Abstract
High-dose ionizing radiation is an established risk factor for glioma, but it remains unknown whether moderate- and low-dose radiation increase glioma risk. In this analysis, we assessed the evidence that self-reported exposures to diagnostic ionizing radiation, including computerized tomography (CT) scans, is associated with increased risk of adult glioma. While no independent association was observed for CT scans alone (3+ scans compared to none P = 0.08 and 1–2 scans compared to none P = 0.68), our findings suggest an increased risk of adult gliomas with cumulative exposure to three or more CT scans to the head and neck region (OR = 1.97, 95% CI: 0.92–4.23) limited to those who reported a family history of cancer: the P value for the interaction between having three or more CT scans and family history of cancer was 0.08. The stratum-specific adjusted OR for those with family history of cancer was more than three times that for the sub-group without family history of cancer. While there is some potential for symptom-related bias, one might expect this to be present for all diagnostic procedures rather than specific to one procedure. The interaction between CT scans and glioma with family history of cancer supports the biological plausibility of our findings, because similar results have been found for breast cancer and radiation. This observational data will increase awareness about potential risks associated with CT scans and the need to minimize the use of unnecessary examinations.
INTRODUCTION
Ionizing radiation is a well-studied human carcinogen, and high-dose radiation is an established risk factor for brain tumors (including glioma, a malignant brain tumor) (1–3). This evidence has emerged from a variety of exposure scenarios, including atomic bomb survivors, nuclear weapons testing/production, medical and diagnostic radiation, and occupational and natural environmental exposures (4).
Risks for gliomas are primarily based on quantitative exposures. Studies of the atomic bomb survivors and Israeli tinea capitis cohort provide information suggesting that the risk of developing a glioma among these exposed adult populations compared to unexposed populations is approximately doubled (5). A recent analysis of the atomic bomb survivor cohort suggests that not only high but also moderate doses of radiation (<1 Sv) elevate the risk of nervous system tumors. This effect was most apparent for schwannomas and was present, but not statistically significant, for gliomas (2). This study also noted that excess rates for overall nervous system tumors were larger for children than for adults.
CT scans have become more prevalent, and diagnostic procedures, at an estimated 700,000 per year, now reflect the most common exposure to low- to medium-dose radiation in the general U.S. population (6). Concern about the impact of these diagnostic exposures has been expressed and may be relevant to the brain, given what we know about higher-dose exposures (2, 7). Because information on adult exposures in the low to moderate doses of radiation is limited and inconsistent, an effort was made to assess the evidence that self-reported exposures to ionizing radiation, including CT scans, are associated with increased risk of adult glioma.
METHODS
Study Population
Cases
People diagnosed with glioma were recruited from brain tumor referral clinics at Duke University Medical Center (North Carolina) and Evanston Hospital (Illinois) in collaboration with investigators from the University of Illinois at Chicago. Institutional Review Board approvals were obtained from all three institutions. Eligible glioma cases (n = 1344) were identified between April 2003 and December 2007 with a confirmed new diagnosis of glioma classified using: International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) (www.who.int/classifications/icd/adaptations/oncology) site codes C70.0–C72.9 and C75.1–C75.3 limited to glioblastoma (ICDO-3 histology codes 9440–9442), astrocytoma (9400–9411 and 9420–9421), and oligodendroglioma (9450–9460). Patients who were 18 years or older, English speaking, and residents of the United States were eligible for recruitment. Cases were targeted for recruitment within 3 months from the initial date of diagnosis, and the patients were approached for participation only if they had the mental capacity to consent. Mental capacity was assessed by the Mini-Mental State Examination during the initial physician assessment. Of the eligible cases, 840 consented to study participation and 611 completed the survey (survey response rate of 72%). For this analysis, only those cases who also had at least one eligible friend control consent to the study were included (n = 273) out of 327 glioma patients who provided contact information for at least one friend as a potential control.
