Abstract
A conjunctive variable-interval differential-reinforcement-of-low-rate (VI-DRL, n= 18) responding schedule and a stop-signal task (n= 18) were used to evaluate the disinhibiting effects of nicotine on response withholding in rats. Sucrose solution was used to reinforce responding, and after a stable baseline was achieved under saline-administration conditions, 0.3 mg/kg nicotine was delivered before each session. Experiment 1 showed that repeated, but not the initial, administration of nicotine decreased performance on both tasks, and the effect of sensitization followed a similar timeline; 10 consecutive doses resulted in poorer proportion-correct VI-DRL trials and percent correct stop trials than the initial dose of nicotine. Furthermore, sensitization to 0.3 mg/kg nicotine decreased performance regardless of whether a spaced or consecutive-dosing regimen was followed. Experiment 2 was designed to test whether mecamylamine hydrochloride (0.1–1.0 mg/kg) could attenuate the effects of repeated 0.3 mg/kg nicotine administration, and the degree to which mecamylamine attenuation of the effect of nicotine to produce impulsive action was relative to dose. Results from experiment 2 showed that response disinhibition, as evaluated using the VI-DRL and stop-signal tasks, is related in a systematic manner to nicotinic-acetylcholine receptor activation.
Keywords: differential-reinforcement-of-low-rate, impulsivity, impulsive action, inhibition, mecamylamine, nicotine, rat, response disinhibition, sensitization, stop-signal
Introduction
The empirical examination of psychomotor-stimulant sensitization (Robinson and Berridge, 2001) has been fueled by the potential implications for understanding and treating substance-dependence disorders, and earlier investigations using a self-administration paradigm have underscored the relevance of sensitization to continued drug taking (Horger et al., 1990; Schenk and Partridge, 1997; Schenk and Davidson, 1998). The primary objective of this study was to evaluate further whether impulsivity is among the constellation of behaviors that are subjected to a sensitizing effect of psychomotor-stimulant drugs (Balcells-Olivero et al., 1997; Dallery and Locey, 2005; Kirshenbaum et al., 2008, 2009). Different behavioral tasks have been used to determine the degree to which nicotine produces dose-regimen-dependent changes in response to disinhibition (also called ‘impulsive action’, Uslaner and Robinson, 2006), and two of these behavioral tasks are described below.
Differential-reinforcement of low-rate schedule
Differential-reinforcement-of-low-rate (DRL) schedules are used to maintain a low rate of responding because a reinforcer is provided only when a response is made after a specified, predetermined time interval (X) has elapsed. DRL schedules are also referred to as interresponse time (IRT) greater than X-s schedules and the principal performance variable obtained from DRL schedule responding is the proportion of total trials within a session that are reinforced (Bostwick, 1977; Sokolowski and Seiden, 1999; Dekeyne et al., 2002). The presession administration of psychomotor-stimulants produces poor performance and subjects fail to meet the IRT requirement imposed by the DRL-schedule criterion (Schuster and Zimmerman, 1961; Segal, 1962; Zimmerman and Schuster, 1962; Morrison, 1968; Pradhan and Dutta, 1970; Sanger, 1978; Balcells-Olivero et al., 1997; McClure and McMillan, 1997; Popke et al., 2000; Wiley et al., 2000; Saulsgiver et al., 2007; Kirshenbaum et al., 2008, 2009).
Kirshenbaum et al. (2008, 2009) showed that repeated, consecutive, once-daily nicotine administrations (0.3mg/kg) produced a sequential disruption of DRL 29.5-s schedule performance. These deteriorations in performance on the DRL 29.5-s schedule were taken as support of sensitization of response disinhibition. However, the acute, presession administration of nicotine and other stimulant drugs leads to a reliable decrement in the rate or frequency of reinforcing events within a session. This finding is an even greater problem for investigations involving repeated drug dosing (e.g. Kirshenbaum et al., 2008) than experiments in which the acute effects of a single administration are evaluated (e.g. Popke et al., 2000). That is, as performance worsens across repeated drug testing, fewer reinforcers are obtained, such that these reduced levels of reinforcement could contribute to a performance decrement. Thus, the effect of repeated nicotine dosing reported by Kirshenbaum et al. (2008, 2009) could have been due to diminishing reinforcer density rather than an effect of drug sensitization, or perhaps an interaction of these two variables. The series of investigations presented here represents an attempt to eliminate the rate-of-reinforcement confound inherent in DRL-schedule evaluations, by standardizing the rate of reinforcement across nicotine-dosing regimens using a procedure similar to that reported by Sagvolden and Berger (1996).
Stop-signal task
The stop-signal task (SST, also called the stop-signal reaction-time task; Logan and Cowan, 1984; Logan, 1994; Eagle and Robbins, 2003; Winstanley et al., 2006) has been developed as a means of assessing response disinhibition, and a major advantage of this task, in comparison with the DRL schedule, is that the SST does not involve timing behavior. A reinforcer is provided after a subject has made two successive responses (a left-right response sequence on two different manipulanda), and on some occasions, to receive a reinforcer, the subject is required to withhold the second response after a ‘stop signal’ is presented. The stop signal is presented at some point in the interval between the first and second response, and response disinhibition is measured by the proportion of stop-signal failures proportionate to the total number of stop-signal trials. The effects of presession, acute nicotine administration on SST task performance have neither been characterized previously in rodents, nor there is evidence in the literature relevant to the effects of repeated drug administration on SST-task performance.
There is a consensus in the literature that repeated, intermittent dosing of psychomotor stimulants may lead to sensitization from response disinhibition (Balcells-Olivero et al., 1997) whereas repeated, once-daily consecutive dosing leads to tolerance (Schuster and Zimmerman, 1961; Zimmerman and Schuster, 1962; Pearle and Sieden, 1976). This study (experiment 1) was designed to evaluate the effect of repeated nicotine administration on DRL schedule and SST-task performance when both intermittent and consecutive dosing regimens were used. In place of a traditional DRL schedule, a conjunctive variable-interval (VI)-DRL schedule was used because the VI portion helped to standardize the reinforcement rate across dosing days. Experiment 2 involved the use of the noncompetitive nicotinic α2–α6 antagonist mecamylamine (Shytle et al., 2002; Bacher et al., 2009) to investigate whether nicotine-induced response disinhibition is mediated through acetylcholinergic neural pathways. Mecamylamine has been shown to inhibit the activation of nicotinic acetylcholine receptors (nAchRs) by nicotine (Varanda et al., 1985) and thereby block many of the effects of nicotine on behavior. For instance, in rats, mecamylamine (0.25–5.0 mg/kg) has been shown to reduce intravenous self-administration of nicotine (DeNoble and Mele, 2006), to enhance preference for high-concentration nicotine (32 μg/kg) and reduce preference for low-concentration nicotine (4 μg/kg; Glick et al., 1996), to attenuate nicotine-induced locomotor activity (Coolon and Cain, 2009), to induce conditioned place aversion associated with nicotine withdrawal (Jackson et al., 2009), and to precipitate other overt signs of nicotine withdrawal (O’Dell and Koob, 2007). Thus, experiment 2 was designed to extend these antagonist studies using mecamylamine and nicotine to encompass response disinhibition.
Methods
Experiment 1
Subjects
Twenty male, experimentally naive, Sprague–Dawley rats (Rattus Norvegicus), approximately 65 days old at the beginning of the experiment were used. The rats were obtained from Charles River Laboratories (Montreal, Quebec). Twelve rats were trained on the conjunctive VI-DRL schedule (six in each group: spaced or consecutive dosing) and eight rats were trained on the SST (four in each group: spaced or consecutive). All rats were housed in groups of two or three, and each was fed a restricted diet (18 g per day per rat) of rat chow to maintain initial body mass. The rats were housed in a room with a 12 : 12-light : dark cycle. A 12 × 6-inch polyvinyl chloride tube and Nylabone chew also were placed in the home 24″ × 30″ × 14″ opaque plastic home cages and all care and experimental procedures were approved by the Saint Michael’s College Institutional Animal Care and Use Committee.
