to be extremely effective, it has the highest adverse event rate of any FDA-approved vaccine and was associated with a number of severe adverse side effects, such as progressive vaccinia, eczema vaccinatum, postvaccinial encephalitis, fetal vaccinia, peri-/myo-carditis, generalized vaccinia, autoinoculation and erythema multiforme among other more common but less severe reactions [20,21]. The overall adverse event rate is approximately 1000 per million vaccinations. Between 14 and 52 per million vaccinations will be potentially life threatening. In a 1968 survey, vaccination was found to result in one to two deaths per million vaccinations [22], although a more recent analysis suggests that the adverse event rate following vaccination was strain dependent and that there may have been as many as eight deaths per million vaccinations with the commonly used Lister strain [23].
Now that smallpox is no longer an endemic threat, the risks of vaccination outweigh the benefit of protection and the vaccine is no longer administered to the general population. Considering that the vaccine should not be administered to those that are specifically at risk for adverse events, nor should it be given to anyone with a close household contact that is at risk for adverse events, the vaccine is currently contraindicated for approximately 25% of the population [21]. The risk of adverse events is highest in those with a history of eczema/atopic dermatitis or in the immunocompromised. Even after the events of the 9/11 attacks, efforts to vaccinate the ‘primary responder’ and military personnel were unsuccessful due in part to perceived and real dangers of vaccination [8]. Nevertheless, in the event of smallpox exposure, the vaccine is indicated for all exposed and their contacts. There is evidence that the vaccine may be administered immediately following exposure to smallpox and provide some level of protection against contracting the disease [3].
Current efforts to generate safer smallpox vaccines include the use of live but attenuated strains of vaccinia such as modified vaccinia Ankara or a defective Lister strain, LC16m8 [24–31]. Each of these viruses has proven to be safe in humans and no adverse events are associated with their use. Current studies in animals have demonstrated that modified vaccinia Ankara is safe even in immunocompromised animals. Neither virus is as potent as its respective parent and therefore must be used at higher doses and/or in prime/boost vaccination regimens. Furthermore, in the event of a smallpox outbreak, these vaccines may not be suitable for postexposure vaccine therapy considering the time required for prime and boost vaccinations to be effective. In addition, spontaneous revertants of LC16m8 have been observed in which LC16m8 exhibits neurovirulence similar to the parental virus [32]. Acambis (MA, USA; recently acquired by Sanofi Pasteur) has been contracted by the US government to produce vaccine to replace the now-discontinued Dryvax vaccine and has provided approximately 200 million doses of ACAM2000™ to the Strategic National Stockpile (SNS). ACAM2000 was clonally derived from the original Dryvax vaccine [33–35]. The vaccine stock is maintained and propagated entirely in cell culture rather than calf lymph and, thus, is presumed to be safer. Acambis has demonstrated that ACAM2000 exhibits reduced neurovirulence in animal models relative to Dryvax. Immune responses and protective immunity in animals were very similar to Dryvax and immune responses in human clinical trials were similar as well. Despite an improved safety profile in experimental animals relative to Dryvax, ACAM2000 appears to harbor some of the same potential for adverse reactions in humans (e.g., peri-/myo-carditis) as the original vaccine (ACAM2000 Medication Guide [105]).
The need for a smallpox antiviral
In 2004, then President George W Bush signed into law Project BioShield [106]. The purpose of Project BioShield is to:
-
▪
Expedite the conduct of NIH research and development on medical countermeasures that are based on the most promising recent scientific discoveries;
-
▪
Give the FDA the ability to make promising treatments quickly available in emergency situations – this tightly controlled new authority will enable access to the best available treatments in the event of a crisis;
-
▪
Ensure that resources are available to pay for ‘next-generation’ medical countermeasures. Project BioShield will allow the government to buy improved vaccines or drugs. The fiscal year 2004 appropriation for the Department of Homeland Security included US$5.6 billion over 10 years for the purchase of next- generation countermeasures against anthrax and smallpox as well as other chemical, biological, radiological and nuclear (CBRN) agents.
The Biomedical Advanced Research and Development Authority (BARDA) manages Project BioShield. BARDA is within the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services. BARDA procures medical countermeasures and funds/supports advanced development of medical countermeasures for CBRN agents. In addition, BARDA manages the Public Health Emergency Medical Countermeasures Enterprise (PHEMCE). The PHEMCE Implementation Plan for Chemical, Biological, Radiological and Nuclear Threats (2007) listed smallpox as a ‘Top Priority’ CBRN threat and the acquisition of a smallpox antiviral as a ‘Projected Future Top Priority Medical Countermeasure’. In March 2009, BARDA announced a request for proposal (RFP-09–35) to supply a smallpox antiviral to the SNS [107]. SIGA Technologies (OR, USA) has submitted a proposal to supply ST-246 to the SNS.
Discovery of ST-246 & its mechanism of action
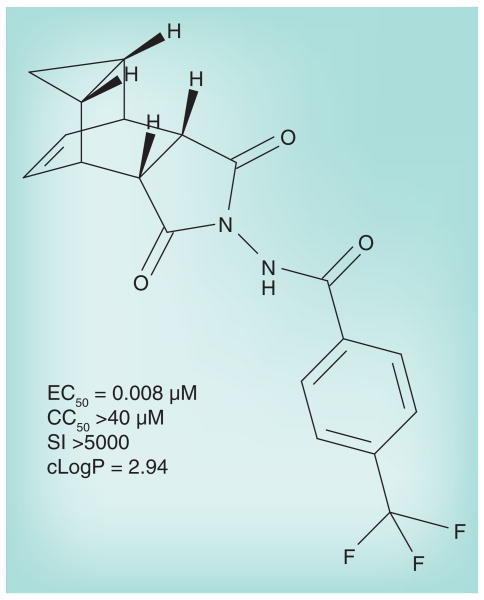
ST-246 was identified in a high-throughput screen of 356,240 compounds in a chemically diverse library [36]. A total of 759 compounds were found to be capable of inhibiting the poxvirus cytopathic effect on tissue culture cells in vitro. The effective concentration capable of inhibiting 50% of the cytopathic effect seen in untreated infected cells (EC50) was calculated for all ‘hits’. They were grouped into nine distinct chemical series based upon the structure of their parent scaffolds. Several chemical series were optimized further based on nascent structure–activity relationships of related analogs. Among these were the tricyclononene carboxamides with EC50 values that ranged from 20 nM to the upper limit of measurement (>20 μM). The 4-trifluoromethyl-phenol derivative (ST-246; shown in Figure 1) was selected for further characterization from a group of analogs based on a low EC50 and relative metabolic stability (see below for stability data). ST-246 was tested against numerous viruses in vitro and found to be potently active and specific for orthopoxviruses (Table 2). Replication of other DNA and RNA viruses tested was completely unaffected by ST-246. ST-246 also inhibited cidofovir-resistant cowpox virus, indicating that its mechanism of action was different from that of cidofovir, which is a nucleoside analog that inhibits DNA replication. It is most notable that ST-246 was highly active against pathogenic variola and monkeypox. ST-246 has been evaluated for poxvirus inhibition in primary cell lines including human embryonic lung fibroblasts, primary human keratinocytes and organotypic endothelial raft cultures [37] as well as standard tissue culture cells such as Vero, BSC-40 and SIRC [36], and was found to display similar activity regardless of cell type.
Figure 1. Structure of anhydrous ST-246 (C19H15F3N2O3).
CC50: Concentration of ST-246 in μM that is cytotoxic, causing a 50% reduction in the viability of cells in culture; cLogP: Logarithm of ST-246 partition coefficient between n-octanol and water (log [coctanol/cwater]), a measure of the ST-246's hydrophilicity; EC50: Effective concentration of ST-246 in μM capable of reducing the cytopathic effect of vaccinia virus in cell culture by 50%; SI: Specificity index, CC50/EC50.
Table 2. ST-246 antiviral specificity.
| Virus family | Strain | EC50 |
|---|---|---|
| Orthopoxviridae | Vaccinia | 0.01 |
| Variola | 0.02–0.05 | |
| Cowpox | 0.05 | |
| Ectromelia | 0.07 | |
| Monkeypox | 0.01 | |
| Camelpox | 0.01 | |
| Herpesviridae | Herpes simplex virus | >40 |
| Cytomegalovirus | >40 | |
| Paramyxoviridae | Respiratory syncytia virus | >40 |
| Reoviridae | Rotavirus | >40 |
| Flaviviridae | Bovine viral diarrhea virus | >40 |
| Bunyaviridae | Rift valley fever virus | >40 |
| Arenaviridae | Tacaribe | >40 |
| Lymphocytic choriomeningitis virus | >40 |
EC50: Effective concentration of ST-246 in micrograms capable of reducing the cytopathic effect of vaccinia virus in cell culture by 50%.
Adapted from [36].
