Summary
Patients with systemic lupus erythematosus (SLE) have an impairment in phenotype and function of endothelial progenitor cells (EPCs) which is mediated by interferon α (IFN-α). We assessed whether murine lupus models also exhibit vasculogenesis abnormalities and their potential association with endothelial dysfunction. Phenotype and function of EPCs and type I IFN gene signatures in EPC compartments were assessed in female New Zealand Black/New Zealand White F1 (NZB/W), B6.MRL-Faslpr/J (B6/lpr) and control mice. Thoracic aorta endothelial and smooth muscle function were measured in response to acetylcholine or sodium nitropruside, respectively. NZB/W mice displayed reduced numbers, increased apoptosis and impaired function of EPCs. These abnormalities correlated with significant decreases in endolthelium-dependent vasomotor responses and with increased type I IFN signature in EPC compartments. In contrast, B6/lpr mice showed improvement in endothelium-dependent and endothelium-independent responses, no abnormalities in EPC phenotype or function and downregulation of type I IFN signatures in EPC compartments. These results indicate that NZB/W mice represent a good model to study the mechanisms leading to endothelial dysfunction and abnormal vasculogenesis in lupus. These results further support the hypothesis that type I IFNs may play an important role in premature vascular damage and, potentially, atherosclerosis development in SLE.
Keywords: endothelial progenitor cells, endothelium, interferon-alpha, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects primarily young women and is highly heterogeneous in its clinical presentation. Importantly, SLE is associated with a striking increase in the risk of premature cardiovascular (CV) complications due to accelerated atherosclerosis,(1) which significantly contributes to morbidity and mortality in this patient population.(2) While traditional risk factors may play a role in this increased propensity, they do not seem to fully account for this complication.(3) Indeed, the current proposal is that immune dysregulation characteristic of lupus plays the dominant role in the development of premature atherosclerosis. However, the exact etiology of this increased CV risk remains unclear.
A significant proportion of individuals with SLE have evidence of subclinical vascular disease which may precede the development of atherosclerosis. These subclinical abnormalities include the development of endothelial dysfunction (with preservation of vascular smooth muscle function), (4) arterial stiffness(5) and coronary perfusion abnormalities.(6) Endothelial dysfunction is a state wherein the vasodilatory, anticoagulant or anti-inflammatory properties of the endothelium are impaired and predisposes individuals to the development of atherosclerotic lesions.(7) As a potential mechanism explaining the development of endothelial dysfunction and accelerated atherosclerosis in SLE, our group and others have recently reported that patients with lupus develop a striking imbalance between vascular damage (apoptosis) and repair (vasculogenesis).(4,8,9) Indeed, SLE patients display decreased numbers and abnormal function of the cells involved in adult vasculogenesis: the endothelial progenitor cells (EPCs).(8) Importantly, similar abnormalities in EPCs have clearly been identified by numerous groups in various diseases characterized by an increased CV risk including diabetes mellitus, hypertension, tobacco use, aging and rheumatoid arthritis.(10–14) Furthermore, low EPC levels predict the occurrence of CV complications in the general population.(10) Therefore, the evidence that lupus EPCs are aberrant in their phenotype and function suggests that abnormal vascular repair may play a role in the development of premature vascular damage in this disease. Furthermore, we have recently reported that IFN-α, recently implicated as a major player in SLE disease pathogenesis and severity,(15) promotes abnormal EPC phenotype and function in human lupus.(8)
Ideally, lupus murine models would contribute to understanding the role of abnormal vascular repair and type I IFNs in the development of endothelial dysfunction and atherosclerosis in SLE. Unfortunately, the study of plaque formation in mice is complicated by the fact that this species is, in general, fairly resistant to the development of atherosclerotic lesions.(16) This general assessment also applies to murine lupus. Indeed, in contrast to human SLE, lupus-prone mice appear to be genetically-resistant to overt and florid diet-induced atherosclerosis, unless they are crossed to well-established murine models of atherosclerosis like the apolipoprotein E (ApoE) knock-out mouse or the low density lipoprotein (LDL) receptor knockout mouse.(17–20) In these settings, synergism in plaque development is observed, further indicating that lupus immune dysregulation may accelerate vascular damage in susceptible individuals. While florid atheroma formation has not been reported in murine lupus, a recent report indicates that the New Zealand Black/New Zealand White F1 lupus model (NZB/W) may be more prone to develop endothelial dysfunction than control mice.(21) This finding suggests that this murine model is prone to developing vascular damage. However, whether abnormal EPC phenotype and function are also present in lupus-prone mice and are correlative to endothelial dysfunction has not been investigated.
The current study has evaluated the association of abnormal EPC phenotype and function with the development of endothelial dysfunction in murine lupus models. The studies were performed in two well-characterized lupus-prone mouse strains: NZB/W, a model where type I IFNs appear to play an important role in lupus development and severity,(22–27) and the B6.MRL-Faslpr/J (B6/lpr) model, where type I IFNs appear to be protective of disease severity and development.(28,29)
Materials and methods
Mice and tissue harvesting
Six- to eight- week old NZB/W, BALB/c, B6.MRL-Faslpr/J (B6/lpr) and C57BL/6J (C57BL/6) females were obtained from the Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free barrier facility. Mice were killed at two time points: early disease before proteinuria development (labeled in the text as pre-nephritic or early disease stage), and active disease with proteinuria (labeled in the text as nephritic or active disease stage). Proteinuria was assessed using Uristix 4 (Siemens Healthcare Diagnostics Inc., Tarrytown, NY) following manufacturer’s instructions. For NZB/W and control BALB/c, the early and late time points were 20 and 36 weeks, respectively; while for B6/lpr and control C57BL/6 this corresponded to 8 and 16 weeks of age, respectively. All animal protocols were reviewed and approved by the University of Michigan’s Committee on Use and Care of Animals.