Apparatus
Behavioral assessment was recorded by four identical operant test chambers [MED Associates, Inc., St Albans, variable-time (VT), model number ENV-007]. Chambers were housed in polyvinyl chloride sound-attenuating boxes with built-in fans for ventilation. Each chamber contained a houselight located on the center of the back wall, a reinforcer delivery well in the center of the front wall, and two tricolor nose-pokes on either side. Each aperture consisted of an infrared photobeam interrupt used to detect responses in the two tricolored nose pokes (MED Associates, model number ENV-114M) and head-entry responses in the dipper well (model number ENV-254). The reinforcer delivery well consisted of a liquid dipper (ENV-202M) for sucrose-solution delivery (26.62% solution). The dipper arm contained a cup that delivered 0.03 ml of solution per reinforcing event. All four chambers were operated simultaneously during experimental sessions and were interfaced with a PC. Data interpretation was done using MED-PC IV software (MED Associates, Saint Albans, Vermont, model SOF-735).
Conjunctive variable-interval, differential-reinforcement- of-low-rate schedule
DRL-schedule training
All rats were placed on a VT 15-s schedule for two consecutive daily sessions of 15 min. During these VT-schedule sessions, the houselight was illuminated and sucrose solution was available for 5 s after a consumption response to feed from the dipper; the consumption response was monitored through the photo-beam interrupt in the dipper well. On day 3 of training, nose poking was shaped on a fixed-ratio (FR) 1 schedule with sucrose reinforcement. A trial began when the right nose-poke green light emitting diode (LED) illuminated. After a rat responded in the right nose poke, the LED switched to red and the dipper arm delivered the sucrose reinforcer. In addition, the rats had 5 s after the head-entry was detected in the dipper well to consume the reinforcer. Once each rat had earned 50 reinforcers within 30min (typically after two daily 30-min sessions), DRL-schedule shaping began.
The DRL-schedule shaping procedure used an FR 2 schedule with an imposed delay such that a response on the nose poke initiated a timer. On day 1 of DRL-schedule shaping, the interresponse timer was set to 0.5 s; therefore, a nose-poke response was made, 0.5 s elapsed, and a second response resulted in the reinforcer delivery. If rats failed to wait 0.5 s, no reinforcer was provided, and the right nose-poke green LED and the houselight were turned off for 10 s. For every five reinforcing events, the interresponse timer increased by 0.5 s to shape, waiting 29.5 s between responses. The sessions lasted 80 min, and each daily session began with the previous session’s ending IRT value. The DRL-schedule shaping program was used for 17 consecutive sessions until all rats were earning reinforcers reliably when the IRT was 29.5 s. At this point, a static DRL 29.5-s schedule was used, and sessions were shortened to 60 min each.
Introduction of the VI schedule
The VI schedule was introduced after 10 daily 60-min sessions of responding on the static DRL 29.5-s schedule. Fleshler and Hoffman (1962) calculations were used to generate VI values. A VI of 120-s schedule was added to the beginning of the DRL, such that the green LED was illuminated in the right nose poke (the same operant described above for the DRL schedule), and a response on that nose poke initiated the VI. Once the VI timer elapsed, a response was required to initiate the DRL 29.5 s portion. Responses before the VI timer elapsing had no programmed consequences. The green LED remained illuminated until the delivery of the reinforcer; thus, the VI and DRL-schedule portions were not differentiated by a stimulus cue or a change in stimulus conditions. If the rat waited 29.5 s between responses in the DRL component, then again the red LED was illuminated in the nose poke to signal the availability of reinforcement in the dipper well. If the rat failed to wait 29.5 s between responses on the DRL portion of the schedule, then the houselight was terminated for 10 s. Thus, responses before the VI timer elapsing produced no programmed consequences; however, responses before the DRL criterion produced this 10-s timeout period. Rats returned to the VI-schedule portion of the schedule regardless of whether the reinforcer was delivered or not.
An auto-adjusting VI schedule was used in this design to equate reinforcement rate across conditions of varying performance. Poorer performance on the DRL schedule is typically evidenced by responses occurring before the IRT criterion has elapsed, and this performance would yield a lower reinforcement rate. The auto-adjusting VI schedule shortened or lengthened by a mean value of 40 s; therefore, if performance was poor, the VI-schedule value shortened automatically within the session from a mean of 120 s to a mean of 80 s. Theoretically, the shorter VI-schedule duration would provide more opportunities to obtain reinforcers within a 60-min session than would a longer VI-schedule value. Conversely, better DRL-schedule performance would be accompanied by an adjusted increase in the VI-schedule value, allowing for fewer opportunities to earn reinforcers.
The primary purpose of the auto-adjusting VI-schedule portion of the schedule, therefore, was to equate reinforcement rate across drug-dosing conditions. On the basis of preliminary pilot investigation, it was determined that rats could earn four reinforcers per 60-min session under conditions expected to produce the poorest DRL-schedule performance (i.e. after 10 or more doses of 0.3 mg/kg nicotine). The 60-min conjunctive VI-DRL sessions were divided into four blocks of 15 min each. The VI-schedule value was adjusted based on whether rats had earned fewer than or more than one reinforcer per 15-min block. If the rat earned two or more reinforcers during the first 15-min block, the VI-schedule value increased by a mean of 40 s for the next 15-min block. Alternatively, if a rat failed to earn one reinforcer within the first block, the VI-schedule value was shortened by 40 s. Thus, poor performance would be reflected in a decrease in the mean VI-schedule value and a fewer percentage of trials ended with a reinforcer within a session. Each session began with the previous session’s ending mean VI-schedule value.
Stop-signal task
Head-entry training
The method of training used in this study was modified and adapted from the study by Eagle and Robbins (2003). In the first phase of SST training, experimentally naive rats were shaped to respond to an FR 1 schedule in which a single head-entry response into the dipper-well aperture resulted in the presentation of a sucrose-solution reward. The beginning of a trial was signaled by the illumination of a center stimulus light (ENV-221M) directly above the dipper well, which remained illuminated until the rat made a head-entry response to the dipper aperture. A head-entry response was followed by the delivery of 0.03 ml of sucrose solution, and the dipper remained available for 5 s to provide the rat with the opportunity to consume the sucrose. The head-entry training was conducted for two consecutive days, 30 min per session or until the rat received 50 reinforcers across two consecutive daily sessions.
Left nose poke training
In this phase, rats were trained on an FR 1 schedule in which one response on the left nose poke was required to receive reinforcement. A trial began with the illumination of the red LED within the left nose poke. A left nose poke was followed immediately by the activation of the dipper arm and the stimulus light above the dipper well, and the deactivation of the red nose-poke LED. The stimulus light and dipper arm remained available for 5 s after the rat made a head-entry response into the dipper well to retrieve the reinforcer. Responses on the right nose poke were not followed by scheduled consequences. Rats experienced one daily session that was limited by either 25 reinforcers or 30min, whichever occurred first. Left nose-poke training lasted until 25 reinforcers were earned across two consecutive daily sessions; this training typically lasted two consecutive days.
Go-trial training
The ‘go’ trial was a trial in which a left–right nose-poke response sequence (FR 2) was required to receive reinforcement. Illumination of the left nose-poke red LED signaled the beginning of a trial and it remained illuminated for a maximum of 60 s. If the rat failed to make the initial left nose poke within 60 s, the red LED was terminated for a 10-s period and the event was recorded as a left-omission error. If a left nose poke was made within the 60-s period, the left nose-poke LED was extinguished and, simultaneously, the right nose-poke red LED was illuminated. In addition, the right LED remained illuminated for 60 s and a right nose poke was immediately followed by the activation of the dipper arm and the stimulus light above the dipper well, and the deactivation of the right nose-poke LED. The stimulus light and dipper arm remained available for 5 s after the rat made a head-entry response into the dipper well to retrieve the reinforcer. The elapsed time between the left and right nose-poke responses was measured as the ‘go trial’ signal-reaction time and over the course of each session, these reaction times were averaged together to create a mean go-reaction time (mRT) that was used as a dependent variable. Initially, the right nose poke remained illuminated for 60 s; if rats failed to make a right nose-poke response during this 60-s limited-hold (LHgo) period, a 10-s blackout period ensued and a new trial was initiated. If the rat made the left response, but failed to respond on the right nose poke during the LHgo period, an omission-right was recorded.