ST-246's mechanism of action is to prevent the formation of egress-competent forms of orthopoxviruses [38]. Productive viral replication leads to the formation of a number of phenotypically distinct, infectious virion forms: Intracellular mature viruses (previously referred to as IMVs but now known as MVs) are formed first and are fully infectious but are retained intracellularly until cell lysis. A fraction of the MV particles acquire additional membranes within the cell and are released in a nonlytic fashion. Although at least three enveloped virion forms exist, they are collectively referred to as enveloped virus (EV). Intracellular EV (IEV) is formed by wrapping of MV with intracellular membranes. This form of the virus is egress competent and migrates to the cell periphery on microtubules. At the cell surface, IEV loses its outermost membrane but remains cell associated (cell-associated enveloped virus [CEV]). This form of the virus induces actin tail formation, which pushes the virus away from the cell and into neighboring cells, thus facilitating cell-to-cell spread. A portion of the CEV particles are released from the cell as extracellular EV. This form of the virus is implicated in long-range spread in cell culture and likely in vivo as well [39]. The formation of EV is of particular importance in the virulence of vaccinia virus, the prototypic poxvirus. The closely related viruses, variola (smallpox) and monkeypox, are disseminated via the lymph and blood as cell-associated (monocytes/macrophages) virus [40], suggesting that virus could efficiently disseminate in vivo without the formation of EV, although this may not be the case based on studies with vaccinia. In the absence of EV formation, the virus poorly disseminates from the site of original inoculation [39,41]. Mutant viruses defective for EV production show reduced virulence and poor dissemination in vivo and display a small-plaque phenotype in tissue culture [42–45]. While other viral and perhaps cellular proteins are involved, the poxvirus p37 protein plays a central role in the formation of EV [42]. As shown in Figure 2, the effect of ST-246 on virus in culture is to prevent virus envelopment and cell-to-cell spread and thus block the formation of plaques in cell monolayers. In vivo, the effect is to prevent the dissemination of virus from the original site of inoculation, thus reducing viremia and skin pock formation (see also Figure 3), and since the virus is restricted to local replication, clinical signs of disease are also reduced.
Figure 2. ST-246 inhibits enveloped virus formation and prevents dissemination of virus in vitro and in vivo.
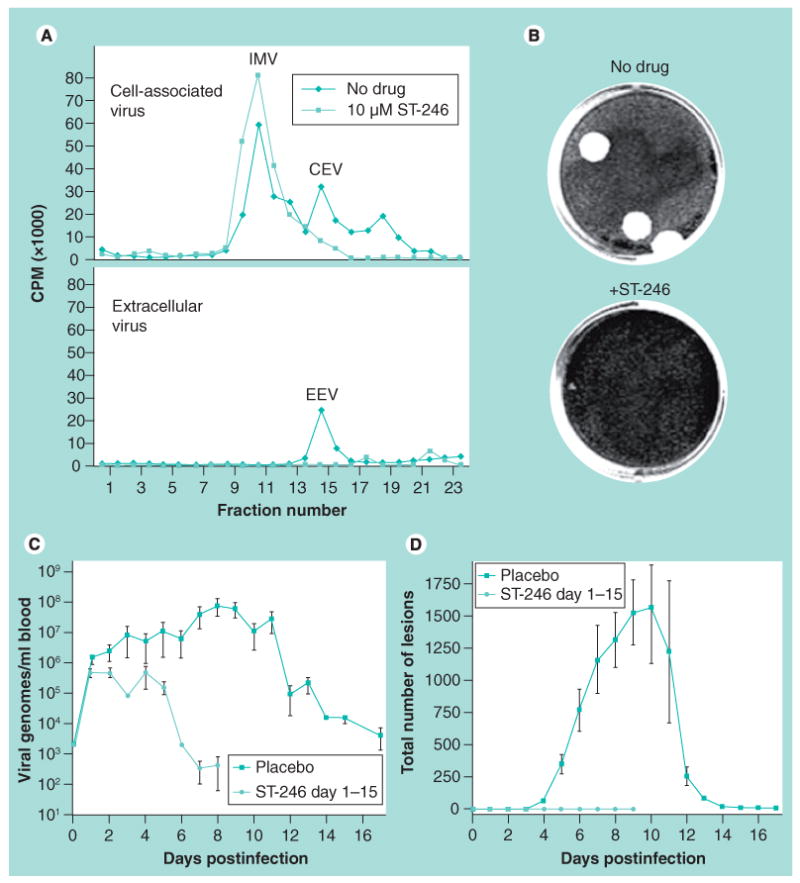
(A) ST-246 prevents the formation of enveloped virus in tissue culture. Virus was purified from tissue culture cells (CEV) and culture supernatants (EEV) and separated by centrifugation through CsCl gradients. IMV, CEV and EEV peaks are noted in untreated cultures. Note the reduction/absence of CEV and EEV in ST-246-treated cultures. (B) Inhibition of virus envelopment reduces cell-to-cell spread in tissue culture cell monolayers. Note the large plaques in the ‘No drug’ culture, while the ST-246-treated cultures do not contain detectable plaques. (C) ST-246 inhibition of virus envelopment results in reduced viremia in vivo. Cynomolgus monkeys were infected with variola virus by intravenous injection. One group was treated with vehicle while the other was treated with ST-246 at 300 mg/kg/day for 14 days starting 24 h postinfection. Viremia was assessed by quantitative PCR detection of viral genomes. (D) ST-246 inhibition of virus envelopment reduces clinical symptoms of disease. Animals as described in (C) were monitored for the formation of dermal lesions (pocks) for up to 17 days postinfection. Note that the ST-246 dosing regimen utilized in the animal study that generated data used in (C & D) is not reflective of current dose evaluations in preclinical or clinical studies. It is presented here only for proof-of-concept: ST-246 inhibition of virus envelopment reduces cell-to-cell spread, which translates to reduced viremia and lesion counts in vivo.
CEV: Cell-associated enveloped virus; CPM: Counts per minute; EEV: Extracellular enveloped virus; IMV: Intracellular mature virus.
Figure 3. Progression of monkeypox in untreated and ST-246-treated nonhuman primates.
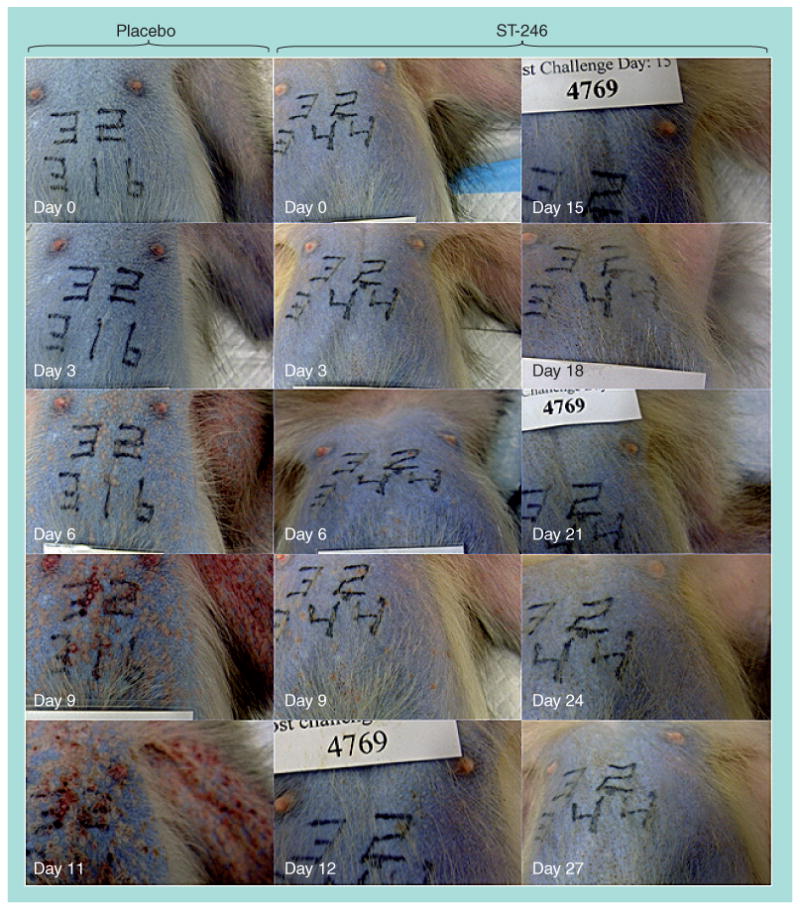
Cynomolgus macaques were infected with monkeypox virus by intravenous injection with 5 × 107 plaque-forming units. Animals were treated with a placebo or ST-246 (10 mg/kg/day) starting on day 4 postinfection, when all animals had already begun to form pocks, until day 17 postinfection. Animals were photographed every 3 days. The photographs are of a representative animal for each treatment group and show the chest and left axillary areas. The placebo-treated animal was euthanized on day 11 due to severe disease while the ST-246-treated animal survived the challenge.