Assessment of vascular function
Post mortem, aortas were excised, cleaned and cut into 2-mm length rings. Endothelium was left intact and aortic rings were mounted in a myograph system (DMT-USA, Inc., Atlanta, GA). Vessels were bathed with warmed, aerated (95% O2/5% CO2) physiological salt solution (PSS). Aortic rings were set at 700 mg passive tension, equilibrated for 1 h and washed every 20 min. Prior to performing concentration response curves, the vessels were contracted with PSS containing 100 mmol/L potassium chloride (KPSS). Vessels were washed and contracted again with KPSS. After washing out excess potassium, vessels were contracted with phenylephrine (PE; 10−6 mol/l) and subsequently treated with acetylcholine (Ach; 10−7 mol/l) to test the integrity of the endothelium. Cumulative concentrations of PE (10−9 mol/l to 10−5 mol/l) were added to the bath to establish a concentration-response curve. The PE contraction was washed out with PSS, and the vessels were recontracted with PE at a concentration calculated to correspond to the EC80 and allowed to reach a stable plateau in the contraction. Ach (10−10 mol/l to 10−5 mol/l) was added cumulatively to the bath to examine endothelium-dependent relaxation. PE and Ach were washed out of the vascular preparation at the end of the concentration response, and the aortic rings were again recontracted with the PE EC80 and allowed to reach a stable plateau in the contraction. Endothelium-independent relaxation was assessed by the cumulative addition of sodium nitroprusside (SNP; 10−11 mol/l to 10−6 mol/l) to the bath. Ach and SNP relaxation were expressed as a percentage of PE EC80 contraction.(30,31)
Quantification of EPCs
Spleens and long bones were harvested post mortem. Femurs and tibias were washed and epiphyses were excised and flushed with ice-cold MACS buffer (Miltenyi Biotech, Auburn, CA). Bone marrow cells and spleens were filtered through a 40 μm cell strainer (BD Bioscience, Bedford, MA) to obtain a single cell suspension. Bone marrow cells (30–60 × 106) were depleted of lineage-positive cells using a mouse lineage depletion kit (Miltenyi), following the manufacturer’s recommendations. Spleen cells were depleted of B and T cells using anti-CD3 (eBioscience San Diego, CA) and anti-CD19 (Biolegend San Diego, CA) monoclonal antibodies (mAbs), respectively, using a similar protocol that used for bone marrow depletion. Mononuclear cells were obtained from cardiac puncture blood by Histopaque 1083 density gradient (Sigma Aldrich, St. Louis, MO) and RBCs were lysed with 172 mM NH4Cl2 and 83.9 mM KHCO3.
Approximately 1 × 106 lineage-depleted cells were incubated with mAbs against murine CD34 and murine VEGF-R2 (flk-1) (eBioscience) to determine the total number of EPCs, as described previously.(32) Similar experiments were performed with blood mononuclear cells obtained from cardiac puncture. In additional experiments, bone marrow EPCs were further characterized by co-staining lineage-negative cells with mAbs to murine Sca-1 (eBioscience) and CD117 (Biolegend), as described previously.(1) EPC apoptosis was assessed by Annexin-V staining (BD Bioscience) following the manufacturer’s recommendations. Fluorescence-activated cell sorter (FACS) was carried out using a FACSCalibur (BD Biosciences), followed by analysis with FlowJo (Treestar, Ashland, OR).
Assessment of EPC differentiation into mature endothelial cells
Bone marrow or spleen EPCs, isolated as described above, were plated onto fibronectin coated plates (BD Biosciences) at a density of 1 × 106 cells/cm2 in EGM-2 Bulletkit media (Lonza Allendale, NJ) supplemented with 5% heat inactivated FBS. Media was changed after 72 h in culture, then every 48 h. When indicated, graded concentrations of recombinant murine IFN-α (PBL InterferonSource, Piscataway, NJ) were added to the freshly plated cells for the initial 72 h of culture. On day 7, cells were incubated with FITC-conjugated Bandeiraea (Griffonia) Simplicifolia Lectin I (Isolectin B4) (BS-1; Vector Laboratories Burlingame, CA) and 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (diI)–acetylated LDL (ac-LDL; Biomedical Technologies, Stoughton, MA) for 4 h. Cells were analyzed by fluorescent microscopy using a Leica DMIRB fluorescent inverted microscope (Bannockburn, IL). Images were acquired with an objective magnification of × 10, (× 100 total magnification) using an Olympus DP30BW camera (Olympus Corporation, Tokyo, Japan) and the acquisition software Olympus-BSW (Olympus). Final processing was performed with Adobe Photoshop CS2 (San Jose, CA). Mature endothelial cells (ECs) were designated as those cells co-staining with BS-1 and ac-LDL, and were quantified in five to six random fields per well. Similar experiments were performed using anti-murine CD31 and anti-murine vascular endothelial growth factor receptor-2 (VEGFR2) (both from Biolegend) to confirm endothelial-cell specificity.
RNA isolation, real-time polymerase chain reaction and enzyme-linked immunosorbent assay for quantification of type I IFN-iinducible genes and proteins
RNA was isolated from bone marrow and spleen EPCs using Tripure (Roche Indianapolis, IN) following manufacturer’s recommendations. cDNA was synthesized using MMLV RT (Invitrogen Carlsbad, CA) and 1 μg of RNA using a MyCycler thermocyler (Bio-Rad, Hercules, CA). Six type I-IFN sensitive genes (ISGs) and one house-keeping gene (β-actin) were quantified by real-time polymerase chain reaction (PCR) using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA) as described previously.(24) Primer sequences were as follows:
Monocyte chemoattractant protein-1 (MCP-1)
AGGTCCCTGTCATGCTTCTG (forward),
GGATCATCTTGCTGGTGAAT (reverse);
Myxovirus (influenza virus) resistance 1 (M×1)
GATCCGACTTCACTTCCAGATGG (forward),
CATCTCAGTGGTAGTCAACCC(reverse);
IFN regulatory factor 7 (IRF-7)
TGCTGTTTGGAGACTGGCTAT (forward),
TCCAAGCTCCCGGCTAAGT (reverse);
IFN-inducible protein 10 (IP-10)
ATCATCCCTGCGAGCCTAT (forward),
ATTCTTGCTTCGGCAGTTAC (reverse);
ISG15 ubiquitin-like modifier (ISG15)
CAGAAGCAGACTCCTTAATTC (forward),
AGACCTCATATATGTTGCTGTG (reverse);
IFN-γ
AGCGGCTGACTGAACTCAGATTGTA (forward),
GTCACAGTTTTCAGCTGTATAGGG (reverse);
β-Actin
TGGAATCCTGTGGCATCCTGAAAC (forward),
TAAAACGCAGCTCAGTAACAGTCCG (reverse).