Controls
Three groups of controls were selected for this case-control study: friends of brain tumor patients, siblings of the brain tumor patients, and clinic-based controls recruited primarily from an orthopedic surgery clinic. For this analysis, we used friend controls because of concern for potential biases in the other two potential comparison groups: siblings may have similar cancer predisposition and conditions leading to exposure to medical radiation, and clinic-based controls may have increased exposure to diagnostic radiation due to trauma or due to the nature of the condition bringing them to the clinic. Therefore, friend controls were selected because they are more likely than a random sample to represent the underlying referral population for the two study clinics without having the aforementioned biases. Of the consented cases, 273 cases (33%) had at least one eligible friend control who consented to the study, but some had multiple matched controls. We did not calculate a participation rate for friend controls, because the effort was made to recruit at least one friend control per case. Therefore, although several potential friend controls per case were contacted, we ceased our effort to recruit additional friend controls after at least one was recruited to match a specific case.
Ultimately, some cases and controls failed to complete the survey by the end of the study (January 2010). Therefore, our analytical sample included only cases that had a completed survey and had at least one control that completed the survey: 205 cases and 333 friend controls.
Data Collection
Subjects who consented to participate completed either a web-based or a telephone survey. A resurvey of a subset of questions suggested that the resulting data were reasonably reliable (8). This survey focused on occupational and environmental exposures associated with known animal neurocarcinogens. In addition, the survey collected information on demographics, family history, medical history and history of diagnostic and therapeutic radiation exposures as potential confounding and modifying variables.
Exposure to diagnostic ionizing radiation was assessed by questions specific to pre-diagnostic procedures (as an adult, more than 2 years ago), including dental (X rays and full-mouth or panorex X rays) and head, face, neck or upper spine procedures (diagnostic X rays, MRIs and CT scans). To calculate individual doses to the brain, we assigned an average organ dose of radiation as cited by the Health Physics Society (http://www.hps.org/documents/meddiagimaging.pdf and http://www.hps.org/hpspublications/articles/dosesfrommedicalradiation.html): dental X ray = 0.02 mSv; panorex = 0.09 mSv; head, neck, face, upper spine X ray = 0.01–0.03 mSv; head, neck, face, upper spine CT scan (CT-head) = 2.0 mSv. Taking into account the frequency range options for each procedure, we assigned the following values for the frequency categories: 0 = never, 1–2 = 1.5, 3–5 = 4, 6–9 = 7.5, and ≥10 = 10. The exposure scores for each type of procedure were calculated, based on the average organ dose and the number of procedures (procedure average dose × procedure frequency). These scores were summed to create an overall exposure score for diagnostic ionizing radiation to the brain. Procedures with missing values for any of the exposures were given a score of zero for that exposure. Based on an observed break between 4.89 and 8.31 in the exposure distribution, this score was categorized into two groups, <8.0 mSv and 8.0+ mSv.
Statistical Analysis
The association between diagnostic exposure to radiation and adult gliomas was examined by calculating crude and adjusted odds ratios (ORs) and 95% confidence intervals (CI) from conditional logistic regression models that included cases and matched friend controls. The match ratio for cases to controls ranged from 1:1 (n = 98 cases) to 1:4 (n = 1 case). The models for adjusted ORs also included age and gender. Each exposure variable was treated as a categorical variable for the calculation of odds ratios, with a common referent group representing the lowest level of exposure. Additional models were run treating exposure variables as ordinal to test the significance of a possible trend across increasing exposure levels. An interaction term between exposure and family history of cancer was tested in each model to determine whether the relationship between radiation exposure and gliomas differed significantly for those with and those without a family history of cancer.