ST-246 targets the poxvirus p37 protein, which is required for the production of extracellular forms of virus. P37 was identified as the target of ST-246 based on genetic mapping of ST-246-resistant mutant viruses [36]. It is highly conserved in all orthopoxviruses and has no mammalian homologs. As depicted in Figure 4, vaccinia p37 nucleates the formation of a wrapping complex derived from late endosomal (LE) membranes that catalyzes the envelopment of IMV particles to produce EV [38]. In an uninfected cell, LE-derived transport vesicles shuttle cellular ‘cargo’ between the LE and Golgi compartments. LE-derived transport vesicles assemble around cargo proteins through specific interactions with the Rab9 GTPase, and TIP47, a Rab9-specific effector protein. In an infected cell, the wrapping complex is formed through interactions of p37 with components of LE-derived transport vesicles. Poxvirus virus p37 acts like cellular cargo and interacts with Rab9 and TIP47 to nucleate a virus-specific wrapping complex required for assembly of EV. ST-246 blocks interaction of p37 with Rab9 and TIP47. Thus, ST-246 prevents wrapping complex formation by inhibiting interaction of p37 with components of LE-transport vesicle biogenesis.
Figure 4. ST-246 mechanism of action.
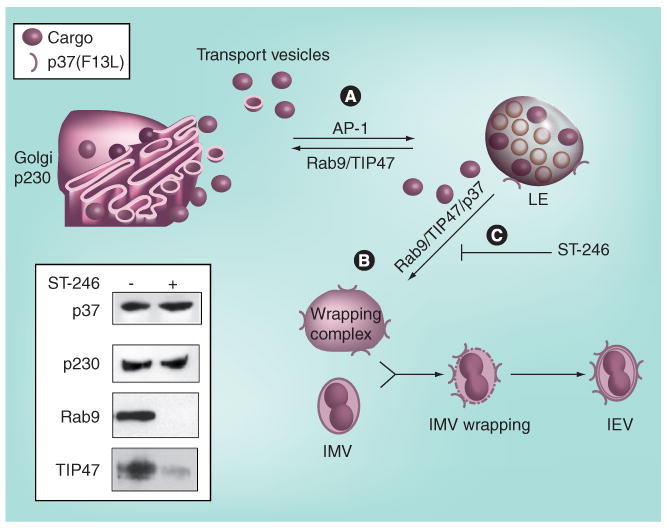
Vaccinia p37 nucleates formation of a wrapping complex derived from LE membranes that catalyzes the envelopment of IMV particles to produce IEV. (A) Normally, LE-derived transport vesicles shuttle cellular ‘cargo’ between the LE and Golgi compartments. LE-derived transport vesicles assemble around cargo proteins through specific interactions with the Rab9 GTPase, and TIP47, a Rab9-specific effector protein. (B) The wrapping complex is formed through interactions of p37 with components of LE-derived transport vesicles. Vaccinia virus p37 acts like cellular cargo and interacts with Rab9 and TIP47 to nucleate a virus-specific wrapping complex required for assembly of IEV. (C) ST-246 blocks interaction of p37 with Rab9 and TIP47 (but not p230, a Golgi-specific marker protein), and prevents wrapping complex formation. The inset shows a western blot analysis of proteins that coimmunoprecipitated with p37 in the presence and absence of ST-246.
IEV: Intracellular enveloped virus; IMV: Intracellular mature virus; LE: Late endosome.
Chemistry, manufacturing & control
ST-246 is a white to off-white powder manufactured via a four-stage convergent process consisting of three bond-forming chemical reactions together with four solid isolations, one of which is highly purifying. Its molecular weight is 376.33 g/mol. Its melting point is 196°C. ST-246 is very soluble in organic solvents but is nearly insoluble in water. It is nonhygroscopic. ST-246 exhibits polymorphisms and exists as three chemical forms: anhydrous, monohydrate and hemihydrate. Their physicochemical properties are very similar. Based on its low solubility in aqueous fluid and good partition coefficient, ST-246 can be classified as a biopharmaceutical classification system class II drug per FDA definition. The monohydrate was chosen for advanced development due to its chemical stability. The ST-246 drug product consists of hard gelatin capsules containing 200 mg of active ingredient along with few inactive ingredients. All inactive ingredients and excipients are generally recognized as safe by the US Pharmacopeia – National Formulary. SIGA has produced several small batches, three new drug application one-tenth scale commercial registration batches and is in the process of full commercial-scale process validation.
Metabolism & pharmacokinetics
ST-246 is essentially insoluble in aqueous solutions and simulated gastric fluids, yet in Caco-2 assays, it was found to be very permeable, suggestive of good gastric uptake. Indeed, ST-246 is orally bioavailable. In in vitro absorption, distribution, metabolism and excretion studies, ST-246 has moderate-to-high plasma protein binding and is not metabolized to a significant degree by cytochrome P450 enzymes. The potential for drug–drug interactions is low, considering that ST-246 did not cause induction of cytochrome P450 at a 10-μM concentration, but at 100 μM induction was observed for 2B6, 2C9, 3A4 and 2C19. Inhibition of nine CYP enzymes that were tested (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4) was less than 50% at a concentration of 300 μM. Interaction with hERG was very low and only 7% inhibition was achieved at a concentration of 30 μM [SIGA Technologies, Unpublished Data].
The metabolic stability of ST-246 was evaluated in isolated microsomes from mice, rats, dogs, monkeys and humans. Small amounts of ST-246 were cleaved, liberating 4-trifluoromethylbenzoic acid from the parent compound in rats, mice and dogs, but these metabolites were not seen in monkeys and humans. An in vivo mass balance study in mice showed that ST-246 was nearly completely eliminated within 96 h after oral administration. Prior to clearance, ST-246 was broadly distributed to all organs, including the brain, with the highest concentration outside of the intestinal tract observed in the gallbladder. ST-246 is primarily eliminated through the feces with a smaller amount eliminated by urine. ST-246 eliminated by feces is intact ST-246, while that eliminated by urine is metabolized (trifluorobenzoic acid and glucuronidated ST-246 metabolites were the only compounds identified).
The oral bioavailability of ST-246 is increased when taken with food. Plasma exposures are not dose proportional suggesting that absorption is rate limiting at higher doses [SIGA Technologies, Unpublished Data].
Drug substance & drug product stability
Each drug substance batch was placed on a 60-month stability protocol, per International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines. The stability conditions were long term (25°C/60% relative humidity [RH]), intermediate (30°C/65% RH) and accelerated (40°C/75% RH). To this point, the drug substance is stable under accelerated conditions for 6 months and under long-term and intermediate conditions for 24 months. No new impurities or degradation products have been observed. The proposed storage condition is controlled room temperature (15–30°C) and a retest date of 36 months from the date of manufacture is projected. The proposed shelf-life is expected to be more than 3 years.
Various packaging configurations for the final drug product have been considered. Each packaging configuration for each batch was staged on a 60-month stability program. The staging conditions for this stability program were long term (25°C/60% RH), intermediate (30°C/65% RH) and accelerated (40°C/75% RH). The drug product is stable for 6 months in all packaging configurations at accelerated conditions, and for 18 months in all packaging configurations at long-term and intermediate conditions. The proposed storage condition of the final drug product is controlled room temperature (15–30°C) and the proposed shelf-life is more than 2 years.
Safety/toxicology
The cytotoxicity of ST-246 was measured in selected cell lines from mouse, rabbit, monkey and humans. The cytotoxic concentration of ST-246 that inhibited cell proliferation by 50% in comparison to untreated controls cells (CC50) values were found to be >50 μM in all cell lines tested, including human embryonic lung fibroblasts and primary human keratinocytes [37]. ST-246 exhibits no bone marrow toxicity and caused no chromosomal aberrations in the mouse micronucleus test, and additionally is not genotoxic in bacterial and mammalian genotoxicity assays [SIGA Technologies, Unpublished Data].
SIGA has conducted safety evaluations in multiple species: mice, rats, rabbits, dogs and nonhuman primates (NHPs) [46]. ST-246 is a well-tolerated compound and seems to elicit very little toxicity at doses that are much higher than those required for the antiviral activity. In mice, the no observable effect level was determined to be 2000 mg/kg, which was the highest dose evaluated. The maximum tolerated dose in mice was 2000 mg/kg in both single-dose and 28-day repeat-dose studies. At this dose, there were no CNS abnormalities, and no functional or behavioral changes. A 3-month study evaluating a top dose of 1000 mg/kg of ST-246 in mice resulted in a slight increase in liver weight with no accompanying histopathology observed. The no observable adverse effect level (NOAEL) was 1000 mg/kg for the 3-month study. Cardiovascular safety evaluation in NHPs showed no prolongation of QTc at the highest dose administered in repeat-dose studies, using a dose of 300 mg/kg. In dogs, a 30-mg/kg dose resulted in behavioral and encephalogram evidence of seizures, although follow-up studies in NHPs showed no evidence of seizures even after repeat dosing with 300 mg/kg. The increased sensitivity of dogs to ST-246 is likely attributable to the increased exposure of the brain and cerebral spinal fluid to ST-246, suggesting that dogs are unique in this sense. The highest single dose administered to NHPs was 2000 mg/kg, which resulted in decreased activity and ataxia, which was also observed after single-dose administration of 1000 mg/kg. At 300 mg/kg, ataxia was observed 4 h post-dose in one female NHP. Owing to these observations the highest dose used in multiple dose safety studies in NHPs was 300 mg/kg. This was used for both the 28-day and 3-month safety studies and the no observable effect level was the top dose of 300 mg/kg in both of these studies.