Each go-trial session was 60 min long with no limit on the number of reinforcers earned. Initially during the 60-min session, the LHgo for each rat was 60 s. The LHgo was shortened to 2.5 s after eight consecutive sessions because all rats were responding with a mRTof less than 2.5 s. After another four 60-min daily sessions, the LHgo was further reduced to 1.5 s because all rats were responding with a mRT of less than 1.5 s. The LHgo remained at 1.5 s for the remainder of experimentation, and the go-trial training procedure lasted for another eight consecutive sessions.
Stop-trial training
‘Stop’ trials were introduced to the go-trial sequence. Stop trials were trials in which a tone (40 ms, 4000 Hz, 75 dB) was presented (Med-Associates model number ENV-223) immediately on detection of a response on the left nose poke. For a rat to earn reinforcement on a stop trial, it must omit its right nose-poke response for 2.5 s; thus, the tone served as a stop signal for the left–right behavioral sequence. On a successful stop trial, a left response was made, the tone was presented, and then no right response occurred for 2.5 s. A reinforcer was delivered only if the right responses did not occur within the 2.5 s. Once the 2.5 s elapsed, the dipper was activated, the stimulus light above the dipper well was illuminated, and the right nose-poke LED was simultaneously deactivated. The stimulus light and dipper arm remained available for 5 s after the rat made a head-entry response into the dipper well to retrieve the sucrose reinforcer. Stop trials were interspersed across 60-min go-trial sessions, and occurred in approximately 20% of trials. The presentation of stop or go-trials was pseudorandomly determined by the computer program, which would sample (without replacement) from a binary array of 10 characters at the beginning of each trial to determine whether it was to be a stop or go trial. If a rat completed the left–right response sequence after the presentation of the tone, the trial was recorded as an error in percent correct inhibition and a 10-s blackout ensued.
Stop-task testing
After six consecutive 60-min daily sessions of stop-trial training, each session was divided into four blocks of 15 min each. The first block operated in a manner that mirrored stop-trial training in which the tone was presented immediately after the left nose-poke response. The delay between the left nose-poke response and the presentation of the tone was titled the stop-signal delay (SSD), and the SSD was altered for the three remaining blocks of the daily session. The SSD for each block was catered to each rat such that the three remaining blocks included SSDs that were 30, 60, and 90% of the rat’s mRT for the first block (in which the tone was presented immediately on completion of the left nose poke, and the SSD for this initial block would be ‘zero’). Therefore, for the 90% SSD block, the tone on stop trials would be presented in closest temporal proximity to the right nose-poke response compared with other SSD blocks; in other words, if the rat’s mRT=1 s, then on stop trials for the 90% SSD block, the tone would be presented 0.9ms after the left nose-poke response was made. According to Logan (1994), the probability that rats should fail to inhibit the left–right response sequence is greatest at longer delayed SSDs.
All 60-min sessions began with the zero SSD block to obtain the mean go-reaction times (mRTs) per rat per day. The order of the three successive blocks (SSDs: 30, 60, and 90%) was counterbalanced across daily sessions so that each SSD had to occupy a different position in the block sequence on each of three consecutive days.
Drug dosing
All rats (conjunctive VI-DRL schedule and SST groups) experienced 10 days in which 1 ml/kg physiological saline (0.9% solution) was administered subcutaneously immediately before each session. Nicotine was administered in 0.3 mg/kg doses, dissolved as a base saline solution, from nicotine ditartrate dihydrate (162.21g per mole, Sigma Chemical Co., St Louis, Missouri, USA); this concentration yielded doses of 1 ml/kg. Immediately after all injections, each rat was placed into the respective operant chamber for a 5-min blackout period.
Rats in each study were placed on two different dosing regimens: (i) spaced or (ii) consecutive dosing. All rats (n=20) received the same number of nicotine injections; rats maintained on the conjunctive VI-DRL schedule received 10 doses and rats trained on the SST received 14 doses. Rats in the spaced-dosing group (n=6 VI-DRL, and n=4 SST) were administered nicotine once every 3 days, such that a dose of nicotine before one daily session was followed by two daily sessions in which only saline was administered. Rats in the consecutive dosing group (n=6 VI-DRL, and n=4 SST) received a dose of nicotine before each consecutive daily session.
Experiment 2
Subjects
Sixteen male, experimentally naive, Sprague–Dawley rats (Rattus Norvegicus), approximately 65 days old at the beginning of the experiment were used. Six rats were trained on the conjunctive VI-DRL schedule and 10 rats were trained on the SST. Housing and diet restriction protocol mirrored that of experiment 1.
Conjunctive variable-interval, differential-reinforcement- of-low-rate schedule
The procedures for training the rats on the conjunctive VI-DRL schedule followed that of experiment 1 with two exceptions; (i) the daily sessions for experiment 2 were 20min rather than 60 min in duration, and (ii) a VI of 120-s schedule component was used in place of the auto-adjusting VI-schedule component. The static VI 120-s schedule was used instead of the auto-adjusting procedure because the session durations were not long enough for the auto-adjusting VI schedule to make accommodations based on DRL-schedule performance. The justification for reducing the session lengths comes from preliminary experimentation with mecamylamine and nicotine, in which it was discovered that across the range of doses used, the effects of mecamylamine were most prominent in the first 20–30 min.
Stop-signal task
The SST training and testing procedures for experiment 2 were identical to the procedures established for experiment 1, with the exception that rats experienced 30-min, rather than 60-min daily experimental sessions. These 30-min sessions were divided into two blocks of 15 min each; in the first block, rats experienced the 0% SDD followed by the 60% SSD in the second block. The design of experiment 2 used the 0% SSD to obtain the mRT per rat per day, and the 60% SSD because effects of dose regimen in experiment 1 were not apparent on the 30 or 90% SSD blocks.
Drug dosing: mecamylamine
Drug dosing for both groups (VI-DRL and SST) followed the same schedule, as shown in Table 1. All rats (n=16) were administered saline for 5 days to establish a baseline and then given 10 consecutive daily doses of nicotine (0.3 mg/kg, dosing concentrations equivalent to experiment 1). After the tenth daily dose of nicotine, five sessions were conducted to recover baseline. Intraperitoneal injections of mecamylamine hydrochloride were used for the series of antagonist evaluation (dissolved as base, 167.29 g per mole, Sigma Aldrich, St Louis, Missouri, USA). Once the dose of mecamylamine was administered, rats were placed back into home cages for 10 min before receiving a 0.3 mg/kg nicotine dose, delivered subcutaneously. Immediately after the nicotine dose, all rats were placed into the operant chambers for a 5-min presession blackout. Rats were randomly assigned for a different dose of mecamylamine (doses ranging from 0.1 to 1.0 mg/kg) on any given experimental day, such that the day-to-day sequence of doses was counterbalanced across subjects and across daily sessions. Each rat received each dose combination of mecamylamine twice. Furthermore, each daily mecamylamine/nicotine session was followed by 2 days of saline/saline (saline delivered intraperitoneally 10 min before a second injection of saline). On two occasions, intermixed within the mecamylamine/nicotine sessions, nicotine sensitization was re-evaluated with a saline/nicotine coadministration. In addition, on two occasions, intermixed within the mecamylamine/nicotine sessions, 1.0 mecamylamine/saline was administered to assess the effect of mecamylamine only on responding.
Table 1.