RESULTS
The matched analysis includes 205 cases and 333 friend controls. Cases had higher proportions of males compared to friend controls, but no differences were seen with regard to age, race, income or family history of cancer (Table 1). Adjusted ORs in Table 2 reflect similar frequencies of reporting having dental X rays and diagnostic X rays to the head/face/neck/upper spine, and no association was found. However, a twofold increase (adjusted OR = 1.97, 95% CI: 0.92–4.23) in glioma risk was noted for those reporting having three or more CT scans compared to those who reported no CT scans; as mentioned in the Methods section, only CT scans involving the head and neck were considered in this analysis. This finding prompted further analyses to focus on CT scans.
TABLE 1.
Distributions of Selected Characteristics of Cases and Matched Friend Controls
| Characteristic | Cases (n = 205)
|
Matched friend controls (n = 333)
|
||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| Gender | ||||
| Male | 114 | 55.6 | 155 | 46.6 |
| Female | 91 | 44.4 | 178 | 53.5 |
| P valuea | 0.01 | |||
| Age (mean/SD) | 48.5 (12.3) | 49.8 (12.5) | ||
| P valuea | 0.13 | |||
| Race | ||||
| White | 197 | 96.1 | 321 | 97.0 |
| Non-white | 8 | 3.9 | 10 | 3.0 |
| Missing | 0 | — | 2 | — |
| P valuea | 0.64 | |||
| Education | ||||
| 12 years or less | 32 | 15.6 | 37 | 11.1 |
| 13–16 years | 82 | 40.0 | 151 | 45.4 |
| 16+ years | 91 | 44.4 | 145 | 43.5 |
| P valuea | 0.19 | |||
| Annual income | ||||
| <$50,000 | 35 | 17.2 | 48 | 14.6 |
| $50,000–99,999 | 77 | 37.9 | 124 | 37.6 |
| $100,000+ | 91 | 44.8 | 158 | 47.9 |
| Missing | 2 | — | 3 | — |
| P valuea | 0.79 | |||
| Family history of any cancer | ||||
| Yes | 87 | 42.4 | 159 | 47.8 |
| No | 118 | 57.6 | 174 | 52.3 |
| P valuea | 0.28 | |||
P value for beta estimate from crude conditional logistic regression model predicting case/control status.
TABLE 2.
Distributions of Diagnostic Radiation Related Exposures in Cases and Friend Controls and Their Association with Gliomas Using Adjusted OR (adjOR) Estimates and 95% CI Estimated from a Multivariable Conditional Logistic Regression Model Controlling for Age and Gender
| Exposure | Cases (n = 205)
|
Friend controls (n = 333)
|
Estimates
|
P value for trendb | |||
|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | adjOR | 95% CI | ||
| Dental X rays | |||||||
| Never | 8 | 3.9 | 8 | 2.4 | Ref | 0.52 | |
| Less frequently than 1× per year | 117 | 57.4 | 193 | 58.0 | 0.58 | 0.20, 1.67 | |
| At least 1× per year | 79 | 38.7 | 132 | 39.6 | 0.60 | 0.21, 1.73 | |
| Missing | 1 | — | 0 | — | — | — | |
| Panorex examinations | |||||||
| Never | 84 | 50.3 | 126 | 45.3 | Ref | 0.23 | |
| 1–2 | 47 | 28.1 | 81 | 29.1 | 0.95 | 0.57, 1.58 | |
| 3+ | 36 | 21.6 | 71 | 25.5 | 0.70 | 0.40, 1.21 | |
| Missing/don’t know | 38 | — | 55 | — | — | — | |
| Diagnostic X raya | |||||||
| Never | 111 | 55.5 | 177 | 54.8 | Ref | 0.43 | |
| 1–2 | 50 | 25.0 | 75 | 23.2 | 1.05 | 0.68, 1.64 | |
| 3+ | 39 | 19.5 | 71 | 22.0 | 0.79 | 0.49, 1.28 | |
| Missing | 5 | — | 10 | — | — | — | |
| CT scana | |||||||
| Never | 148 | 72.6 | 260 | 78.3 | Ref | 0.12 | |
| 1–2 | 39 | 19.1 | 59 | 17.8 | 1.10 | 0.69, 1.76 | |
| 3+ | 17 | 8.3 | 13 | 3.9 | 1.97 | 0.92, 4.23 | |
| Missing | 1 | — | 1 | — | — | — | |
To the head, face, neck or upper spine.