Real time PCR was carried out using an ABI PRISM 7900HT (Applied Biosystems). Transcripts were normalized using the β-actin gene. Expression of normalized genes was compared using a two-tailed Student’s t-test. Data is shown as the relative fold change over control using 2−ΔΔCt method as reported previously.(34)
To confirm findings at the protein level, an enzyme-linked immunosorbent assay (ELISA) was performed to quantify circulating MCP-1 (Biolegend) and IP-10 (RayBiotech Norcross GA) following manufacturer’s recommendations.
Statistical Analysis
Unless otherwise specified, results represent mean ± standard error of the mean (SEM) and all statistics were calculated using Student’s t-test with GraphPad prism 5 (GraphPad Software, La Jolla, CA). For measurements of endothelial function, median effective concentration (EC80) for agent-induced relaxation in aortic rings was calculated by non-linear regression analysis (GraphPad Prism, San Diego, CA). Comparisons in the dose response to EC80 contractions were analyzed using a Student’s t-test for each concentration of agonist.
Results
NZBW mice have impaired endothelium-dependent vasorelaxation
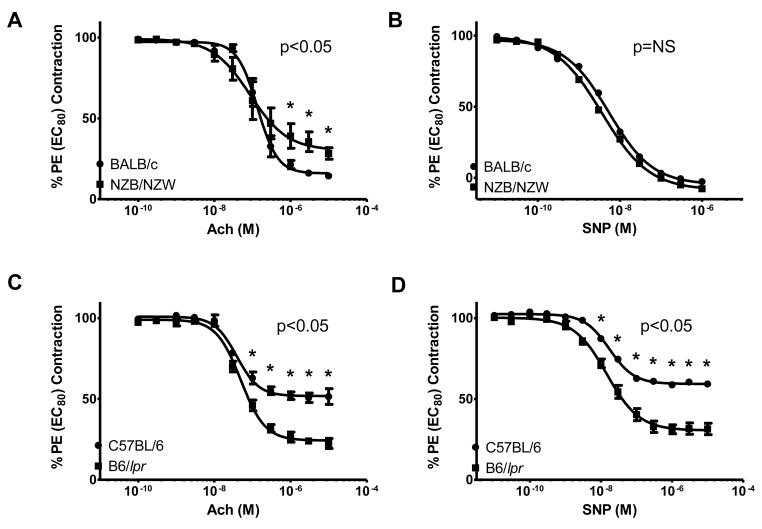
Endothelium-dependent and endothelium-independent responses were similar between NZB/W and BALB/c mice at 20 weeks of age (not shown). However, similar to what has been reported in human SLE(4) and a previous study in these mice,(21) 36-week-old NZB/W mice display impaired endothelial-dependent vasorelaxation of pre-contracted thoracic aortas, as assessed by blunted responses to Ach when compared with age-matched BALB/c mice (Figure 1A). Endothelium-independent vasorelaxation in the NZB/W mice, assessed by response of pre-contracted thoracic aortas to SNP, was not impaired at the same time-point (Figure 1B).
Figure 1. Decreased endothelial-mediated vasorelaxation in NZB/W mice.
Results assess acetylcholine (Ach)-mediated endothelium-dependent relaxation and sodium nitroprusside (SNP)-mediated endothelium-independent relaxation in aortic rings from (A), (B) 36-week-old NZB/W and BALB/c mice and (C), (D) 16-week-old B6/lpr and C57BL/6 mice. NZB/W mice have impaired Ach-mediated responses, while B6/lpr mice showed enhanced ACh-mediated and SNP-mediated responses. Results are mean ± SEM % phenylephrine (PE) EC80 contraction (n= 5–8 mice/group). *p<0.05; NS=not significant.
In contrast, at both early and active disease time-points, B6/lpr mice displayed significant increases in endothelial-dependent and endothelial-independent vasorelaxation when compared to control C57BL/6 mice (Figure 1C and D and not shown). These improvements were seen even in those mice with well-established renal disease, as assessed by proteinuria development. When examined during the active disease time-point, NZB/W and B6/lpr mice did not defer in their degree of proteinuria (625 ± 460.8 and 520 ± 372 mg/dl, respectively, p = not significant). These results indicate that nephritic-stage NZB/W mice, but not B6/lpr mice with similar degrees of lupus disease activity develop significant endothelial dysfunction with preservation of vascular smooth muscle function.
NZB/NZW mice display reduced numbers and increased apoptosis of EPCs
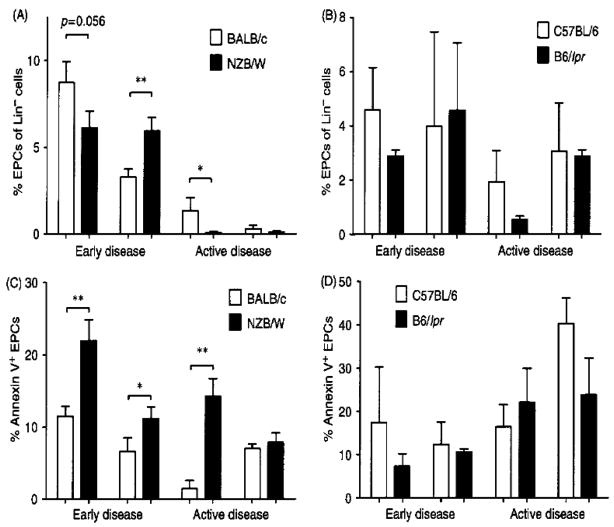
At 20 weeks of age, EPCs were decreased in the bone marrow but increased in the spleen of NZB/W mice, when compared to BALB/c mice (p<0.01). The depletion was enhanced by 36 weeks of age, when NZB/W EPCs were significantly decreased in both the bone marrow and spleen compartments (p<0.03; Figure 2A). In contrast, B6/lpr EPC numbers did not significantly differ from control C57BL/6 mice when quantified in bone marrow and spleen at pre-nephritic and nephritic stages (Figure 2B). Circulating EPC numbers did not significantly differ between NZB/W or B6/lpr mice and control mice at either stage of the disease (not shown).