Description of the design of experiment 2
| Subject | Phase
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Establish baseline | Sensitization | Recover baseline | Antagonist evaluations (all doses in mg/kg) | ||||||||||
| 1 | Five sessions, saline dosing | Ten sessions, consecutive doses of 0.3 mg/kg nicotine | Five sessions, saline dosing | 0.1 meca/0.3 nic | Two sessions, saline | 0.5 meca/0.3 nic | Two sessions, saline | 1.0 meca/0.3 nic | Two sessions, saline | 1.0 meca/saline | Two sessions, saline | Saline/0.3 nic | Two sessions, saline |
| 2 | 0.5 meca/0.3 nic | 1.0 meca/0.3 nic | 1.0 meca/saline | Saline/0.3 nic | 0.1 meca/0.3 nic | ||||||||
| 3 | 1.0 meca/0.3 nic | 1.0 meca/saline | Saline/0.3 nic | 0.1 meca/0.3 nic | 0.5 meca/0.3 nic | ||||||||
| 4 | Saline/0.3 nic | 0.1 meca/0.3 nic | 0.5 meca/0.3 nic | 1.0 meca/0.3 nic | 1.0 meca/saline | ||||||||
Each drug-dose combination during the antagonist-evaluation phase was delivered on two occasions; therefore, the five drug combinations were repeated (not listed on the table).
meca, mecamylamine; nic, nicotine.
Data analysis
Conjunctive VI-DRL schedule
Mixed-design analyses of variance (ANOVAs) were used to evaluate the effects of dose regimen on general DRL-schedule performance variables, including: (i) proportion-correct trials (number of reinforcing events divided by the number of total trials), (ii) mean VI value, (iii) absolute number of reinforcers delivered per session, and (iv) response rate (per minute) on the active nose-hole poke, see Figure 1. Given that proportion-correct trials were based on a total number of trials that was free to vary from session-to-session, these data were arcsine transformed to improve homogeneity of variance for the parametric analyses (Kirshenbaum et al., 2008, 2009); but the raw data are depicted in Figure 1.
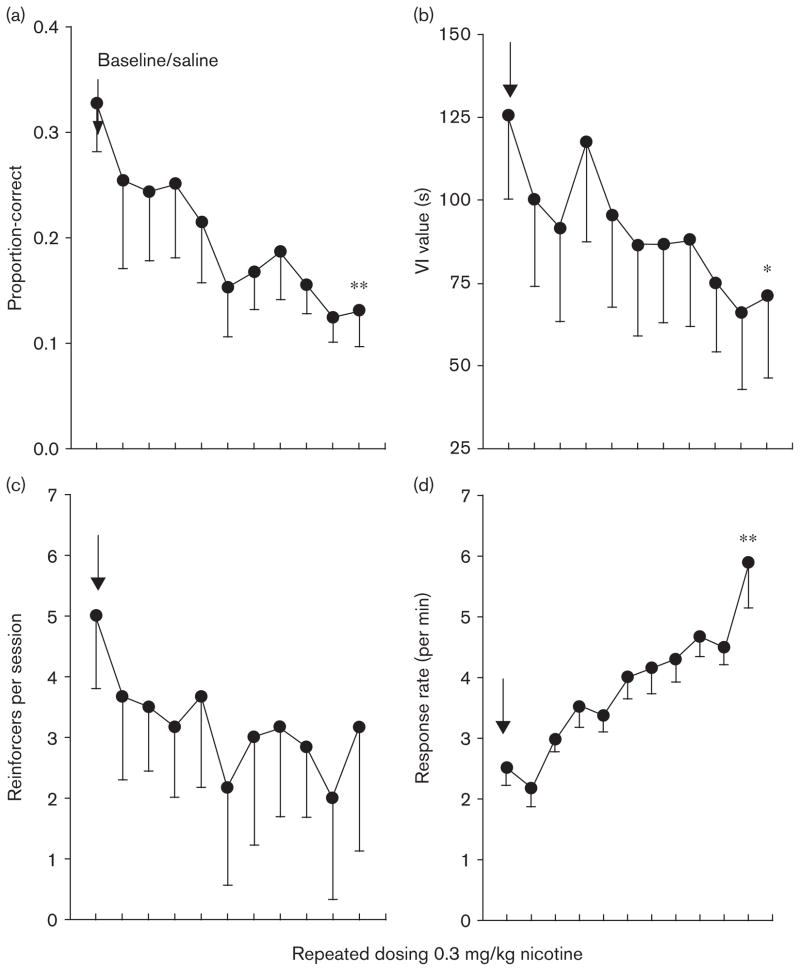
Fig. 1.
Results from the variable-interval differential-reinforcement-of-low-rate (VI-DRL) evaluation, experiment 1. All figures represent means and standard errors, and the arrow indicates the mean from the collapsed baseline under saline-administration conditions. Repeated 0.3 mg/kg nicotine dosing resulted in significant changes to proportion-correct trials, mean VI value, and response rate. The adjusting VI portion of the conjunctive VI-DRL schedule prevented significant deterioration in the number of reinforcing events per session across dosing days (c). *P<0.05, **P<0.01, compared to baseline/saline.
To determine when to initiate nicotine dosing, stability in conjunctive VI-DRL-schedule responding was evaluated across five consecutive baseline/saline sessions using a repeated-measures multivariate ANOVA (MANOVA). The analysis was performed using all the general performance variables outlined above.
The distribution of IRTs is an important measure of DRL-schedule performance because it allows for a more detailed understanding of how performance is altered by the presence of nicotine. Naturally, percent correct trials and the IRT distribution should correspond with one another such that a higher proportion of short IRTs (or IRTs emitted before the schedule criterion of 29.5 s) should also result in fewer percent of total trials that are reinforced. However, the percent correct trials variable does not provide information about how exactly the presence of a drug interferes with accurate DRL-schedule performance. Therefore, an IRT-distribution analysis is warranted, and a cumulative IRT frequency distribution (McClure and McMillan, 1997; Sanabria and Killeen, 2008; Kirshenbaum et al., 2008, 2009) was used in this study. For a description of how the cumulative IRT frequency distributions were constructed the studies by Kirshenbaum et al. (2008, 2009) are suggested. Cumulative frequency distributions were used rather than relative or probability IRT distributions because drug effects are more easily compared with baseline/saline performance when the distribution is expressed in a cumulative manner.
To analyze differences across the baseline/saline and nicotine-dose regimens, a nonlinear regression analysis was performed on each rat’s cumulative IRT frequency distribution, using means obtained across the last 3 days of baseline, the first three doses of nicotine, and the last three doses of nicotine (doses 8, 9, and 10). This portion of the analysis provided information about how nicotine and the dose regimen altered the distribution of IRTs. Furthermore, mean IRT distributions for each rat were obtained for both the VI-schedule and DRL-schedule portions; these data were used to determine whether the distribution of IRTs differed across schedules. A nonlinear regression was performed using a cumulative Gaussian distribution-for-proportions as the model (Kirshenbaum et al., 2008, 2009), illustrated by:
where μ and σ (the mean and standard deviation) are for the cumulative distribution function Φ(x) of the Gaussian distribution. The median IRT time bin between 0.0 s and the asymptote of the curve is represented by μ (0.5 on the y axis). Smaller μ values indicate poorer DRL-schedule performance, or shorter average IRTs. Furthermore, shorter mean IRTs are also represented as smaller values for the standard deviation (σ) in which smaller numbers yielded steeper slopes.
Prism 5 (Graphpad Software, San Diego, California, USA, 2007) was used to perform the nonlinear regression analysis, which yielded values of both free parameters (μ and σ) and a goodness-of-fit value (R2). The values of μ, σ, and R2 did not violate the homogeneity-of-variance assumption of parametric statistics (based on Mauchly’s test of sphericity), and a repeated-measures ANOVA was performed to determine whether μ, σ, and R2 values differed across conditions.