P value for significance of exposure when modeled as an ordinal variable.
We made an attempt to create a unifying scale for all medical radiation items included in this study. Using our crude exposure score that incorporated all the ionizing radiation items available, we compared cumulative radiation exposure reported by the participant for different medical procedures (Fig 1). As shown in Fig. 1, a single CT scan produced generally higher radiation scores compared to the summary score from all other assessed radiation exposure procedures. Moreover, subjects who received radiation from three or more CT scans have much higher radiation scores than all other participants. Since there was practically no overlap between the radiation scores for CT scans and for other procedures, using radiation scores for further analysis of the association with CT scans was not justified.
FIG. 1.
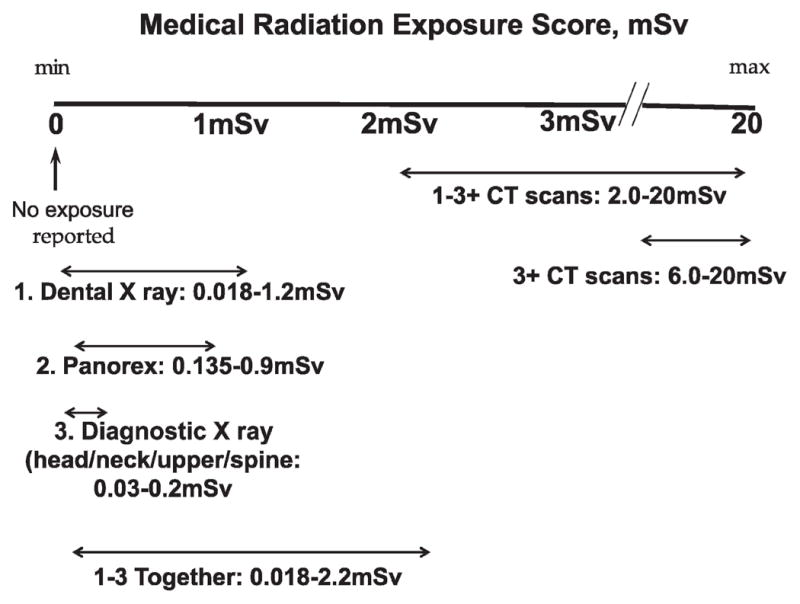
Ranges of cumulative radiation exposure reported by the participant for different medical procedures.
Further analyses found that the elevated OR between the number of CT scans received (3+ compared to none) and glioma was limited to those who reported a family history of cancer. The P value for the interaction term between having three or more CT scans and family history of cancer was 0.08. The stratum-specific adjusted OR for those with family history of cancer was more than three times that for the sub-group without family history of cancer. Although the trend test is borderline statistically significant among those with a family history, the difference in adjusted ORs between the 1–2 CT scans and the zero category is small (1.05 compared to 1.0), suggesting that this result may in fact be a threshold effect driven by the 3+ CT-scan category (Table 3).
TABLE 3.
Distributions of CT Scan Exposure in Cases and Friend Controls and Their Association with Gliomas, Stratified by Family History of Any Cancera, Using Adjusted OR (adjOR) Estimates and 95% CI Estimated from a Multivariable Conditional Logistic Regression Model Controlling for Age and Gender
| Number of CT scansb | Family history of cancer = Yes
|
Family history of cancer = No
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n = 117) No. (%) | Controls (n = 173) No. (%) | adjOR (95% CI) | P value for trendc | Cases (n = 87) No. (%) | Controls (n = 159) No. (%) | adjOR (95% CI) | P value for trendc | |
| 3+ | 13 (11.1) | 5 (2.9) | 3.74 (1.24, 11.28) | 0.06 | 4 (4.6) | 8 (5.0) | 0.81 (0.23, 2.92) | 0.94 |
| 1–2 | 24 (20.5) | 37 (21.4) | 1.05 (0.58, 1.91) | 15 (17.2) | 22 (13.8) | 1.14 (0.54, 2.42) | ||
| 0 | 80 (68.4) | 131 (75.7) | Ref | 68 (78.1) | 129 (81.1) | Ref | ||
P value for overall interaction effect (2 df) = 0.21; P value for family history interaction with 1–2 CT scans = 0.86 and with 3+ CT scans = 0.08.