Figure 2. EPCs are decreased in NZB/W mice and show higher levels of apoptosis.
EPCs were quantified in the bone marrow and spleen in pre-nephritic (early disease) and nephritic (active disease) NZB/W (A) and B6/lpr mice (B), as well as control mice. A significantly higher number of NZB/W EPCs were apoptotic, as determined by Annexin V expression at (C) pre-nephritic (early) time-point, both in the bone marrow and the spleen and at the nephritic (late) time point in the bone marrow. The B6/lpr mice showed no significant increases in EPC apoptosis at D) the early or active disease time points. Results are mean ± SEM % lineage-negative EPCs (n= 5–8 mice/group). *p<0.05; **p<0.01.
The decrease in bone-marrow EPCs in NZB/W mice at 20 and 36 weeks of age was associated with a significant increase in EPC apoptosis in this compartment, when compared with BALB/c mice (p = 0.004 and p = 0.005, respectively, Figure 2C). At 20 weeks of age, apoptotic EPCs also were increased in NZB/W spleen (p = 0.05), while no evidence of increased EPC death was detected in the blood of these mice. In contrast, B6/lpr mice showed no increases in EPC apoptosis during early or active disease in any of the compartments analyzed (Figure 2D). Similar results were observed when EPCs were quantified as lineage negative/Sca-1+/CD117+ cells (not shown).
EPCs from NZB/W mice are impaired in their capacity to differentiate into mature ECs
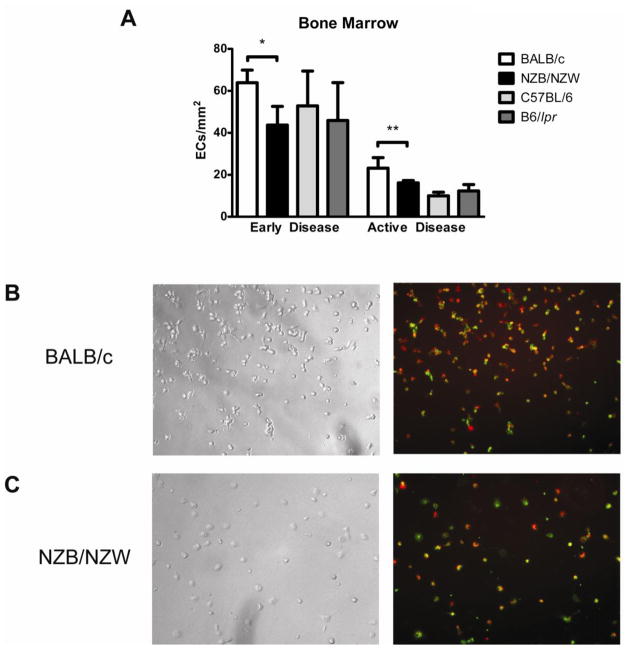
The functional ability of murine EPCs was assessed by quantifying their capacity to differentiate into mature endothelial-like cells under in vitro proangiogenic stimulation. After plating equal numbers of cells, NZB/W bone marrow EPCs displayed a decreased capacity to differentiate into cells that express mature EC markers, both at 20 weeks and at 36 weeks (Figure 3), when compared to BALB/c mice. Similar to what we previously reported in human SLE,(8) 7-day NZB/W cultures typically displayed scattered or clustered ECs but no EC monolayer formation, while BALB/c EPCs clearly differentiated into mature ECs with significant ac-LDL uptake and co-expression of BS-1. These findings were confirmed when other mature EC markers, CD31 and VEGFR-2 were examined (not shown). In contrast, B6/lpr bone marrow EPCs displayed no significant impairment in their capacity to differentiate into mature ECs, when compared with control C57BL/6 mice (not shown).
Figure 3. NZB/W EPCs exhibit impaired capacity to differentiate into mature ECs.
Bone marrow-derived EPCs were cultured under proangiogenic conditions and incubated at different time-points during culture with Dil-ac-LDL and BS-1-FITC. Mature endothelial cells were identified by co-staining of BS-1 and ac-LDL. (A) Bar graphs represent the number of mature endothelial cells per centimeter at day 7, when comparing NZB/W and B6/lpr bone marrow-derived EPCs with control EPCs, at early and active disease time-points. Results are mean ± SEM of 3 or 4 independent experiments; *p<0.05, **p<0.01. (B), (C) Results are representative images obtained from BALB/c (B) and NZB/W (C) EPCs cultured under proangiogenic conditions for 7 days. Left panels show bright field images and right panels show images obtained by fluorescent microscopy. NZB/W EPCs show decreased ability to differentiate into endothelial cells, when compared to BALB/c mice. DiI-Ac-LDL is red while BS-1 lectin is green. Total magnification is ×100.