Definitive segment I, II and III reproductive toxicology studies were conducted in both mice and rabbits. Segment I studies involved evaluation of the potential of ST-246 to exert toxicity on general fertility and early embryonic development (i.e., sperm count, sperm motility, sperm morphology, sperm viability in males and number of corpora lutea and implantation sites per female). Segment II studies were conducted during major organogenesis (implantation to the closure of the hard palate). These studies evaluated the potential toxicity of ST-246 on embryo–fetal development (potential effects on visceral and skeletal development fetal viability, appearance of morphology and weight) and maternal function. Segment III studies spanned the F0, F1 and F2 generations: F0-maternal function, F1- and F2-developmental (skeletal and visceral), behavioral and functional patterns. At a dose of 100 mg/kg, maternal toxicity was observed in rabbits. Very low levels of ST-246 were observed in the placenta of pregnant mice and breast milk of nursing mice. There was no evidence in either mice or rabbits of decreased fertility or fetal resorptions, fetal abnormalities or toxicity.
In vivo efficacy evaluation according to the ‘animal rule’
It would be neither feasible nor ethical to conduct clinical trials to evaluate the efficacy of ST-246 versus smallpox in humans. Other orthopoxviruses such as vaccinia or cowpox, which occasionally infect humans, are not appropriate as surrogates for smallpox considering that they are much less pathogenic than smallpox and the mechanism of smallpox virulence is not fully understood. In order to develop medical countermeasures for those agents that cannot be tested in humans, the ‘animal rule’ was enacted (Box 1) (see 21 CFR 314.600 for drugs or 21 CFR 601.90 for biological products [108]). The animal rule allows the FDA to approve drugs or vaccines based on efficacy data in animals coupled with safety and pharmacokinetic data in humans. Animal efficacy data allow the selection of the human dose. If the drug or vaccine is shown to be safe in humans and plasma exposure levels are achieved that mimic efficacious dosing in animals, then one may reasonably conclude that the drug will be efficacious in humans.
Box 1. The animal rule.
-
▪
The ‘animal rule’ is a colloquial term for US FDA regulations (21 CFR 314.600 for drugs; 21 CFR 601.90 for biologics [108]) that concerns the approval of drugs or biologics when human studies are neither ethical nor feasible, as is the case for smallpox therapeutics. The regulations state: the FDA may grant marketing approval for a new drug product for which safety has been established (in humans) and for which the requirements of 314.600 are met based on adequate and well-controlled animal studies when the results of those animal studies establish that the drug product is reasonably likely to produce clinical benefit in humans. In assessing the sufficiency of animal data, the agency may take into account other data, including human data, available to the agency. The FDA will rely on the evidence from studies in animals to provide substantial evidence of the effectiveness of these products only when:
– There is a reasonably well-understood pathophysiological mechanism of the toxicity of the substance and its prevention or substantial reduction by the product;
– The effect is demonstrated in more than one animal species expected to react with a response predictive for humans, unless the effect is demonstrated in a single animal species that represents a sufficiently well-characterized animal model for predicting the response in humans;
– The animal study end point is clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity;
– The data or information on the kinetics and pharmacodynamics of the product or other relevant data or information, in animals and humans, allow selection of an effective dose in humans.
-
▪
Approval according to the animal rule is subject to three additional requirements;
– Postmarketing studies (e.g., specific plans to conduct field trials during a smallpox outbreak);
– If warranted, restrictions may be enacted in order to ensure safe use (e.g., restrictions on distribution to certain facilities and individuals, and conditional distribution based on specified medical procedures and record keeping);
– Specific labeling of the product to inform the patient that the drug or biologic was approved based on animal studies.
SIGA Technologies has conducted more than 50 studies in animals to evaluate the efficacy and safety of ST-246. One of the requirements of the animal rule is that “there is a reasonably well-understood pathophysiological mechanism of the toxicity of the substance and its prevention or substantial reduction by the product.” That is to say that the human disease must be understood and that natural history studies must be conducted in animals to establish that the model recapitulates at least some aspects of human disease. These may act as end points for evaluating efficacy [109]. No one animal model fully recapitulates human smallpox (see the aforementioned smallpox disease description). Smallpox is strictly a human disease. There are no animal reservoirs for variola and the virus replicates poorly in animals. In order to establish reproducibly lethal disease in NHPs, a very high dose of the virus must be administered by intravenous injection [40]. Animals often die of a ‘hemorrhagic’ disease (a very rare form of smallpox in humans) prior to the formation of any pock lesions. The dose, route and clinical manifestations of disease do not model human smallpox. Therefore, other animal models were used for evaluating ST-246 efficacy. The primary end point for all efficacy evaluations is survival. While human smallpox had a 30% fatality rate, animal models in which mortality approaches 100% are preferable. This ensures reproducibility and provides statistical power while using the smallest number of animals possible. Secondary end points include quantifIable aspects of disease such as lesion formation, viremia and clinical observations. SIGA has evaluated ST-246 in mice infected with vaccinia, cowpox or ectromelia viruses [36,47–49], prairie dogs and golden ground squirrels infected with monkeypox virus [50], rabbits infected with rabbitpox virus [51] and cynomolgus monkeys infected with monkeypox virus or variola virus (smallpox) [52,53]. In each model, an efficacious dose was determined that prevented mortality and prevented or significantly reduced clinical symptoms of disease (Table 3).
Table 3. Summary of animal efficacy.
| Animal model | Challenge virus | ST-246 dose (mg/kg) | Treatment delay (hours postinfection) | Survival (%) | Ref. |
|---|---|---|---|---|---|
| Mouse | Vaccinia | 100 | 72 | 100 | [36,49] |
| Cowpox | 100 | 48 | 93 | [49] | |
| Ectromelia | 100 | 72 | 100 | [49] | |
| Squirrel | Monkeypox | 100 | 72 | 100 | [50] |
| Rabbit | Rabbitpox | 40 | 48 | 100 | [51] |
| Monkey | Monkeypox | 3 | 96 | 100 | [52,53] |
| Variola | 10 | 24 | 100 | [52] |
Data taken from [46].
It was established in mice that dosing may be delayed until after clinical signs of disease (therapy) and that daily dosing for a minimum of 5 consecutive days was necessary to prevent mortality. In immunodeficient mice we demonstrated that ST-246 serves to hold the virus in check until the remaining components of the immune system are activated to clear the virus. Ectromelia infections in mice and rabbitpox infection in rabbits were useful models since they involve host-adapted viruses that are extremely virulent for their natural hosts. In those models, a single infectious virus may cause lethal disease. This is similar to the very low infectious dose for smallpox in humans. Monkeypox infections of prairie dogs and ground squirrels provided the opportunity to evaluate ST-246 against a virus known to be lethal in man. Monkeypox is actually somewhat of a misnomer, as its natural host(s) is (are) rodent species. Infection of monkeys or humans is a zoonosis. Ground squirrels and prairie dogs are artificial hosts, although they likely share a similar physiology with the (unknown) natural host for monkeypox. Indeed, they were found to be susceptible to monkeypox. ST-246 was effective against monkeypox in these hosts. In the prairie dog, ST-246 treatment could be delayed until 10 days postinfection and still provide complete protection against mortality [SIGA Technologies, Unpublished Data]. The small animal models were useful in demonstrating that orally delivered ST-246 could prevent mortality in an animal lethally challenged with a poxvirus even after the first manifestations of clinical disease were evident. The small animal models are not as useful when evaluating the quantifiable aspects of disease progression and resolution, which are needed to establish the human dose.