To the head, face, neck or upper spine.
P value for significance of exposure when modeled as an ordinal variable.
DISCUSSION
The largest source of man-made radiation exposure is now medical X rays, which are being used with increasing frequency in many countries, including the U.S. Data indicate that while only 5–10% of all imaging is performed using CT, 40–67% of all exposure to medical radiation derives from this procedure (9, 10). The use of CT scans has rapidly increased in developed countries, and some forms of scans involve absorbed doses as great as 15 mSv. In the present analysis, when cumulative exposure to diagnostic X ray has been scored, exposure to CT scans to the head/face/neck/upper spine involved overwhelmingly higher doses than all other procedures combined. The higher doses and the wide prevalence of CT scans in current medical practices make it important to understand whether radiation exposure from CT scans influences the risk of cancer and gliomas specifically. If established, such connection would argue for the limited use of CT scans to those cases when they are absolutely necessary. Currently, in the U.S., it has been estimated that about one-third of CT scans are medically unnecessary (11).
Our findings suggest that an increased risk of adult gliomas may be present with cumulative exposure to three or more CT scans to the head and neck region (Table 2) and that this association is limited to the subgroup with a family history of cancer (Table 3). The interaction with family history of cancer suggests biological plausibility of these findings, because similar results have been found for breast cancer. Specifically, the association between ionizing radiation and breast cancer risk is stronger (~3-fold greater) among breast cancer patients who reported having a first- or second-degree relative diagnosed with breast cancer (12). The data from the latter study may more strongly implicate genetic susceptibility because, unlike the current results, both main and interaction effects were observed (12). Such interaction, along with other data, implies that genetic predisposition may play an important role in sensitivity to the damaging effects of low or moderate doses of radiation (13).
The present study has two limitations that deserve careful consideration: the type of control group used and the potential for information bias. The preferred controls for cancer case-control studies are population-based controls. There are two reasons why the population-based controls were not the preferred method of selection in the present study. First, brain tumors tend to be referred to highly specialized clinics for diagnostic workup and treatment. Identifying the underlying referral population for the two clinics in this project (individuals who would have been referred to these clinics if they had a brain tumor) is extremely problematic. The second reason is that response rates for population-based recruitment (such as random digit dialing) have declined over time and are frequently at less than 50%, which is well below that considered appropriate for a generalizable sample. Because these two clinics draw referrals from a wide geographical region, we elected to identify and recruit self-identified friends of patients. Given that friends may have similar socioeconomic status (SES) and that SES may influence access to diagnostic procedures, it is possible that friend cases and controls may have similar access to diagnostic radiation exposure experiences, causing overmatching by SES. When the analyses controlled for SES (using education and income as surrogate measures), the ORs comparing exposure from three or more CT scans to no CT scans increased from 1.97 (0.92, 4.23) to 2.14 (0.99, 4.66). Thus the choice of friends as a control group, if it influenced the results, is likely to have deflated the detected ORs.