Type I IFN signatures are increased in NZB/W EPC compartments
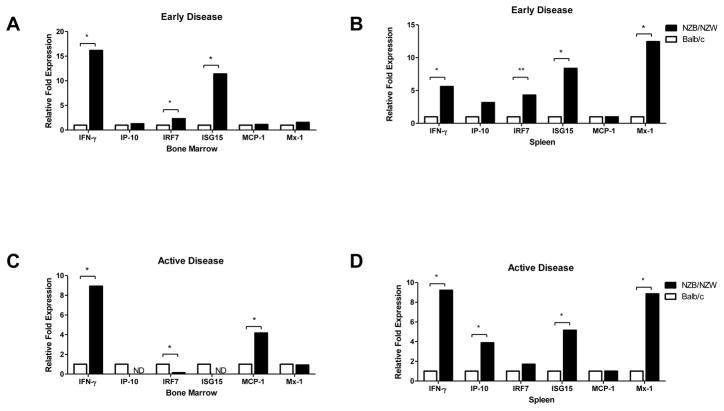
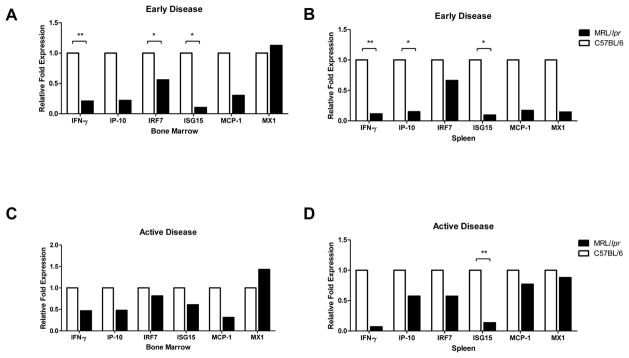
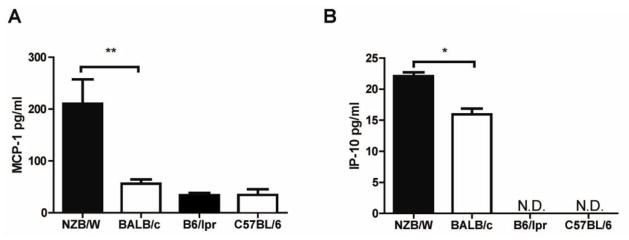
To assess whether the increased apoptosis and decreased numbers of EPCs in NZB/W mice could be associated with their enhanced exposure or increased sensitivity to type I IFNs, the level of expression of six type I-IFN regulated genes was examined in the bone marrow and spleen EPCs of lupus-prone and control mice. As shown in Figure 4, 20- and 36-week old NZB/W mice display significant increases in expression of type I IFN inducible genes in both compartments, when compared with BALB/c mice. In contrast, B6/lpr mice display downregulation of various IFN-responsive genes in those same compartments, both at 8 and 16 weeks of age, when compared with control C57BL/6 mice (Figure 5). Increased systemic exposure to type I IFNs in NZB/W mice was confirmed at the protein level, as elevated expression of both circulating MCP-1 (Figure 6A, p<0.0081) and IP-10 (Figure 6B, p<0.0113) was detected in these mice, but not in B6/lpr mice, when compared with controls (Figure 6). These results indicate that NZB/W EPCs are exposed in vivo to increased levels of type I IFNs and/or are more sensitive to the effects of these molecules when compared to control mice.
Figure 4. Type I IFN-inducible genes are increased in NZB/W EPC compartments.
Results are displayed as relative fold change of type I IFN-inducible genes over control BALB/c mice (n=5 mice/group) in bone marrow and spleen EPC compartments during (A), (B) early and (C), (D) active disease stage. Transcripts were normalized to β-actin; *p<0.05; **p<0.01; ND = not detected.
Figure 5. B6/lpr EPCs display decreased expression of type I IFN-inducible genes.
Results are displayed as relative fold change of type I IFN-inducible genes over control C57BL/6 mice (n=3 mice/group) in bone marrow and spleen EPC compartments during (A), (B) early and (C), (D) active disease stage. Transcripts were normalized to β-actin; *p<0.05; **p<0.01.
Figure 6. Increased circulating levels of type I-IFN inducible proteins in NZB/W mice.
Levels of the type I IFN-sensitive chemokines MCP-1 and IP-10 were quantified in the plasma of lupus-prone mice with active disease. Results represent mean ± SEM (n = 3–4 mice per group). *p<0.05.
IFN-α induces cytotoxicity of murine EPCs
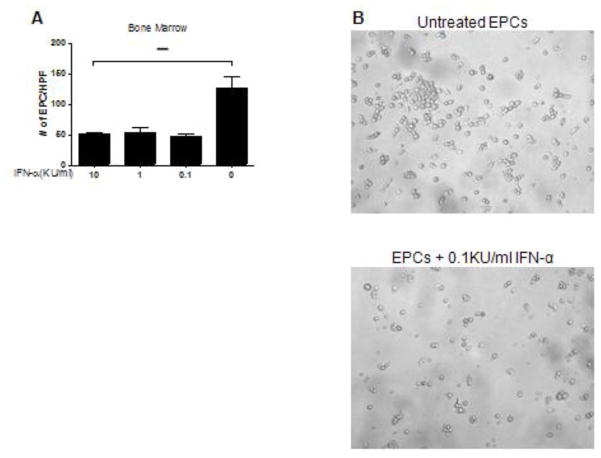
As type I IFNs are toxic to human EPCs and induce significant apoptosis of these cells,(8) we examined whether murine EPCs showed a similar sensitivity. Murine bone marrow (Figure 7) and spleen (not shown) EPCs from control BALB/c mice showed loss in their capacity to differentiate into mature ECs and increased death after in vitro exposure to recombinant IFN-α, recapitulating the phenotype observed in NZB/W EPCs (Figure 7).
Figure 7. IFN-α is toxic to murine EPCs.
Bone marrow EPCs from BALB/c mice were cultured in proangiogenic media in the presence or absence of graded concentrations of recombinant IFN-α for 3 days. Numbers of mature ECs were quantified at 7 days in culture. Exposure to IFN-α resulted in a significant loss of EPC ability to differentiate into mature endothelial cells. (A) Bar graph displays 1 representative experiment and represent mean ± SEM of 5 high-power fields; n=3, ***p<0.001. (B) Representative bright field images of untreated BALB/c bone marrow EPCs (top) and BALB/c bone marrow EPCs treated with 0.1KU/ml IFN-α. IFN-α–treated EPCs from BALB/c controls acquired the phenotype of lupus cells and were unable to form an endothelial cell monolayer.