Nonhuman primates, particularly cynomolgus macaques, have served as the most relevant model for human smallpox. In the FDA Guidance for Industry, which expounds on the animal rule [109], it is stated that when seeking FDA approval via the animal rule, candidate drugs should be evaluated against the authentic etiological agent (variola in this case) following exposure of animals by the natural route of infection (aerosol) and in an animal model that closely recapitulates human disease. Ideally, then, an animal would be exposed to a low dose of virus by the aerosol route, followed by an incubation period in which no disease was evident. During this time, the virus would replicate locally in the lungs and lymph nodes and establish a disseminated infection via a primary viremia. Then, over the course of a few days, a secondary viremia would establish widely systemic disease, animals would become febrile and pocks would form on the face and extremities. Early attempts to develop a variola model in monkeys were suggestive in some aspects of human smallpox [40]. Although a high dose of virus administered intravenously was needed to establish infection, and the disease course was significantly shortened, the monkeys formed numerous lesions and 33% of them died. Further investigations in this model demonstrated considerable variability in disease progression and mortality [52]. Nevertheless, ST-246 provided 100% protection from morbidity and mortality in this model when administered at 24 h postinfection at a dose of 300 mg/kg/day for 14 consecutive daily doses while all control animals died (100 % mortality, not 33% as reported earlier) [52]. This dose level is not achievable in humans and treatment was initiated prior to the development of lesions and thus could not be considered therapeutic dosing, so another study was conducted in which monkeys were intravenously challenged with variola and then treated with ST-246 (10 mg/kg) starting on the day that lesions first appeared (typically day 4 postinfection). All ST-246-treated animals survived, as did the control monkeys that were treated with placebo [SIGA Technologies, Unpublished Data]. The 100% survival of the control monkeys in this experiment along with the 0% survival of the control monkeys in the previous experiment highlights the inconsistencies with this model and the need for further refinement in order to establish its utility. On the other hand, monkeypox virus infection of cynomolgus macaques is a very reliable model that captures many of the aspects of human smallpox. Aerosol exposure results in rapid disease onset and mortality sometimes prior to the formation of skin lesions [54] but intra venous challenge results in a disease that is extremely similar to human smallpox after the secondary viremia stage of the disease [25,29,53,55–61]. Animals develop fever within 2 days of challenge, and develop a skin rash and lesions on the face and extremities reminiscent of the centrifugal rash in human smallpox (Figure 3). The pocks that form are numerous (>800 total body count by the time of death) and progress through the same stages as human smallpox. Animals develop increasingly high titers of virus in the blood. Mortality in this model is >99%. The mean time to death is approximately 14 days postinfection. In this model we have established that ST-246 treatment may be delayed until the onset of lesions (day 4 postinfection), that 3 mg/kg is the minimally efficacious dose, that a minimum of five consecutive daily doses after the formation of lesions is necessary to prevent mortality, and that an ongoing poxvirus infection does not adversely impact the pharmacokinetics of ST-246 [52,53]. ST-246 provides 100% protection from mortality in this model. Additionally, ST-246 significantly reduces viremia and pock number (Figure 3) as well as reducing or preventing clinical signs of disease. The intended human dose of 400 mg/day for 14 days is based on our observations of efficacy, in addition to safety/toxicology and pharmacokinetic studies in this model.
Clinical experience with ST-246
ST-246 has been evaluated in three clinical trials and has been used under Emergency-Investigational New Drug (E-IND) approvals in three separate cases [62,63] – two of which are presented here. In two Phase I clinical trials [110,111], ST-246 was found to be safe and well tolerated after single and multiple doses [64]. The human dose-selection process for Phase I clinical trials was based on the NOAEL for the monkey converted to the human equivalent dose calculated based on body surface area, not direct mg/kg weight, per FDA guidance on estimating the maximum safe starting dose in initial clinical trials. The rationale for this is that rates of metabolism are thought to be more linearly related to body surface area than weight. The starting single dose of 500 mg in the first Phase 1 study was calculated to be within a tenfold safety margin. Pharmacokinetic evaluations showed plasma drug exposures in the range predicted to be sufficient for inhibiting orthopoxvirus replication based on animal studies. No severe adverse events were observed, and no subject was withdrawn due to ST-246. The most commonly reported drug-related adverse event was neutropenia, which was found, upon further analysis, not to be treatment related. ST-246 was readily absorbed following oral administration, with mean times to maximum concentration from 3 to 4 h. Fasted individuals did not absorb ST-246 as readily as nonfasted individuals. In determining the human dose that would be reasonably expected to provide protection from smallpox, NHP data were used. A 3-mg/kg dose was sufficient to protect NHPs from a lethal monkeypox virus challenge, thus establishing a plasma exposure level likely to provide protection in humans. Pharmacokinetic analysis shows that a human dose of 400 mg/day is very similar to NHP dosing at 10 mg/kg/day – a dose well above the minimal effective dose yet far below the NOAEL. In a Phase II clinical trial in which nonfasted subjects were dosed at 400 or 600 mg/day for 14 days, ST-246 was found to be safe and well tolerated. In addition, pharmacokinetic evaluation showed that essentially all subjects, regardless of age, weight or sex, attained plasma exposure levels above the minimally effective dose levels in NHPs (Figure 5).
Figure 5. A comparison of ST-246 exposure in monkeys and humans.
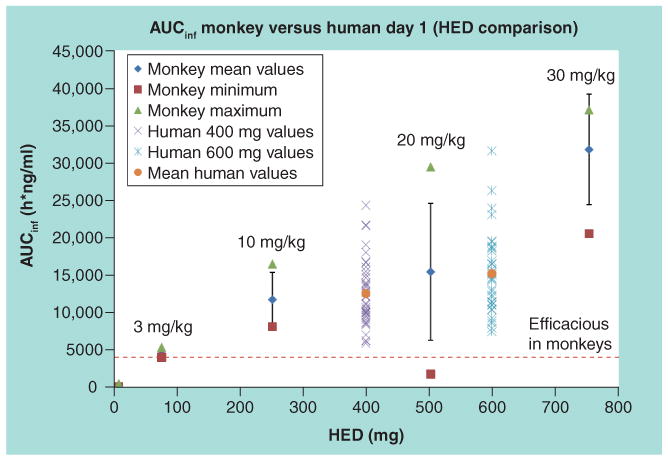
Monkey doses were converted to HEDs by multiplying first by 0.32 in accordance with US FDA guidance for industry (entitled Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, published July 2005) and then multiplying by the average weight of humans (78.6 kg) from the clinical study. The maximum, minimum and mean AUC for monkeys are shown in the green triangles, brown squares and blue diamonds, respectively. The range in AUC values in humans is indicated by the symbols. The red dashed line indicates the dose of ST-246 that protects nonhuman primates from lethal infection. AUC: Area under the concentration–time curve; AUCinf: Area under the concentration-time curve with the last concentration extrapolated based on the elimination rate constant; HED: Human equivalent dose.
In the first E-IND case [63], ST-246 was administered to a severely ill child who had developed eczema vaccinatum after exposure to the virus through contact with a recent vaccinee. The child had a previous history of eczema/atopic dermatitis and failure to thrive. The child first presented at the emergency room with fever and a skin rash thought to be severe eczema. This was later determined to be approximately 2 weeks following exposure to vaccinia via contact transfer from a relative who had recently received the smallpox vaccine. Eczema vaccinatum was confirmed by PCR positive for orthopoxvirus. The child was initially treated with vaccinia immune globulin intravenous (VIGIV; 6000 U/kg) on hospital day 6 and two separate doses of VIGIV (4000 and 14,000 U/kg) on hospital day 7, but his condition continued to deteriorate. He exhibited progressive metabolic then respiratory acidosis, hypoalbuminemia, hypothermia and hypotension. The subject also received one dose of cidofovir (5 mg/kg) on hospital day 8. ST-246 (5 mg/kg) was orally administered via a nasogastric tube on hospital day 9 and 10 followed by 7.5 mg/kg on days 11 and 12 and then 10 mg/kg on days 13–22. Clinical signs of the child's improvement were observed within 1 week of the antiviral intervention (VIGIV, cidofovir and ST-246). The child did not experience any adverse events due to ST-246 treatment and fully recovered without sequelae.
A second E-IND was issued for a recent vaccinee who had received the smallpox vaccine in preparation for a military deployment [62]. Approximately 2 weeks following vaccination, the vaccinee was diagnosed with acute myeloid leukemia and induction therapy was initiated immediately to save his life, although this rendered him immunodeficient. The vaccine lesion failed to resolve and progressed to 4 cm in size. At that time the patient was diagnosed with progressive vaccinia and ST-246 was requested. In addition to ST-246, the patient was treated with topical imiquimod (5%, 12.5 mg once daily for 7 weeks), periodic infusions of VIGIV at varying doses (6000 U/kg, eight doses; 18,000 U/kg for one dose; 24,000 U/kg for five doses) and CMX001, a lipid prodrug of cidofovir (200 mg as a ‘loading’ dose, followed by five maintenance doses of 100 mg every 6 days). In addition, an E-IND was issued for the use of a topical formulation of ST-246 (0.5 ml 1% solution in gel, applied directly to the lesion twice daily initially, then once daily). Oral ST-246 dosing started at 400 mg/day (15 daily doses), then was increased to 800 mg/day (five daily doses) and then to 1200 mg/day (until treatment cessation). The oral ST-246 dose was increased due to the observation that ST-246 exposure levels were lower than those observed in healthy individuals treated at the same doses. ST-246 treatment continued for 2 months at which time the patient's lymphocyte count increased to a level that was capable of mounting an immune response that cleared the virus. The patient experienced numerous serious complications as a result of his disease and therapy but experienced no adverse events attributable to ST-246.
In each of the E-IND cases described here, disease occurred as a result of smallpox vaccination in a setting of immune dysregulation. In treating those cases, substantial measures were taken to preserve the lives of these individuals, which included the combined use of a number of experimental therapies. It is possible that each contributed to the successful treatment of these individuals. This may raise questions regarding the efficacy of the singular use of these drugs. Is it sufficient to stockpile a single drug for use in a national smallpox emergency? ST-246 is being developed as a standalone treatment for postexposure therapy of smallpox in an otherwise healthy population. In an otherwise healthy host, based on animal efficacy and pharmacokinetic and safety studies in humans, there is a reasonable expectation that the singular use of ST-246 would be sufficient to hold the virus in check until the immune system has been activated to clear residual virus. If the indications for ST-246 use are to be expanded into comorbid populations (including immunodeficient individuals, among others), additional studies are necessary. In studies performed in immunodeficient mice, we have demonstrated that ST-246 provides complete protection from a lethal intranasal challenge with vaccinia virus in all immunodeficient settings except when both CD4+ and CD8+ T cells are absent [48]. It would be of interest to explore combination therapies in this setting. Furthermore, a number of immunodeficient NHP models are available that would facilitate studies in higher-order animals.