A potential information bias may involve over-reporting of diagnostic X-ray procedures by patients and/or inaccuracy of reporting. Over-reporting of diagnostic X-ray procedures may arise from awareness by glioma patients of reports in the literature on this topic. These patients may be more conscious of this type of exposure and therefore may have put more individual effort into recalling their diagnostic X-ray procedures when filling out the questionnaire, and/or they may have had a tendency to exaggerate the procedures they did experience. This recall bias could inflate the estimated odds ratio. However, it is unlikely that such inflation of the estimates would be specific to over-reporting CT scans specifically. Information about dental and other head and neck X rays has been reported in the literature. Therefore, one would expect reporting biases to have affected our self-report measures for all these diagnostic procedures. The fact that we do not see an association with diagnostic procedures other than CT scans argues against such a reporting bias.
Another source for over-reporting from information bias may be the increased use of CT scans due to pre-diagnosis symptoms related to the brain tumor. One might argue that those with more CT scans were under clinical observation and more likely to have had a tumor present with symptoms that triggered these examinations. While imperfect, our questions focused the respondent on recalling CT scans experienced more than 2 years ago. Because we were unable to acquire and review these examinations, we cannot rule out the potential for some symptomatic indication of a tumor being present at the time of the scan. We stratified the data by high-grade and low-grade tumors, thinking that if symptom-related biases were present the ORs in the lower-grade tumors may be elevated. The adjusted OR for high-grade (1.75, 95% CI 0.72–4.22) and low-grade tumors (1.63, 95% CI, 0.31–8.67) were similar, providing no indication of a diagnostic bias. Had bias been overriding our observations, we would also expect to see an elevation in both those with and those without a family history of cancer. The lack of this observation also argues against this type of bias and gives some credence to the observed association with CT scans among those with family history of cancer.
For bias to explain this result one would need to argue that individuals with a family history of brain tumors are more likely to have CT scans either through physician knowledge, family pressure or being proactive as an individual. We think it is unlikely that CT scans to the head would be recommended by a physician for routine cancer screening in individuals with a family history of a tumor with such a poor prognosis. However, private whole-body scans are becoming more available for disease screening, making a family history bias possible because family history of cancer is more prevalent in the case group than in the control group. These data were collected between 2003 and 2007, before widespread use of private facilities, which may minimize this potential in these data.
It is also important to note that 8.5% of cases without friend controls (excluded from the current analysis) compared to 8.3% of cases with friend controls reported 3+ CT scans in the period 2 years prior to diagnosis. As such, the prevalence of CT-scan exposure was similar among all cases, not just those with friend controls included in this study sample. In further comparing cases included in (n = 406) and excluded from (n = 205) the analysis, we did find that there were no apparent differences by gender or age. However, there were some differences in direction that would be expected: cases in the analysis were more likely to have more education and higher incomes than those not included in the analysis. While of borderline statistical significance, there was a tendency for cases included in the analysis to have family history of cancer and to be white and non-Hispanic compared to those not included in the analysis.
We also evaluated the use of MRI 2 or more years prior to diagnosis. These data were not included in the tables because MRIs do not emit ionizing radiation. However, there was a strong correlation between the two procedures, making data on MRI procedures uninformative with respect to the exposure or potential reporting biases in these data.
While we concur with the sentiment that studies with good dosimetry will provide much-needed information (11), we believe these observational data have face validity, particularly when the number of CT scans discussed in the literature as having the potential to cause tumors is similar to that reported here as being associated with gliomas (7). We also concur with the literature that the exposures of primary concern are in the pediatric population, although this study suggests that consideration also be given to exposures in the adult population.
Epidemiological data on diagnostic X-ray exposures with respect to brain tumors (or glioma) have been inconsistent (4). There are five published case-control studies of brain tumors that have addressed diagnostic radiation exposures, specifically in the head and neck region (Table 4) (14–18). To reconcile the results of these studies and those of our study, it is important to consider two factors – the country where the study has been conducted and the study period. With different countries, comparing results is highly problematic due to differences in the health care systems and how they introduce and use radiologic diagnostic procedures. The study period is important, because it entails temporal trends in medical diagnostic procedures. These trends include the following: (a) individual exposures to diagnostic radiation declined with the introduction of the ALARA principle (as low as reasonably achievable) in the 1970s; and (b) CT scans were first used in the 1970s, but the pace at which this technology was integrated into clinical care in different regions or countries may have varied, which would further complicate the comparison of the results from different studies. These temporal trends may explain the difference in the results at least for the American studies (Table 4).