Discussion
The results from this study suggest that the NZB/W murine model recapitulates the abnormal findings in EPC phenotype and function reported recently in human SLE.(8,9,35,36) Indeed, these mice have decreased numbers of EPCs in various compartments, increased bone marrow and spleen EPC apoptosis and decreased capacity of these cells to differentiate into mature ECs. Decreases in EPCs in NZB/W mice were already apparent in the bone marrow before overt clinical disease developed. The reasons for the initial increases in EPC numbers in the spleen of these mice before overt disease develops are unclear but may reflect aberrant homing of bone marrow EPCs or a differential response to ongoing vascular damage. Furthermore, even if EPCs were increased in the spleens of the mice at early disease time-points, a significantly higher number of these cells were apoptotic. The significant decreases in bone marrow and splenic EPCs that ensues as disease progresses could indicate exhaustion of the EPC pool in NZB/W mice in the context of accelerated apoptosis, similar to previously reported murine models of atherosclerosis.(37) The present study cannot assess whether the impairment in the differentiation of EPCs into a mature endothelium is solely secondary to accelerated EPC apoptosis or also results from functional abnormalities with regards to differentiation and this should be further explored in future studies.
The results from this study also support previous findings that EPCs play a key role in vascular health,(10,11,38,39) as the development of endothelial dysfunction in the NZB/W mice correlates with decreased EPC numbers and enhanced EPC death. While we did not evaluate atherosclerosis as an outcome in these mice, given the relative resistance of most murine lupus strains to the development of this complication, NZB/W mice did exhibit impaired endothelium-dependent vasorelaxation with preserved endothelium-independent vascular smooth muscle relaxation, similar to what has been reported observed in human SLE.(4) This was observed without exposing the mice to a Western diet which could have further enhanced these abnormalities.
Endothelial dysfunction is considered to be one of the earliest events in the development of atheroma and a promoter of disease progression and trigger for CV events.(7,40,41) Abnormal vasomotor responses of the endothelium may be attributable to possible alterations in the underlying physical properties of the vessel wall and/or the vascular tone in the setting of chronic inflammation, as proposed to occur in various human systemic autoimmune diseases.(5) While renal dysfunction has been associated with the development of endothelial dysfunction and abnormal EPC phenotype and function(42–45) it is unlikely that lupus nephritis significantly contributes to these abnormalities in the NZB/W mice, as B6/lpr mice with similar degrees of renal dysfunction and established SLE features did not display these vascular abnormalities. Further, while the B6/lpr phenotype can enhance the development of atherosclerosis when compounded by ApoE deficiency,(18) our results indicate that these mice are not impaired in their vasoregulatory function and display normal markers of vasculogenesis, unlike what has been described in human SLE.(4,8) The lack of endothelial and EPC dysfunction in B6/lpr mice in the active disease stage also indicates that the abnormalities observed in NZB/W mice are not primarily explained by lupus disease activity. This is consistent with the human model where, even lupus patients with no evidence of disease activity, display significant abnormalities in EPC phenotype and function as well as striking endothelial dysfunction.(4,8)
The mechanisms that lead to abnormal EPC phenotype and function in the NZB/W mice but not in the B6/lpr mice remain to be defined and may be multifactorial, including an enhanced resistance to apoptosis in the latter. However, B6/lpr mice are also known to have increased spontaneous apoptosis of peripheral blood mononuclear cells through nitric oxide(46) and to generate increased levels of nucleosomes.(47) This suggests that other apoptotic pathways are likely preserved and functional in these mice, as supported by previous studies.(48) Genetic differences, autoantibodies, immune complexes and other immunological abnormalities could certainly play differential roles in vascular damage in both models and need to be further examined. The differences in endothelium-dependent vasorelaxation observed between the NZB/W and B5/lpr mice may also be related to differences in other factors, including inducible and endothelial nitric oxide synthase expression and potential variablities in autoantibody profiles. While these possibilities were not assessed directly in this study, future studies should test the effects of non-selective and iNOS-selective inhibitors on endothelial function in lupus-prone mice.(49,50)
Type I IFNs have been proposed to play an important role in disease development and progression in NZB/W mice and derived congenic strains,(23) whereas the B6/lpr mice do not appear to depend on type I IFNs for their disease progression and may actually be protected by these cytokines.(28) Our group has shown previously that, upon exposure to recombinant IFN-α, human bone marrow and circulating EPCs undergo striking increases in apoptosis and a decreased capacity to differentiate into mature ECs upon proangiogenic stimulation.(8) Indeed, the abnormalities observed in human control EPCs upon exposure to IFN-α recapitulate the phenotype observed in human SLE EPCs.(8) Furthermore, neutralization of type I IFNs in vitro results in restoration of the normal capacity of human lupus EPCs to differentiate into mature ECs.(8) While the precise mechanisms leading to aberrant EPC phenotype and function and endothelial dysfunction in the NZB/W mice remain to be determined and may be multifactorial, an attractive hypothesis is that, similar to human lupus, type I IFNs are main players in the induction of the observed EPC dysfunction and vasomotor abnormalities. Indeed, the presence of endothelial dysfunction was associated with an increased type I IFN signature in the NZB/W EPC compartments, while decreases in these signatures were observed in B6/lpr mice. This is consistent with a recent report where splenic mononuclear cells from NZB/W, but not B6/lpr, lupus-prone mice at the pre-autoimmune stage were found to have increased expression of many known type I IFN-regulated genes.(26) Further, control bone marrow murine EPCs lose the ability to differentiate into a mature endothelium when exposed to IFN-α, and acquire a similar phenotype to what we have now observed in NZB/W mice and what has been described previously in human lupus EPCs.(8) IFN-α also has been shown to decrease colony formation of hematopoietic progenitor cells,(51) further supporting its potential implication in EPC decreases in SLE. Indeed, similar to what we now report in NZB/W EPCs, increased apoptosis of bone marrow
CD34+ cells has been reported in human SLE, but the precise etiology was not characterized.(52) It is possible that the effect of type I IFNs on NZB/W EPCs could be related to not only the exposure to increased levels of these cytokines, but also to increased sensitivity to type I IFN effects, as recently proposed in human SLE.(53) Among various antiangiogenic effects, type I IFNs may promote enhanced in situ bone marrow EPC apoptosis, with a subsequent decrease in total numbers of these cells. This could lead to decreased mobilization and homing into damaged vascular structures, therefore promoting endothelial dysfunction. The lack of apparent decrease in circulating EPCs despite decreased levels in the bone marrow and spleen requires further investigation but may be related to different kinetics with regards to mobilization and homing between murine and human EPCs. It is also possible that the effects of type I IFNs are enhanced in specific organs such as bone marrow or spleen. Indeed, a recent report indicates expansion of bone marrow IFN-α producing dendritic cells in NZB mice which may potentially indicate that the bone marrow niche could be exposed to even higher levels of this cytokine than other peripheral compartments.(22) This hypothesis needs to be investigated in future studies.