Stop-signal task
Percent inhibition, or percent correct performance on stop trials, has been the primary dependent measure used in the literature for SST performance. Other variables of interest include mRTs (the latency between the left and right response on go trials), and percent correct go-trial performance. On go trials, once the left response was made, the right nose poke was available for 2.5 s and this limited-hold procedure allowed for the examination of percent correct go performance. Furthermore, if a subject failed to initiate a trial on the left nose poke within 1.5 s, then this omission was not factored into either stop or go trials. Earlier researchers have argued that these omission trials should not be included in the percent stop-trial performance (e.g. Eagle and Robbins, 2003). Investigators have also advocated that percent correct performance at the zero SSD ought to be 100% because performance at a zero delay is not indicative of response inhibition, but rather, given that the stop signal is delivered immediately on an initiative response, it may be construed as a discriminative stimulus. Therefore, performance errors at the zero delay represent something other than a failure of inhibition. Earlier researchers (Tannock et al., 1989; Solanto et al., 2001; Eagle and Robbins, 2003; Eagle et al., 2007) have used equations to adjust percent correct stop performance for such zero-delay failures, and thus, for this analysis:
For the purposes of the mixed-design ANOVAs, the adjusted percent inhibition scores were arcsine transformed. Raw adjusted percent inhibition scores are depicted in Figures 3 and 4.
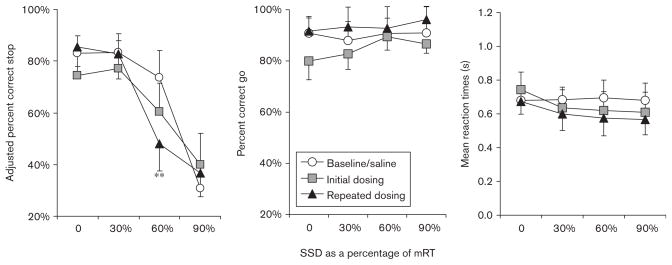
Fig. 3.
Results from the stop-signal task (SST) evaluation, experiment 1. Baseline/saline: data from saline-administration sessions, three pre and three postnicotine dosing sessions. Initial dosing: means from the first 3 days of 0.3 mg/kg nicotine dosing. Repeated dosing: final 3-day nicotine-dosing means. Repeated nicotine administration did not change in mean reaction times or mean percent correct go-trial performance. A significant disruption in percent correct stop-trial performance was noted for the 60% stop-signal delay (SSD) after consecutive dosing with 0.3 mg/kg nicotine. mRT, mean go-reaction time. **P<0.01.
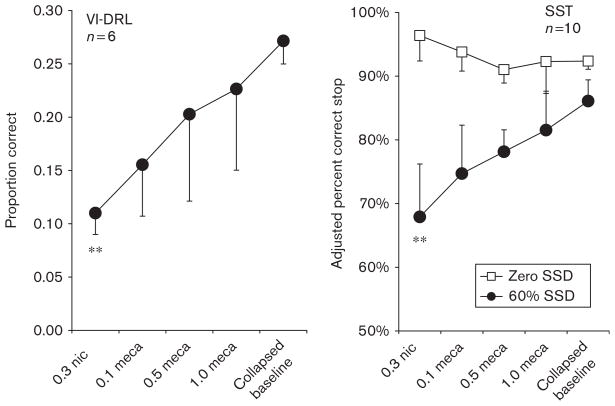
Fig. 4.
Results from experiment 2, variable-interval differential-reinforcement-of-low-rate (VI-DRL) schedule and stop-signal task. The figures depict mean proportion (VI-DRL) or percent (stop-signal task) inhibition across the range of nicotine (nic)+saline or nicotine+mecamylamine (meca) combined doses. No differences were evident between 1.0 mg/kg mecamylamine-only and saline-only conditions; therefore, data from all saline administrations and the saline+1.0 mecamylamine condition were combined to form the ‘collapsed baseline’ condition. For both tasks, the addition of mecamylamine attenuated the disinhibiting effects of 0.3 mg/kg nicotine in a dose-dependent manner. For the VI-DRL schedule, the addition of mecamylamine to nicotine also introduced greater variability. SSD, stop-signal delay. **P<0.01.
Results
Experiment 1
Conjunctive variable-interval, differential-reinforcement- of-low-rate schedule
General performance measures
Using the number of obtained reinforcers, total number of operant responses, mean VI-schedule value, and the arcsine-transformed proportions of correct responding, a MANOVA was performed to assess whether monotonic increases or decreases were significant across five sessions and they were not (P>0.1, F of approximately 1). Furthermore, a similar MANOVA was performed to compare the baseline performance before nicotine dosing to the data obtained once dosing had concluded, and baseline performance was recovered within four sessions on the termination of nicotine dosing and was (P>0.1, F of approximately 1); therefore, the data used for ‘baseline’ in the following analyses includes the data from collapsed pre and postnicotine dosing sessions.
A 2 (group: spaced vs. consecutive) × 2 (timepoint: baseline vs. tenth session of nicotine dosing) mixed-design ANOVA was performed on (i) proportion correct trials, (ii) mean VI-schedule value, (iii) absolute number of reinforcers delivered per session, and (iv) response rate (per minute) on the active nose-hole poke (Fig. 1). A significant main effect of timepoint was discovered for proportion correct trials and response rate [F(1,10)= 15.98, 33.56, respectively, P<0.05, partial η2>0.61]. Neither significant main effect of group was discovered for any variable nor there was any significant group × timepoint interaction. Therefore, no differences were discovered between intermittent/spaced dosing and consecutive dosing, and thus, the groups were collapsed for the remainder of the analyses.
A repeated-measures ANOVA across three timepoints (baseline vs. first session vs. tenth session of dosing) showed a significant main effect for proportion correct trials, operant responses, and mean VI-schedule value; F(2,22)=30.66, 32.13, 14.34, P value of less than 0.05, partial η2 greater than 0.71, 0.87, and 0.49, respectively. There was no significant effect of the number of reinforcers, although this difference approached significance (P=0.12). Least significant difference (LSD) post-hoc pairwise multiple comparisons were made on the data for all dependent measures. Post-hoc comparisons for the proportion correct trials and operant response variables showed significant differences between baseline and final-dosing conditions (P<0.001). Differences between baseline and initial dosing were only apparent for the operant response measure (P<0.05), and significant differences were found for mean VI-schedule value across all time-points/dosing conditions (P<0.05).
IRT bin analyses
The parameter values or Gaussian distribution means (μ) and standard deviations (σ) were obtained for each rat at each timepoint (baseline vs. first session vs. tenth session of dosing). In addition, the goodness-of-fit (R2) values were calculated for each curve for each rat, and these parameter and R2 values were used in a 2 (VI vs. DRL) × 3 (timepoint) repeated-measures ANOVA. No significant differences were found for either μ, σ, or R2 at any condition (P>0.05: Fig. 2). For the remainder of the analyses, the IRTs were collapsed across the VI-schedule and DRL-schedule portions.
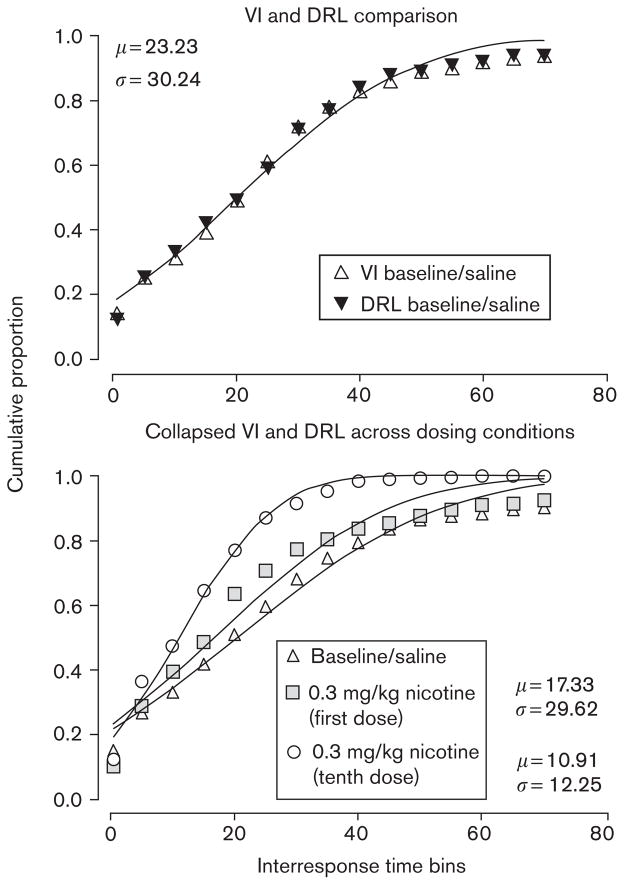
Fig. 2.