TABLE 4.
Brain Cancer Studies Including Diagnostic X-Ray Exposures to the Head and Neck
| Study | Study period | Odds ratio (95% CI where available) | Exposure lag (20 years) |
|---|---|---|---|
| Preston-Martin, 1989 (14) (U.S.) | 1980–1984 | Glioma 3.0 (1 X ray/year) Meningioma 2.5 (statistically significant trend) |
1960–1964 |
| Ryan, 1992 (15) (Australia) | 1987–1990 | No association | 1967–1970 |
| Hu, 1998 (16) (Canada) | 1989–1995 | 5.5 (1.3–32) | 1969–1970 |
| Wrensch, 2000 (17) (U.S.) | 1991–1994 | No association | 1971–1974 |
| Blettner, 2007 (18) (Germany) | 2000–2003 | Meningioma 2.3 Acoustic neuromas 6.5 |
1980–1983 |
| Current (U.S.) | 2005–2009 | Gliomas 2.0 (0.9–4.2) CT scans With family history 3.9 (1.3–1.8) |
1985–1989 |
While the latency between radiation exposure and cancer has not been well characterized in the general population, it is worth noting that an excess of solid tumors did not appear until 15 years after exposure in the atomic bomb survivor cohort (19). Assuming a 20-year lag time between exposure and development of brain tumors, the diagnostic exposures in the U.S. studies took place in different decades. Considering the known temporal trends, the exposure to diagnostic radiation reported in the study of Preston-Martin et al. (14) likely took place during the 1960s – before the move to minimize exposures. The study of Wrensch et al. (17) took place a decade later with exposures that likely reflect the 1970s movement to minimize individual exposure. Finally, the current study likely reflects exposures in the 1980s, when the use of CT scans had been widely integrated into the diagnostic practices. In this context, the results of these three studies suggest an association with general diagnostic procedures to the head and neck conducted in the 1960s (14) but not in the 1970s (17) or the 1980s (current study). The current results may be a reflection of the rise in use of procedures for CT scans consistent with more recent clinical practice.
In previous studies of medical radiation-exposed cohorts, the time between exposure and disease varied by brain tumor subtype. The risk of gliomas seems to increase within 10 years after exposure, and the risk of meningiomas seems to increase within 20 years after exposure (20). If this is the case and the results observed here reflect what is occurring at the population level, we may expect to see an increase in meningiomas associated with the use of CT scans in the next decade. It is important to note that the relative risks associated with high levels of radiation exposures for meningiomas (7.0) are much higher than those for gliomas (2.0), but the risks for exposures at low levels are currently unknown (4).
In summary, this study suggests that exposure to multiple CT scans to the head and neck region may increase the risk of adult gliomas, particularly in individuals who may be more susceptible to a brain tumor through their family history. This is consistent with work showing that organ doses for radiation from two or three typical CT scans are in the range of exposure where evidence of an association with cancer has been observed (7). Because of the potential for symptom and recall biases inherent in case-control studies, this result needs to be confirmed in prospective studies of adults. Consideration should also be given to assessing the risk of meningiomas that may be more sensitive to diagnostic procedures involving the brain in adults. Studies in the pediatric population, a group that is more sensitive to radiation exposures, are ongoing and planned (11). It is hoped that these observational data will enhance appropriate decisions regarding the use of CT scans involving the head and neck and increase awareness about potential risks associated with unnecessary examinations for all age groups.
Acknowledgments
This work was supported by the following NIH grants: NINDS Grant 5P50 NS20023, NCI SPORE Grant 5P50 CA108786, and NCI Merit Award R37 CA 011898. Funding was also received from the American Brain Tumor Association.