In conclusion, the NZB/W lupus-prone mice is characterized by the development of endothelial dysfunction and abnormal phenotype and function of EPCs, which is associated with in vivo exposure of these cells to increased levels of and/or higher sensitivity to type I IFNs. NZB/W mice therefore represent a good model to study the mechanisms leading to endothelial dysfunction and abnormal vasculogenesis in lupus. Future studies specifically examining the role of type I IFNs in premature cardiovascular disease and endothelial dysfunction in murine and human SLE are warranted.
Acknowledgments
This work was supported by a grant from the Biomedical Research Council at the University of Michigan, Public Health Service grant from the National Institutes of Health (NIH) 5R01 HL088419-02 and by the Anthony S Gramer Fund in Inflammation Research (all to MJK). The study was also funded (in part) by NIH through the University of Michigan’s Cancer Center Support Grant (P30 CA46592), the Rheumatic Disease Core Center Grant (P30 AR048310) and Training Grant T32 AI007413. We are grateful to Dr George Tsokos for providing additional murine serum.
Abbreviations used
- Ach
acetylcholine
- ac-LDL
1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (diI)–acetylated LDL
- ApoE
apolipoprotein E
- BS-1
Bandeiraea (Griffonia) Simplicifolia Lectin I (Isolectin B4)
- C57BL/6
C57BL/6J
- CV
cardiovascular CV
- EPCs
endothelial progenitor cells
- IFN-α
interferon α
- ISGs
type I-IFN sensitive genes
- KPSS
PSS containing 100 mmol/L potassium chloride
- LDL
low density lipoprotein
- PE
phenylephrine
- PSS
physiological salt solution
- B6/lpr
B6.MRL-Faslpr/J
- NZB/W
New Zealand Black/New Zealand White F1
- SLE
systemic lupus erythematosus
- SNP
sodium nitroprusside
Footnotes
Competing interests
The author(s) declare that they have no competing interests
Authors’ contributions
ST, DD and JP performed all experiments and analyzed the data. ST drafted the manuscript. MJK conceived and designed the study and helped to draft the manuscript. All authors read and approved the final document.
Contributor Information
Seth Thacker, Email: seththac@umich.edu.
Damon Duquaine, Email: duquaine@gmail.com.
James Park, Email: james.park@comcast.net.
Mariana J Kaplan, Email: makaplan@umich.edu.
References
- 1.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997 Mar 1;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 2.Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9(3):166–9. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 3.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001 Oct;44(10):2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Somers EC, Brook RD, Kehrer C, Pfenninger D, Lewis E, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004 May 15;103(10):3677–83. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 5.Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005 Jul;46(1):194–9. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 6.Bruce IN, Gladman DD, Ibanez D, Urowitz MB. Single photon emission computed tomography dual isotope myocardial perfusion imaging in women with systemic lupus erythematosus. II. Predictive factors for perfusion abnormalities. J Rheumatol. 2003 Feb;30(2):288–91. [PubMed] [Google Scholar]
- 7.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995 Feb 23;75(6):71B–4B. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 8.Denny MF, Thacker S, Mehta H, Somers EC, Dodick T, Barrat FJ, et al. Interferon-alpha promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007 Oct 15;110(8):2907–15. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007 Nov;56(11):3759–69. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 10.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005 Sep 8;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 11.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001 Jul 6;89(1):E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 12.Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005 Jan 18;111(2):204–11. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 13.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008 May 6;51(18):1760–71. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008 Dec;8(8):754–67. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006 Sep;25(3):383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007 May 25;100(10):1415–27. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Li H, Rumbin AA, Wang X, La Cava A, Brechtelsbauer K, et al. ApoE−/−Fas−/− C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J Lipid Res. 2007 April 1;48(4):794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Choudhury A, Kang SA, Monestier M, Cohen PL, Eisenberg RA. Accelerated atherosclerosis in ApoE deficient lupus mouse models. Clin Immunol. 2008 May;127(2):168–75. doi: 10.1016/j.clim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, et al. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004 Apr 19;199(8):1121–31. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanic AK, Stein CM, Morgan AC, Fazio S, Linton MF, Wakeland EK, et al. Immune dysregulation accelerates atherosclerosis and modulates plaque composition in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2006 May 2;103(18):7018–23. doi: 10.1073/pnas.0602311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007 Feb;292(2):R736–42. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 22.Lian ZX, Kikuchi K, Yang GX, Ansari AA, Ikehara S, Gershwin ME. Expansion of bone marrow IFN-alpha-producing dendritic cells in New Zealand Black (NZB) mice: high level expression of TLR9 and secretion of IFN-alpha in NZB bone marrow. J Immunol. 2004 Oct 15;173(8):5283–9. doi: 10.4049/jimmunol.173.8.5283. [DOI] [PubMed] [Google Scholar]