Cumulative interresponse time (IRT) distributions from the variableinterval differential-reinforcement-of-low-rate (VI-DRL) evaluation, experiment 1. The upper panel illustrates the similarity of baseline temporal-patterning across the two portions (VI and DRL) of the conjunctive schedule. The lower panel shows the effect of repeated nicotine dosing on the temporal patterning of responses; repeated 0.3 mg/kg dosing produced an increased frequency of short IRTs (a leftward shift in the distribution) compared with initial dosing.
Using the parameter values μ and σ, a repeated-measures ANOVA was again performed across three timepoints (baseline vs. first session vs. tenth session of dosing). This showed a significant main effect of both μ and σ [F(2,22)=7.61 and 3.01, respectively, P<0.05, partial η2=0.52 and 0.31]. A LSD pairwise multiple comparison was made on both parameter values: μ and σ differed significantly (P<0.05) between baseline and final dosing, but significant differences between initial dosing and baseline were not apparent for either parameter value. Furthermore, only μ differed between initial-dosing and final-dosing conditions. Further repeated-measures ANOVA comparing the goodness-of-fit (R2) of the Gaussian distribution to the available data found no significant differences across timepoints (P>0.05), suggesting that the Gaussian distribution was an appropriate model at all dosing conditions. A visual inspection of Figure 2 shows that, compared with baseline, initial dosing resulted in only a minor shift in the IRT distributions; however, the final dose of nicotine resulted in a leftward shift in the IRT distribution.
Stop-signal task
Using the adjusted percent inhibition scores, percent correct go, and mRTs for each SSD, a MANOVA was performed to assess whether stable baseline performance was achieved before nicotine dosing. No significant changes were evident across five consecutive days (P>0.1, F of approximately 1). In addition, a MANOVA was performed to compare the baseline performance before nicotine dosing to the data obtained once dosing had concluded, and baseline performance was recovered within five sessions on the termination of nicotine dosing (P>0.1, F of approximately 1); therefore, the data used for ‘baseline’ in the following analyses include the data from collapsed pre and postnicotine dosing sessions.
Given that the order of presentation of each of the three SSDs (30, 60, and 90% of mRTs) was counterbalanced across consecutive days, the data for the SST were averaged across three consecutive days for dosing timepoints; ‘initial dosing’ includes means from sessions 1 to 3, and final dosing, means from sessions 9 to 12. Using the adjusted percent inhibition means, a 3 (timepoint: collapsed baseline vs. first 3-day nicotine average vs. last 3-day nicotine average) × 2 (group: consecutive vs. spaced dosing) × 4 (SSD: 0 vs. 30 vs. 60 vs. 90%) mixed design ANOVA was performed. Overall stop-trial accuracy at all SSDs differed across all dosing conditions [F(8,20)>2.40, P=0.054, partial η2=0.30]. Given that stop-trial accuracy was expected to deteriorate at longer SSDs, this result was not surprising. Therefore, to determine whether nicotine dosing resulted in performance deteriorations at each SSD, a 3 (timepoint) × 2 (group) mixed-design ANOVA was performed using each SSD as an independent measure. Significant main effects and interactions of timepoint and group were not apparent at the 0, 30, or 90% SSDs [F(2,12) <1.77, not significant]. However, a significant main effect of timepoint was discovered for stop-trial accuracy at the 60% SSD [F(2,12)=4.98, P<0.05, partial η2=0.45; Fig. 3]. Furthermore, no significant group differences or timepoint × group interaction were discovered [F<0.845, P>0.6]. Post hoc LSD multiple-comparison tests (collapsed across groups) showed significant differences between baseline and final dosing in stop-trial accuracy at the 60% SSD (P<0.05); however, no significant differences were discovered in a comparison of initial dosing with either baseline or final dosing (P>0.05); Figure 3.
A 3 (timepoint) × 2 (group) × 4 (SSD) mixed design ANOVA was performed on percent correct go-trial performance and mRTs. Neither variable was significantly altered across timepoints, nor was there a significant difference between groups (P>0.05). Furthermore, no significant differences or interactions were discovered across the range of SSDs, (P<0.05). Visual inspection of Figure 3 shows that initial dosing may have resulted in a slight difference from baseline at the zero delay (zero SSD), but the result was not significant.
Experiment 2
Conjunctive variable-interval, differential-reinforcement- of-low-rate schedule
To evaluate whether a baseline was recovered and whether 1.0 mecamylamine/saline doses differed from saline-only dosing, three-way repeated measures MANOVAs were performed on the (i) proportion-correct and (ii) response-rate variables. The MANOVA included data from (i) the means of the first five sessions of baseline, (ii) the means from the five sessions of baseline recovery, and (iii) the means from the two sessions in which rats received the combination mecamylamine/saline dose. No significant differences were discovered for the combination of variables across the three conditions (P>0.05), so for the remainder of the analyses, the three conditions were averaged to create a ‘collapsed baseline’. Furthermore, to evaluate whether the tenth dose of nicotine resulted in significant differences from the two nicotine-delivery sessions intermixed with the antagonist sessions (see Table 1), a three-way MANOVA was performed. Significant differences across the three nicotine-dosing sessions, described above, were not apparent (P>0.05). Using (i) proportion-reinforced trials, (ii) response-rate, (iii) μ, (iv) σ (v) R2 measures, and (vi) obtained reinforcers per session, a six-way repeated-measures ANOVA was performed across the following conditions: (i) collapsed baseline, (ii) day 1 nicotine-only, (iii) day 10 nicotine-only, (iv) 0.1mg/kg mecamylamine/nicotine, (v) 0.5mg/kg mecamylamine/nicotine, and (vi) 1.0 mg/kg mecamylamine/ nicotine. For all measures, except R2 and reinforcers earned per session, a significant main effect was shown across conditions [all degrees of freedom (5,25), all P <0.05: (i) F=12.09, partial η2<0.70; (ii) F=31.90, partial η2=0.91; (iii) F=4.71, partial η2=0.31; (iv) F= 3.22, partial η2=0.27]. Table 2 provides the results from post hoc LSD multiple comparisons, and differences from the collapsed baseline (P<0.05) are indicated. For the proportion-reinforced trials measure, Figure 4 illustrates that differences were apparent between the tenth dose of nicotine and the collapsed baseline (P<0.05), but all other comparisons with the tenth dose of nicotine were not significant. The LSD comparisons also showed that the collapsed baseline differed from the 0.1 mg/kg mecamylamine/nicotine dose (P<0.05).
Table 2.
LSD multiple comparison results from experiment 2
| Task and measure | Tenth dose of 0.3 nic | 0.1 meca/0.3 nic | 0.5 meca/0.3 nic | 1.0 meca/0.3 nic | Collapsed baseline |
|---|---|---|---|---|---|
| VI-DRL (n=6) | |||||
| Response rate | 4.375** | 1.305 | 1.305 | 1.286 | 1.232 |
| 0.689 | 0.197 | 0.197 | 0.127 | 0.088 | |
| μ | 11.93** | 12.35* | 26.11 | 22.53 | 25.33 |
| 1.563 | 1.395 | 1.741 | 0.411 | 0.608 | |
| σ | 12.11** | 25.99 | 26.49 | 22.3 | 29.62 |
| 0.654 | 1.593 | 2.914 | 2.311 | 3.023 | |
| R2 | 0.980 | 0.991 | 0.982 | 0.975 | 0.991 |
| 0.024 | 0.069 | 0.035 | 0.042 | 0.023 | |
| SST (n= 10) | |||||
| Percent go | 94.80 | 93.10 | 95.73 | 94.11 | 93.48 |
| 2.59 | 3.54 | 1.88 | 4.17 | 2.71 | |
| mRT (s) | 0.981 | 0.977 | 0.976 | 1.031 | 1.090 |
| 0.069 | 0.066 | 0.063 | 0.061 | 0.063 | |
Means (bold) and standard errors (italics) are reported.
mRT, mean go-reaction time; meca, mecamylamine; nic, nicotine; SST, stop-signal task; VI-DRL, variable-interval differential-reinforcement-of-low-rate.