Conclusion
Smallpox remains a credible threat as an agent of terror or warfare. There is an urgent need for a smallpox antiviral drug. Since its discovery in 2002, ST-246 has become the leading candidate for inclusion in the SNS as a smallpox therapeutic. While ST-246 development has not been without issues, the path to FDA approval appears clear. ST-246 fits an ideal product profile for a drug to be used in a smallpox public health emergency (see Box 2). The drug is safe and very effective. It is easy to manufacture and is stable. Oral dosing is similar to standard antibiotic treatment and would not require the supervision of medical personnel. ST-246 would drastically reduce the medical/social/economic impact of a smallpox outbreak.
Box 2. ST-246 fits an ideal product profile for use in a smallpox emergency.
-
▪
ST-246 is potent. The effective concentration capable of inhibiting 50% of the cytopathic effect seen in untreated infected cells is 0.01 μM while the cytotoxic concentration that inhibited cell proliferations by 50% in comparison with untreated controls cells is >40 μM, yielding a therapeutic index of >4000. The minimally effective dose and corresponding plasma exposure has been established in nonhuman primates (NHPs), and clinical trial data suggest that protective blood exposure levels in humans may be easily achieved with 400-mg per day dosing.
-
▪
ST-246 is safe. ST-246 exhibits no genotoxicity. CNS toxicity was observed in dogs, although this has not been observed in mice or NHPs. The no observable adverse effect level in mice is 2000 mg/kg (20 times the effective dose) and the no observable effect level in NHPs was 300 mg/kg (>30 times the effective dose). Additionally, no prolongation in QTc was observed in NHPs at this dose level. ST-246 has been shown to be safe and well tolerated in single- and multiple-dose human clinical trials, while plasma exposure levels are predictive of efficacy.
-
▪
ST-246 dosing regimen is simple. ST-246 is orally bioavailable and is formulated as 200-mg capsules. A therapeutic dosing regimen would include two capsules daily for 14 consecutive days, similar to many standard antibiotic dosing regimens. The drug would be self-administered and does not require supervision by medical personnel.
-
▪
ST-246 does not interfere with the smallpox vaccine. ST-246 reduces the reactogenicity of the smallpox vaccine without inhibiting immune responses elicited by vaccination. Humoral immune responses may be slightly reduced while cellular responses appear to be slightly enhanced by adjunct ST-246 treatment.
-
▪
ST-246 is simple to produce. ST-246 drug substance is manufactured via a four-stage convergent process consisting of three bond-forming chemical reactions together with four solid isolations, one of which is highly purifying. The process is rugged, produces high-quality pure material and has been demonstrated from small scale (gram level) to full commercial scale (>1000 kg). SIGA has produced several small batches, three new drug application (NDA) one-tenth commercial (NDA-registration batches) scale and is in the process of full commercial-scale process validation.
-
▪
ST-246 is stable. Stability studies per International Conference on Harmonisation guidelines are in progress and data available to date demonstrate that ST-246 is stable for up to 2 years at controlled room temperature.
Future perspective
Conservatively speaking, it seems likely that ST-246 will achieve FDA approval as a smallpox therapeutic within the next 5 years. The drug may be supplied to the SNS prior to FDA approval. Future developments with ST-246 include alternate formulations, including intravenous formulations for the severely ill, perhaps in advanced stages of smallpox, and oral (suspension) formulations for those individuals unable to swallow a capsule. In addition to alternate formulations, expanded indications for use of the oral capsule formulation may include postexposure prophylaxis of smallpox. In this situation, ST-246 treatment would be initiated upon suspected exposure to smallpox but prior to the onset of clinical symptoms. In addition, considering that ST-246 has been demonstrated to be effective against all orthopoxviruses tested, both in vitro and in numerous animal models of disease, ST-246 indications for use may be expanded to the treatment of all orthopoxvirus diseases in man. These include emerging zoonoses such as human monkeypox, cowpox and vaccinia. Human monkeypox is seemingly invading the ecological niche previously occupied by smallpox in West and Central Africa [65], and the lack of antipoxvirus immunity has resulted in increases in zoonotic cowpox infections in Germany [66] and vaccinia infections in Brazil [67]. Monkeypox virus causes a disease very similar to smallpox, although the mortality rate is somewhat lower (1–10%) [65,68,69]. Sporadic outbreaks occur in West and Central Africa and an outbreak in the USA occurred in 2003 due to the importing of infected Gambian rats to sell as exotic pets [70]. Cowpox and vaccinia infections are generally restricted to the dermis and are self-limiting in immunocompetent hosts, but the immunocompromised may suffer serious life-threatening disease.
It is also important to consider the impact of additional therapies that may be used in conjunction with ST-246, as was highlighted in the E-IND cases discussed in this manuscript. In the E-IND cases, patients were treated with VIGIV and cidofovir in the first case and VIGIV and CMX-001(a lipid pro-drug of cidofovir) in the second case. Each has a separate mechanism of action. VIGIV is thought to work by neutralization of both the MV and EV forms of the virus, thus blocking infection and inhibiting dissemination [71], and possibly antibody- dependent cellular cytotoxicity to virus-infected cells. Cidofovir and CMX-001 are nucleoside analogs that act by inhibition of the viral polymerase [72], thus inhibiting replication. In a mouse model, ST-246 and CMX-001 were demonstrated to act synergistically against a lethal poxvirus challenge [73] and it is quite possible that all three therapeutics contributed to resolution of infection in the E-IND cases. Although ST-246 is currently being developed as a standalone therapeutic for the treatment of smallpox in otherwise healthy individuals, it is certainly possible that ST-246 could be utilized ‘off-label’ in immunosuppressed/immunodeficient individuals infected with smallpox/monkeypox/cowpox or suffering adverse effects of vaccination as was seen for the E-IND cases. Therefore, it would be of interest to further characterize the activities of these therapeutics used in combination.
Related to this, ST-246 may also be used to treat or prevent complications from the smallpox vaccine. The smallpox vaccine is a live vaccinia virus. Although the vaccine is attenuated, it is contraindicated for use in approximately 25% of the US population due to direct risk to the vaccinee or indirect risk to close contacts of the vaccinee [21]. Contraindication to receiving the vaccine include: compromised immunity (congenital, acquired or induced – such as in cancer or autoimmune disease therapy), skin disorders such as atopic dermatitis and eczema, and heart disease/cardiac risk factors, among other contraindications. In our initial studies to evaluate the efficacy of ST-246 in animals, we demonstrated that animals were fully protected from lethal poxvirus challenge and that, if re-exposed to the virus, animals were immunologically protected from the rechallenge [36]. ST-246, while providing protection from morbidity and mortality, did not appear to inhibit the induction of a protective immune response elicited by the challenge virus. This suggested to us that ST-246 could be used as an adjunct to smallpox vaccination to:
-
▪
Provide protection from smallpox until full protective immunity has been elicited by the vaccine;
-
▪
Reduce the reactogenicity of the vaccine and thus prevent or treat adverse reactions known to be associated with vaccination, particularly in those populations known to be at increased risk.
In order to evaluate the appropriateness of ST-246 as an adjunct to vaccination, two main criteria must be considered: first, it must be demonstrated that there are no drug–vaccine interactions that would adversely impact the efficacy of the vaccine or ST-246; that is to say that ST-246 must not inhibit the induction of protective immune responses elicited by vaccination and that vaccination concomitant to ST-246 treatment must not inhibit the efficacy of the drug in protecting from a lethal challenge. Second, it must be demonstrated that ST-246 improves the likelihood of safe vaccination in currently contraindicated populations and that vaccine-elicited immunity is protective in those populations.
We have demonstrated that Dryvax and ACAM2000 vaccine efficacy is not compromised by adjunct ST-246 treatment. Normal immunocompetent mice were vaccinated with Dryvax [74] or ACAM2000 [75] using the standard human dose and route and treated with ST-246 immediately following vaccination. We observed that the vaccine lesion severity and time to resolution were reduced following ST-246 treatment. Furthermore, virus shedding from the lesion site was reduced [47]. Vaccinated animals were evaluated for short- and long-term antipoxvirus immunity. Humoral immune responses may have been slightly reduced by ST-246 treatment but cellular immune responses appear to be slightly enhanced. Animals that were vaccinated and treated with ST-246 were equally protected from a subsequent lethal challenge in both short- and long-term experiments, clearly demonstrating that ST-246 does not adversely impact vaccine efficacy. In additional experiments, normal immunocompetent animals were lethally challenged and treated by postexposure vaccination, ST-246 or the combination of vaccination and ST-246 [SIGA Technologies, Unpublished Data]. We found that postexposure vaccination by itself was ineffective, while ST-246 treatment alone provided full protection when treatment was initiated within 72 h. Interestingly, combination treatment with vaccine and ST-246 extended the therapeutic window to 96 h postchallenge. Therefore, ST-246 does not appear to inhibit (pre-exposure prophylactic) vaccine efficacy, while the vaccine does not appear to inhibit drug efficacy as a postexposure therapeutic, and in fact the combined use may have additive effects in terms of postexposure efficacy.