- 23.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005 Mar 1;174(5):2499–506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 24.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, et al. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007 Nov;56(11):3770–83. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007 Dec;37(12):3582–6. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 26.Lu Q, Shen N, Li XM, Chen SL. Genomic view of IFN-alpha response in pre-autoimmune NZB/W and MRL/lpr mice. Genes Immun. 2007 Oct;8(7):590–603. doi: 10.1038/sj.gene.6364421. [DOI] [PubMed] [Google Scholar]
- 27.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, et al. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989 Jan 5;320(1):8–13. doi: 10.1056/NEJM198901053200102. [DOI] [PubMed] [Google Scholar]
- 28.Schwarting A, Paul K, Tschirner S, Menke J, Hansen T, Brenner W, et al. Interferon-α: A Therapeutic for Autoimmune Lupus in MRL-Faslpr Mice. J Am Soc Nephrol. 2005 November 1;16(11):3264–72. doi: 10.1681/ASN.2004111014. [DOI] [PubMed] [Google Scholar]
- 29.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004 Aug 1;173(3):2134–42. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 30.Park JL, Loberg RD, Duquaine D, Zhang H, Deo BK, Ardanaz N, et al. GLUT4 facilitative glucose transporter specifically and differentially contributes to agonist-induced vascular reactivity in mouse aorta. Arterioscler Thromb Vasc Biol. 2005 Aug;25(8):1596–602. doi: 10.1161/01.ATV.0000170137.41079.ab. [DOI] [PubMed] [Google Scholar]
- 31.Park JL, Heilig CW, Brosius FC., 3rd GLUT1-deficient mice exhibit impaired endothelium-dependent vascular relaxation. Eur J Pharmacol. 2004 Aug 2;496(1–3):213–4. doi: 10.1016/j.ejphar.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008 Apr;118(4):1380–9. doi: 10.1172/JCI33125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman MD, Haas CS, Rad AM, Arbab AS, Koch AE. The role of vascular cell adhesion molecule 1/very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007 Jun;56(6):1817–26. doi: 10.1002/art.22706. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Westerweel PE, Luijten RKMAC, Hoefer IE, Koomans HA, Derksen RHWM, Verhaar MC. Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann Rheum Dis. 2007 July 1;66(7):865–70. doi: 10.1136/ard.2006.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grisar J, Steiner CW, Bonelli M, Karonitsch T, Schwarzinger I, Weigel G, et al. Systemic lupus erythematosus patients exhibit functional deficiencies of endothelial progenitor cells. Rheumatology. 2008 October 1;47(10):1476–83. doi: 10.1093/rheumatology/ken286. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007 Jan;27(1):113–9. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 38.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003 Feb 13;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 39.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003 Jul 25;93(2):e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 40.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002 Aug 6;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 41.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992 Nov 7;340(8828):1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 42.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, et al. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 1996 Aug;50(2):600–8. doi: 10.1038/ki.1996.355. [DOI] [PubMed] [Google Scholar]
- 43.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Curr Opin Nephrol Hypertens. 2005 Nov;14(6):525–31. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 44.Lartaud-Idjouadiene I, Lompre A-M, Kieffer P, Colas T, Atkinson J. Cardiac Consequences of Prolonged Exposure to an Isolated Increase in Aortic Stiffness. Hypertension. 1999 July 1;34(1):63–9. doi: 10.1161/01.hyp.34.1.63. [DOI] [PubMed] [Google Scholar]
- 45.Herbrig K, Pistrosch F, Foerster S, Gross P. Endothelial progenitor cells in chronic renal insufficiency. Kidney Blood Press Res. 2006;29(1):24–31. doi: 10.1159/000092484. [DOI] [PubMed] [Google Scholar]
- 46.Oates JC, Gilkeson GS. Nitric oxide induces apoptosis in spleen lymphocytes from MRL/lpr mice. J Investig Med. 2004 Jan;52(1):62–71. doi: 10.1136/jim-52-01-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Licht R, van Bruggen MC, Oppers-Walgreen B, Rijke TP, Berden JH. Plasma levels of nucleosomes and nucleosome-autoantibody complexes in murine lupus: effects of disease progression and lipopolyssacharide administration. Arthritis Rheum. 2001 Jun;44(6):1320–30. doi: 10.1002/1529-0131(200106)44:6<1320::AID-ART224>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Benihoud K, Bonardelle D, Bobe P, Kiger N. MRL/lpr CD4- CD8- and CD8+ T cells, respectively, mediate Fas-dependent and perforin cytotoxic pathways. Eur J Immunol. 1997 Feb;27(2):415–20. doi: 10.1002/eji.1830270211. [DOI] [PubMed] [Google Scholar]
- 49.Njoku C, Self SE, Ruiz P, Hofbauer AF, Gilkeson GS, Oates JC. Inducible nitric oxide synthase inhibitor SD-3651 reduces proteinuria in MRL/lpr mice deficient in the NOS2 gene. J Investig Med. 2008;56:911–919. doi: 10.231/JIM.0b013e3181889e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okun EB, Szado T, Laher I, McManus B, van Breeman C. Augmented contractile response of vascular smooth muscle in a diabetic mouse model. J Vasc Res. 2003;40:520–530. doi: 10.1159/000075238. [DOI] [PubMed] [Google Scholar]
- 51.Broxmeyer HE, Lu L, Platzer E, Feit C, Juliano L, Rubin BY. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–5. [PubMed] [Google Scholar]
- 52.Papadaki HA, Boumpas DT, Gibson FM, Jayne DR, Axford JS, Gordon-Smith EC, et al. Increased apoptosis of bone marrow CD34(+) cells and impaired function of bone marrow stromal cells in patients with systemic lupus erythematosus. Br J Haematol. 2001 Oct;115(1):167–74. doi: 10.1046/j.1365-2141.2001.03076.x. [DOI] [PubMed] [Google Scholar]
- 53.Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: Autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. J Immunol. 2009 Jan 1;182(1):34–8. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]