P < 0.05.
P < 0.01 compared with collapsed baseline. n = 16.
Stop-signal task
The following series of analyses involve only the data obtained from the 60% SSD. First, to evaluate whether a baseline was recovered and whether 1.0 mecamylamine/ saline doses differed from saline-only dosing, a three-way repeated measures ANOVA was performed on the adjusted percent inhibition scores from (i) the means of the first five sessions of baseline, (ii) the means from the five sessions of baseline recovery, and (iii) the means from the two sessions in which rats received the combination mecamylamine/saline dose. No significant differences were discovered (P>0.1). Thus, for the remainder of the analyses, the three aforementioned conditions were averaged to create a ‘collapsed baseline’ to simplify the analyses. To be consistent with the tandem VI-DRL schedule analyses, a three-way MANOVA was performed to evaluate whether the tenth dose of nicotine resulted in significant differences from the two nicotine-delivery sessions intermixed with the antagonist sessions (see Table 1). Significant differences across the three nicotine-dosing sessions, described above, were not apparent (P>0.05).
The adjusted percent inhibition scores at the 60% SSD were examined using a six-way repeated-measures ANOVA: (i) collapsed baseline, (ii) day 1 nicotine-only, (iii) day 10 nicotine-only, (iv) 0.1 mg/kg mecamylamine/ nicotine, (v) 0.5 mg/kg mecamylamine/nicotine, and (vi) 1.0 mg/kg mecamylamine/nicotine. A significant main effect of dose was discovered across all conditions [F(5,45)=4.17, P<0.01, partial η2=0.32]. The results from the post hoc LSD multiple comparisons show that differences were apparent between the tenth dose of nicotine and baseline and 0.5 mecamylamine/nicotine (P<0.05), but all other comparisons with the tenth dose of nicotine were not significant (Fig. 4). Baseline also differed significantly from the 0.1 mg/kg mecamylamine/ nicotine dose (P<0.05). No significant main effects were apparent for either mRTs or percent go variables.
Discussion
Experiment 1
Repeated, but not initial, dosing with 0.3 mg/kg nicotine resulted in task-performance deteriorations on both the conjunctive VI-DRL schedule and SST; therefore, a history of nicotine exposure is necessary for nicotine administration to result in a significant disruption of task performance that included inhibition. The gradual and sequential disruption created by repeated nicotine administration occurred within the same time frame (10–12 dosing sessions) on both tasks, which suggests that the emergence of sensitization is due to a gradual change in the neurophysiological response to nicotine. There were no significant differences between dosing regimens (repeated-consecutive versus repeated-spaced dosing) on either task; thus, the overall number of nicotine doses seems to be a more robust determinant of sensitization than does the method of spacing the doses in a temporal sequence. However, further parametric study is needed to make more certain claims about the factors that lead to sensitization of response disinhibition. One can assert, from the available data, that spacing doses by 2 days does not encumber the induction of sensitization to nicotine.
The addition of the adjustable VI portion to the DRL 29.5-s schedule successfully equated reinforcement rate across dosing conditions. Nicotine administration resulted in higher response rates, shorter VI-schedule values, shorter median IRTs, and a lower proportion of reinforced trials. These results are congruent with earlier findings (Kirshenbaum et al., 2008) showing sensitization to nicotine on the DRL 29.5-s schedule, and extends those findings by illustrating that reinforcer rate is not a factor governing sensitization. That is, the performance worsening effects of repeated nicotine administration on DRL-schedule responding were observed in this research even when reinforcement rate was held constant by adding the adjustable VI-schedule portion to the DRL 29.5-s schedule. The addition of the VI kept reinforcement rate between 2 and 5 mean-reinforcing events per session. This is a low rate, so the possibility exists that there was a floor effect following repeated dosing that prevented rates of reinforcement from further or a more significant decline.
For the SST, mRTs and go-trial performance failed to differ as a result of repeated nicotine dosing, but stop-trial accuracy was sensitive to repeated dosing. The finding that nicotine delivery failed to alter stop-performance at all SST SSDs is difficult to interpret. Differences in stop-performance due to repeated dosing were apparent only at the 60% SSD. One possible interpretation of this finding is that floor effects were present to prevent nicotine from altering performance at the three other SSDs. When a zero-delay and a 30% SSD were present, performance remained relatively stable and there was no evidence that response withholding was altered by the administration of nicotine. When the 90% SSD was used, this delay caused a significant disruption in performance compared with all other SSDs, and performance was so poor at this delay that the administration of nicotine had no quantifiable detrimental effect. Even though the sequential order of SSD presentations across experimental blocks was counterbalanced across experimental days, one possibility is that the decrement in response withholding at the 60% SSD was an experimental artifact created by presenting multiple SSDs to each subject on any given day. Experiment 2 was performed to evaluate performance only at the 60% SSD to avoid this potential confound. Feola et al. (2000) used a slightly different SST procedure in which the SSD were titrated until stop-trial accuracy met 50%. In other words, their primary dependent variable was not percent correct stop trials, but rather, the SSD (Potter and Newhouse, 2004); thus, at the end of a session, longer SSDs were evidence of inhibition whereas shorter SSDs were evidence of disinhibition. In this experiment, the 60% SSD created stop-trial performance that hovered approximately 50%. It remains a possibility that stop-trial accuracy is sensitive to the effects of drug manipulations only when there is adequate opportunity for that dependent variable to be altered in either an upward or downward manner.
Eagle and Robbins (2003) and Eagle et al. (2007) tend to use multiple SSDs in their investigation of stop performance, and performance at longer delays is typically worse than for shorter SSDs. However, in their study, SSDs are fixed at regular intervals (200, 300, 400ms etc. after the go response, e.g. Bari et al., 2009). In this investigation, percentages of each rat’s mRTs were used to calculate SSD so that performance at any SSD would remain relative to the rat’s mRT. This approach was used because it was unknown how nicotine would alter mRTs. Although dosing with 0.3mg/kg nicotine did not significantly alter mRTs, there was a minor decrease as a result of initial dosing. Perhaps using fixed (e.g. Bari et al., 2009), rather than adjusting, SSDs are adequate for measuring nonpharmacological effects, but phenomena such as tolerance and sensitization that may result in changes in mRT make it necessary to consider alternatives in using the fixed SSD technique.
Experiment 2
Experiment 2 was performed to replicate the effects of repeated 0.3 mg/kg nicotine dosing in experiment 1, and to assess whether response disinhibition on both the tandem VI-DRL schedule and SST is related to the degree of cholinergic activation. Performance, in terms of percent correct (VI-DRL) and stop-trial accuracy (SST), again was found to emerge gradually and sequentially over the course of 10 dosing sessions. In addition, the initial dose of nicotine failed to alter task performance significantly, so a history of nicotine exposure is necessary for a 0.3 mg/kg nicotine dose to result in a significant effect on response disinhibition.