In follow-up experiments, we have demonstrated the efficacy of ST-246 in immunocompromised animals [48]. First, we evaluated the efficacy of ST-246 in lethally challenged mice deficient for B- and/or T-cell function and found that ST-246 was efficacious in all models except in situations of severe combined immunodeficiency. Animals that survived the lethal challenge due to ST-246 treatment were resistant to subsequent rechallenge, suggesting that even in a partially immunodeficient setting, sufficiently robust protective immunity may be elicited. Therefore, we performed a series of experiments in which numerous murine models for immunodeficiency were vaccinated with ACAM2000 using the standard human dose and route and then treated with ST-246 [75]. ST-246 was effective in all models with the exception of those completely deficient for cellular immune responses (combined CD4+ and CD8+ deficiency). ST-246 reduced vaccine reactogenicity and the amount of time necessary for resolution of the vaccine lesion. Virus shedding from the lesion site was also reduced by ST-246 treatment. In models with partial immunodeficiencies, animals were safely vaccinated and able to resist subsequent lethal challenge in short-and long- term experiments, thus demonstrating that even in a partially immunodeficient setting, ST-246 improves vaccine safety while allowing the induction of robust immune responses that are capable of resisting lethal challenge.
At this preliminary stage of development it appears that there are no adverse drug–vaccine interactions and that ST-246 improves the safety of the smallpox vaccine even in immunocompromised animals without inhibiting the induction of protective immunity by the vaccine. Future studies will include immunodeficient NHP models prior to seeking FDA approval to initiate a clinical trial program to evaluate ST-246 as an adjunct to the smallpox vaccine.
Executive summary.
There is a need for a smallpox therapeutic antiviral
-
▪
Smallpox is a horrific disease that kills 30% of its victims and permanently scars and maims survivors.
-
▪
Herd immunity is nonexistent due to cessation of routine vaccination and eradication of naturally occurring disease.
-
▪
A Material Threat Assessment by the Department of Homeland Security states that smallpox may be used as a biological weapon and poses a serious risk to the security of the USA.
-
▪
The Biomedical Advanced Research and Development Agency has requested proposals to supply the Strategic National Stockpile with a smallpox antiviral therapeutic.
ST-246 fits an ideal product profile for use in a smallpox public health emergency
-
▪
ST-246 is potent, specific, safe, easy to manufacture, stable, orally bioavailable, has a straightforward dosing regimen that would not require medical supervision and does not interfere with the vaccine.
SIGA Technologies is developing ST-246 according to the ‘animal rule’ to meet the requirements for US FDA approval
-
▪
Safety/toxicology studies are complete. CNS toxicity was noted in dogs and maternal toxicity was noted in rabbits. No other toxicities have been noted in other animals.
-
▪
ST-246 effectively reduces morbidity and prevents mortality in numerous animal models for therapeutic intervention in orthopoxvirus disease including monkeypox virus and variola virus in nonhuman primates.
-
▪
Pharmacodynamic/pharmacokinetic modeling studies in animals have allowed selection of the human dose (400 mg/day for 14 days).
ST-246 is in late-stage clinical development
-
▪
SIGA Technologies has sponsored three clinical trials (Phase I and Phase II) to evaluate the safety, tolerability and pharmacokinetics of ST-246 in healthy subjects.
-
▪
No serious adverse events were reported in any of the trials and the drug is considered safe.
-
▪
Human plasma exposure levels lead to the reasonable expectation of efficacy against smallpox based on animal data.
Acknowledgments
The authors are employees of SIGA Technologies. The project described was supported by Award Number R44AI075747 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Franz DR, Jahrling PB, Friedlander AM, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278(5):399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 2.Nicas M, Hubbard A, Jones R, Reingold R. The infectious dose of variola (smallpox) virus. Applied Biosafety. 2004;9(3):118–127. [Google Scholar]
- 3▪▪.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its Eradication. WHO; Switzerland: 1988. A must-have reference for all things related to smallpox It describes the history and the eradication of the disease – a singular achievement in human history. [Google Scholar]
- 4.Gellman B. 4 Nations Thought to Possess Smallpox. The Washington Post. 2002 Nov 5; [Google Scholar]
- 5.Strikas RA, Neff LJ, Rotz L, et al. US Civilian Smallpox Preparedness and Response Program, 2003. Clin Infect Dis. 2008;46(Suppl. 3):S157–S167. doi: 10.1086/524751. [DOI] [PubMed] [Google Scholar]
- 6▪▪.Henderson DA, Inglesby TV, Bartlett JG, et al. Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999;281(22):2127–2137. doi: 10.1001/jama.281.22.2127. Highlights the potential of smallpox as a biological weapon as well as current policies to counter such an attack. [DOI] [PubMed] [Google Scholar]
- 7.Weiss MM, Weiss PD, Mathisen G, Guze P. Rethinking smallpox. Clin Infect Dis. 2004;39(11):1668–1673. doi: 10.1086/425745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8▪.Enserink M. Infectious diseases. Smallpox vaccination campaign in the doldrums. Science. 2003;300(5621):880–881. doi: 10.1126/science.300.5621.880b. Documents the failure of the civilian smallpox vaccination program for first-responders. Noncompliance with the requirement for vaccination is blamed on both real and imagined dangers from vaccination. [DOI] [PubMed] [Google Scholar]
- 9.Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. Curr Protoc Mol Biol. 2001;(Chapter 16) doi: 10.1002/0471142727.mb1617s43. Unit 16.17. [DOI] [PubMed] [Google Scholar]
- 10.Earl Pl, Cooper N, Wyatt S, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Mol Biol. 2001;(Chapter 16) doi: 10.1002/0471142727.mb1616s43. Unit 16.16. [DOI] [PubMed] [Google Scholar]
- 11.Andrew ME, Coupar BE. Biological effects of recombinant vaccinia virus-expressed interleukin 4. Cytokine. 1992;4(4):281–286. doi: 10.1016/1043-4666(92)90068-3. [DOI] [PubMed] [Google Scholar]
- 12.Massung RF, Liu LI, Qi J, et al. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994;201(2):215–240. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- 13.Henderson DA. The looming threat of bioterrorism. Science. 1999;283(5406):1279–1282. doi: 10.1126/science.283.5406.1279. [DOI] [PubMed] [Google Scholar]
- 14.Bossi P, Tegnell A, Baka A, et al. Bichat guidelines for the clinical management of smallpox and bioterrorism-related smallpox. Euro Surveill. 2004;9(12):E7–E8. [PubMed] [Google Scholar]
- 15.Kretzschmar M, Van Den Hof S, Wallinga J, Van Wijngaarden J. Ring vaccination and smallpox control. Emerg Infect Dis. 2004;10(5):832–841. doi: 10.3201/eid1005.030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan EH. Preventing second-generation infections in a smallpox bioterror attack. Epidemiology. 2004;15(3):264–270. doi: 10.1097/01.ede.0000121821.02642.a4. [DOI] [PubMed] [Google Scholar]
- 17.Legrand J, Viboud C, Boelle PY, Valleron AJ, Flahault A. Modelling responses to a smallpox epidemic taking into account uncertainty. Epidemiol Infect. 2004;132(1):19–25. doi: 10.1017/s0950268803001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EH, Craft DL, Wein LM. Emergency response to a smallpox attack: the case for mass vaccination. Proc Natl Acad Sci USA. 2002;99(16):10935–10940. doi: 10.1073/pnas.162282799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meltzer MI, Damon I, Leduc JW, Millar JD. Modeling potential responses to smallpox as a bioterrorist weapon. Emerg Infect Dis. 2001;7(6):959–969. doi: 10.3201/eid0706.010607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37(2):251–271. doi: 10.1086/375825. Excellent review of adverse events due to smallpox vacination. [DOI] [PubMed] [Google Scholar]
- 21▪.Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;5(2):84–90. Another good review of expected adverse events following vaccination. However, it is most notable for its conclusion that approximately 25% of the US population is contraindicated to receive the vaccine. [PubMed] [Google Scholar]
- 22.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970;122(4):303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 23.Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3(8):E272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coulibaly S, Bruhl P, Mayrhofer J, Schmid K, Gerencer M, Falkner FG. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005;341(1):91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 26.Empig C, Kenner JR, Perret-Gentil M, et al. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine. 2006:3686–3694. doi: 10.1016/j.vaccine.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Mccurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004;38(12):1749–1753. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa S, Sakiyama T, Hasegawa H, et al. An attenuated LC16m8 smallpox vaccine: analysis of full-genome sequence and induction of immune protection. J Virol. 2005;79(18):11873–11891. doi: 10.1128/JVI.79.18.11873-11891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stittelaar KJ, Van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollmar J, Arndtz N, Eckl KM, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2005;24(12):2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Kidokoro M, Tashiro M, Shida H. Genetically stable and fully effective smallpox vaccine strain constructed from highly attenuated vaccinia LC16m8. Proc Natl Acad Sci USA. 2005;102(11):4152–4157. doi: 10.1073/pnas.0406671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artenstein AW, Johnson C, Marbury TC, et al. A novel, cell culture-derived smallpox vaccine in vaccinia-naive adults. Vaccine. 2005;23(25):3301–3309. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 34.Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain) – a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8(Suppl. 2):S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weltzin R, Liu J, Pugachev KV, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 36▪▪.Yang G, Pevear DC, Davies MH, et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol. 2005;79(20):13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. First description of the discovery and efficacy of ST-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duraffour S, Snoeck R, De Vos R, et al. Activity of the anti-orthopoxvirus compound ST-246 against vaccinia, cowpox and camelpox viruses in cell monolayers and organotypic raft cultures. Antivir Ther. 2007;12(8):1205–1216. [PubMed] [Google Scholar]