References
- 1.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163:424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 2.Preston DL, Ron E, Yonehara S, Kobuke T, Fujii H, Kishikawa M, Tokunaga M, Tokuoka S, Mabuchi K. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94:1555–1563. doi: 10.1093/jnci/94.20.1555. [DOI] [PubMed] [Google Scholar]
- 3.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il’yasova D, Kruchko C, McCarthy BJ, Rajaraman P, Buffler PA. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadetzki S. Association between exposure to ionizing radiation and gliomas. In: Mehta MP, Chang SM, Vogelbaum MA, Guha A, editors. Principles and Practice of Neuro-Oncology: A Multidisciplinary Approach. Demos Medical Publishers; New York: in press. [Google Scholar]
- 5.Ron E, Modan B, Boice JD, Jr, Alfandary E, Stovall M, Chetrit A, Katz L. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ, Brenner D. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 8.Rankin KM, Rauscher GH, McCarthy B, Erdal S, Lada P, Il’yasova D, Davis F. Comparing the reliability of responses to telephone-administered versus self-administered Web-based surveys in a case-control study of adult malignant brain cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2639–2646. doi: 10.1158/1055-9965.EPI-08-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semelka RC, Armao DM, Elias J, Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging. 2007;25:900–909. doi: 10.1002/jmri.20895. [DOI] [PubMed] [Google Scholar]
- 10.Hart D, Wall BF. UK population dose from medical X-ray examinations. Eur J Radiol. 2004;50:285–291. doi: 10.1016/S0720-048X(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 11.Hall EJ, Metting N, Puskin J, Ron E. Low dose radiation epidemiology: what can it tell us? Radiat Res. 2009;172:134–138. doi: 10.1667/RR1777.1. [DOI] [PubMed] [Google Scholar]
- 12.Ronckers CM, Doody MM, Lonstein JE, Stovall M, Land CE. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:605–613. doi: 10.1158/1055-9965.EPI-07-2628. [DOI] [PubMed] [Google Scholar]
- 13.Chistiakov DA, Voronova NV, Chistiakov PA. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809–824. doi: 10.1080/02841860801885969. [DOI] [PubMed] [Google Scholar]
- 14.Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49:6137–6143. [PubMed] [Google Scholar]
- 15.Ryan P, Lee MW, North B, McMichael AJ. Amalgam fillings, diagnostic dental x-rays and tumours of the brain and meninges. Eur J Cancer B Oral Oncol. 1992;28B:91–95. doi: 10.1016/0964-1955(92)90034-x. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Johnson KC, Mao Y, Guo L, Zhao X, Jia X, Bi D, Huang G, Liu R. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev. 1998;22:100–108. doi: 10.1046/j.1525-1500.1998.cdoa22.x. [DOI] [PubMed] [Google Scholar]
- 17.Wrensch M, Miike R, Lee M, Neuhaus J. Are prior head injuries or diagnostic X-rays associated with glioma in adults? The effects of control selection bias. Neuroepidemiology. 2000;19:234–244. doi: 10.1159/000026261. [DOI] [PubMed] [Google Scholar]
- 18.Blettner M, Schlehofer B, Samkange-Zeeb F, Berg G, Schlaefer K, Schuz J. Medical exposure to ionising radiation and the risk of brain tumours: Interphone study group, Germany. Eur J Cancer. 2007;43:1990–1998. doi: 10.1016/j.ejca.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Boice JD, Lan CE, Preston DL. Ionizing radiation. In: Schottenfeld D, Fraumeni J Jr, editors. Cancer Epidemiology and Prevention. 2. Oxford University Press; New York: 1996. pp. 319–354. [Google Scholar]
- 20.Davis F, Tavelin B, Grutsch J, Malmer B. Second primary tumors following a diagnosis of meningioma in Sweden, 1958–1997. Neuroepidemiology. 2007;29:101–106. doi: 10.1159/000109823. [DOI] [PubMed] [Google Scholar]