- 38.Chen Y, Honeychurch KM, Yang G, et al. Vaccinia virus p37 interacts with host proteins associated with LE-derived transport vesicle biogenesis. Virol J. 2009;6:44. doi: 10.1186/1743-422X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(Pt 12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. Along with [41,42], this paper demonstrates the significance of enveloped virus in the pathogenesis of poxviruses, thus providing the rationale for targeting this process in treating disease. [DOI] [PubMed] [Google Scholar]
- 40.Jahrling PB, Hensley LE, Martinez MJ, et al. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc Natl Acad Sci USA. 2004;101(42):15196–15200. doi: 10.1073/pnas.0405954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Payne LG. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. Along with [39,42], this paper demonstrates the significance of enveloped virus in the pathogenesis of poxviruses, thus providing the rationale for targetting this process in treating disease. [DOI] [PubMed] [Google Scholar]
- 42▪.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J Virol. 1991;65(11):5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. Along with [39,41], this paper demonstrates the significance of enveloped virus in the pathogenesis of poxviruses, thus providing the rationale for targeting this process in treating disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurt I, Abdalrhman I, Katz E. Pathogenicity and immunogenicity in mice of vaccinia viruses mutated in the viral envelope proteins A33R and B5R. Antiviral Res. 2005;69(3):158–164. doi: 10.1016/j.antiviral.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Mcintosh AA, Smith GL. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J Virol. 1996;70(1):272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolffe EJ, Isaacs SN, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67(8):4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan R, Leeds JM, Tyavanagimatt S, Hruby D. Development of ST-246® for treatment of poxvirus infections. Viruses. 2010;2:2409–2435. doi: 10.3390/v2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berhanu A, King DS, Mosier S, et al. ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob Agents Chemother. 2009;53(12):4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grosenbach DW, Berhanu A, King DS, et al. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc Natl Acad Sci USA. 2010;107(2):838–843. doi: 10.1073/pnas.0912134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quenelle DC, Buller RM, Parker S, et al. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob Agents Chemother. 2007;51(2):689–695. doi: 10.1128/AAC.00879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sbrana E, Jordan R, Hruby De, et al. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am J Trop Med Hyg. 2007;76(4):768–773. [PubMed] [Google Scholar]
- 51.Nalca A, Hatkin JM, Garza NL, et al. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res. 2008;79(2):121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53(6):2620–2625. doi: 10.1128/AAC.00021-09. The first demonstration of ST-246 efficacy in a nonhuman primate model for therapeutic intervention following lethal poxvirus challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jordan R, Goff A, Frimm A, et al. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother. 2009;53(5):1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81(12):1581–1600. doi: 10.1038/labinvest.3780373. Significant paper for its description of monkeypox disease in nonhuman primates, its similarity to human smallpox and its sufficiency as a model for evaluating smallpox therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 56.Fogg CN, Americo JL, Lustig S, et al. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine. 2007;25(15):2787–2799. doi: 10.1016/j.vaccine.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heraud JM, Edghill-Smith Y, Ayala V, et al. Subunit recombinant vaccine protects against monkeypox. J Immunol. 2006;177(4):2552–2564. doi: 10.4049/jimmunol.177.4.2552. [DOI] [PubMed] [Google Scholar]
- 58.Hooper JW, Thompson E, Wilhelmsen C, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marriott KA, Parkinson CV, Morefield SI, Davenport R, Nichols R, Monath TP. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nigam P, Earl PL, Americo JL, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stittelaar KJ, Neyts J, Naesens L, et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439(7077):745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 62.CDC. Progressive vaccinia in a military smallpox vaccinee – United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(19):532–536. [PubMed] [Google Scholar]
- 63.Vora S, Damon I, Fulginiti V, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46(10):1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 64.Jordan R, Tien D, Bolken TC, et al. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob Agents Chemother. 2008;52(5):1721–1727. doi: 10.1128/AAC.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65▪▪.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. Documents that human monkeypox is invading the niche previously occupied by smallpox. The lack of poxvirus immunity along with cultural and socioeconomic factors plays a role in the increase in human monkeypox cases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vorou RM, Papavassiliou VG, Pierroutsakos IN. Cowpox virus infection: an emerging health threat. Curr Opin Infect Dis. 2008;21(2):153–156. doi: 10.1097/QCO.0b013e3282f44c74. [DOI] [PubMed] [Google Scholar]
- 67.De Souza Trindade G, Drumond BP, Guedes MI, et al. Zoonotic vaccinia virus infection in Brazil: clinical description and implications for health professionals. J Clin Microbiol. 2007;45(4):1370–1372. doi: 10.1128/JCM.00920-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3):434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parker S, Nuara A, Buller RM, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2:17–34. doi: 10.2217/17460913.2.1.17. [DOI] [PubMed] [Google Scholar]
- 70▪▪.Kile JC, Fleischauer AT, Beard B, et al. Transmission of monkeypox among persons exposed to infected prairie dogs in Indiana in 2003. Arch Pediatr Adolesc Med. 2005;159(11):1022–1025. doi: 10.1001/archpedi.159.11.1022. Describes the outbreak of human monkeypox in the USA in 2003. It shows how easily an exotic disease may make its way to a developed country. [DOI] [PubMed] [Google Scholar]
- 71.Shearer JD, Siemann L, Gerkovich M, House RV. Biological activity of an intravenous preparation of human vaccinia immune globulin in mouse models of vaccinia virus infection. Antimicrob Agents Chemother. 2005;49(7):2634–2641. doi: 10.1128/AAC.49.7.2634-2641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magee WC, Hostetler KY, Evans DH. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob Agents Chemother. 2005;49(8):3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quenelle DC, Prichard MN, Keith KA, et al. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51(11):4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grosenbach DW, Jordan R, King DS, et al. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine. 2008;26(7):933–946. doi: 10.1016/j.vaccine.2007.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berhanu A, King DS, Mosier S, et al. Impact of ST-246® on ACAM2000 smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice. Vaccine. 2010;29(2):289–303. doi: 10.1016/j.vaccine.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.WHO Fact Sheet. Smallpox. [Accessed 24 February 2011]; www.who.int/mediacentre/factsheets/smallpox/en.
- 102.CDC. Smallpox vaccination: information for health professionals. www.bt.cdc.gov/agent/smallpox/vaccination.
- 103.McClain D. Smallpox. Office of the Surgeon General, Department of the Army. Virtual Naval Hospital. Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare. 1997 www.bordeninstitute.army.mil/cwbw/default.htm.
- 104.Executive summary of the Smallpox Response Plan. www.bt.cdc.gov/agent/smallpox/response-plan/fles/exec-sections-i-vi.pdf.
- 105.ACAM2000™ medication guide. www.bt.cdc.gov/agent/smallpox/vaccination/pdf/ACAM2000MedicationGuide-31Aug2007.pdf.
- 106.Project BioShield. Progress on the war on terror. [Accessed 26 December 2010];2004 http://georgewbush-whitehouse.archives.gov/infocus/bioshield.
- 107.BARDA request for proposal. RFP-09–35 www.fbo.gov/index?s=opportunity&mode=form&tab=core&id=de86bdf6692468c380b358e99a36158f&_cview=0.
- 108.Code of Federal Regulations Title 21. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=314&showfr=1&subpartnode=21:5.0.1.1.4.9.
- 109▪▪.US FDA. Guidance for Industry: Animal models – essential elements to address efficacy under the animal rule. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078923.pdf Important guidance from the US FDA on the use of animal models to develop drugs that cannot be tested for efficacy in humans.
- 110.Phase I Trial of an Investigational Small Pox Medication. http://clinicaltrials.gov/ct2/show/NCT00728689.
- 111.Phase I, Escalating, Multiple-Dose, ST-246 Safety, Tolerability and Pharmacokinetics 21-Day Trial in Healthy Volunteers (SIGA-246-002) http://clinicaltrials.gov/ct2/show/NCT00431951.