In experiment 2, doses above 0.1 mg/kg mecamylamine hydrochloride attenuated the sensitized effects of 0.3 mg/kg nicotine on both the tandem VI-DRL schedule and SST, and there was a dose-response pattern evident in terms of proportion-correct trials (VI-DRL) and adjusted percent inhibition (SST). The mecamylamine plus nicotine combination dosing sessions were conducted only after predosing baseline performance was recovered following the repeated administrations of nicotine. Furthermore, mecamylamine plus nicotine doses were administered in a counterbalanced manner, and saline-only sessions were conducted between each antagonist-agonist dosing day, to reduce the likelihood that lingering effects of either drug were exerting control over responding. These measures were taken so that a within-subjects dose-response pattern could be evaluated, and to restrict the overall number of subjects used in the investigation. There are, however, potential problems of using the within-subjects approach. Each data point for the combination dose was obtained from an average of two separate sessions, and the possibility exists that these means were affected in some way by the order in which the combination doses were administered. However, given that the order was counterbalanced, this possibility is unlikely. Furthermore, the dose-response pattern is directional and congruent with the theory of cholinergic activation; higher doses of mecamylamine are more likely to disrupt nicotine-induced response disinhibition.
There were differences in the procedures between experiments that needed to be addressed. For the tandem VI-DRL schedule, session duration was reduced from 60min in experiment 1 to 20 min in experiment 2. The decrease in session duration did not result in a differential effect of nicotine on DRL-schedule performance. Furthermore, the auto-adjusting VI schedule (experiment 1) was replaced by a nonadjusting VI 120-s schedule (experiment 2), and the inclusion of the VI schedule equated reinforcement rate across conditions regardless of whether the auto-adjusting or static schedule was used. For the SST, four different SSDs were used in experiment 1, these being a zero-delay, 30, 60, and 90% delay of the mRTs. In experiment 2, only the zero delay and 60% SSD were used for each session because the effects of repeated dosing had been apparent at the 60% SSD only in experiment 1. The zero-delay block was used because the delay for the stop-signal presentation in the 60% SSD block was established based on the mRTs from the zero-delay block for that daily session. In addition, as was found in experiment 1, nicotine sensitization was evident during the 60% SSD blocks, but the zero-delay blocks were unaffected.
General discussion
In experiment 1, repeated dosing with 0.3 mg/kg nicotine led to a gradual decline in performance accuracy on both the conjunctive VI-DRL schedule and SST, and these results were replicated in experiment 2. Furthermore, the performance decrements created by nicotine were attenuated in a dose-dependent manner when mecamylamine was coadministered with nicotine. The pattern of results suggests that nicotine-induced response disinhibition results from cholinergic activation. The SST and VI-DRL schedule appear to measure similar aspects of response disinhibition, given that nicotine and mecamylamine dosing had parallel effects on responding under both tasks. The results suggest that the VI-DRL schedule and SST are potentially interchangeable measures for assessing inhibitory control in response to nicotine administration.
One possible neurophysiological mechanism involved in nicotine sensitization is nAChR upregulation, and in particular, upregulation of sites containing the β2 subunit (McCallum et al., 2006). However, cholinergic desensitization of receptors including the β2 subunit also may play a role (Picciotto et al., 2008). Given that, in this study, mecamylamine dose-dependently blocked the effect of nicotine to induce response disinhibition, then response disinhibition is unlikely to be a product of desensitization. If nicotine-induced impulsivity was related to desensitization, then coadministration with mecamylamine would promote greater disinhibition, rather than the attenuation of disinhibition. The possibility remains that response disinhibition may result from the concurrent upregulation and desensitization at different receptors throughout the nervous system (Picciotto et al., 2008).
In comparison with the extant literature on DRL-schedule performance (Schuster and Zimmerman, 1961; Segal, 1962; Zimmerman and Schuster, 1962; Morrison, 1968; Pradhan and Dutta, 1970; Sanger, 1978; McClure and McMillan, 1997; Balcells-Olivero et al., 1997; Popke et al., 2000; Wiley et al., 2000; Saulsgiver et al., 2007; Kirshenbaum et al., 2008, 2009), minimal evidence exists regarding the effects of acute presession psychomotor-stimulant dosing and SST performance (Feola et al., 2000; Eagle and Robbins, 2003; Eagle et al., 2007) and there is no evidence of investigation using repeated-stimulant dosing. It is presently unknown whether increases in reinforcer magnitude (e.g. Doughty and Richards, 2002), and/or other manipulations of motivational arousal, would induce performance decrements on the SST as they do for the DRL schedule (Kirshenbaum et al., 2008).
Both tasks seem to have good face validity for assessing response inhibition (or impulsive action), but evidence does not exist yet to ascertain whether repeated administration of nicotine induces poor performance on each task in a human population. Potter and Newhouse (2004, 2008) found that nicotine administration decreased, rather than increased, impulsive action, in impulsive action in humans diagnosed with attention-deficit hyperactivity disorder (ADHD). These experiments involved the SST, and the inhibiting effects of nicotine on this population may be attributable to the drug’s effects on dopaminergic activity (Ferrari et al., 2001). The facilitation of dopaminergic activity is thought to be the primary neural mechanism responsible for the efficacy of stimulant medication for ADHD (see Swanson et al., 2007; Volkow et al., 2009; for review) and acute doses of nicotine may serve to reverse a dopamine deficit. However, the efficacy of nicotine to promote inhibition in an ADHD population may be altered as a consequence of repeated dosing, and the possibility exists that chronic self-administration of nicotine may result in very different outcomes on impulsivity than acute dosing.
Observed in this study were results suggesting that a change in the sensitivity to nicotine was evident as a result of repeated dosing with nicotine. Furthermore, the sensitized response to nicotine was attenuated by mecamylamine, and this finding suggests that sensitization may involve upregulation of nAChRs. Future investigations may seek to evaluate the extent and duration to which the change in sensitivity to nicotine is present, and to assess the underlying neural mechanics of robust, long-term changes in relation to response disinhibition.
Acknowledgments
The authors express their gratitude to the members of the Saint Michael’s College Institutional Animal Care and Use Committee, with special appreciation to the extraordinary effort of Angela Irvine and training support by Dr. Ruth Blauwiekel of the University of Vermont. They also would like to thank Dr. Bret Findley for help in the serial dilution of the drugs used in the study. This research report was made possible, in part by the Vermont Genetics Network (Grant Number P20 RR16462) from the IDeA Networks of Biomedical Research Excellence Program of the National Center for Research Resources, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of National Center for Research Resources or National Institutes of Health. The authors express great appreciation for additional funding support from Diane and Michael McGrath of the McGrath Foundation.
References
- Bacher I, Wu B, Shytle D, George T. Mecamylamine-a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert Opin Pharmacother. 2009;10:2709–2721. doi: 10.1517/14656560903329102. [DOI] [PubMed] [Google Scholar]
- Balcells-Olivero M, Richards JB, Seiden LS. Sensitization to amphetamine on the differential-reinforcement-of-low-rate 72-s schedule. Psychopharmacology. 1997;133:207–213. doi: 10.1007/s002130050393. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Adam A, Robinson EJ, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick AD. Comparison of the efficiency and durability of responding effected by two modified DRL procedures with humans. The Psychological Record. 1977;27:225–233. [Google Scholar]
- Coolon R, Cain M. Effects of mecamylamine on nicotine-induced conditioned hyperactivity and sensitization in differentially reared rats. Pharmacol Biochem Behav. 2009;93:59–66. doi: 10.1016/j.pbb.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Gobert A, Auclair A, Girardon S, Millan MJ. Differential modulation of efficiency in a food-rewarded ‘differential reinforcement of low-rate’ 72-s schedule in rats by norepinephrine and serotonin reuptake inhibitors. Psychopharmacology. 2002;162:156–167. doi: 10.1007/s00213-002-1070-x. [DOI] [PubMed] [Google Scholar]
- DeNoble V, Mele P. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184:266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Doughty AH, Richards JB. Effects of reinforcer magnitude on responding under differential-reinforcement-of-low-rate schedules of rats and pigeons. J Exp Anal Behav. 2002;78:17–30. doi: 10.1901/jeab.2002.78-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and D-amphetamine. Behav Neurosci. 2003;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Tufft MRA, Goodchild HL, Robbins TW. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology. 2007;192:193–206. doi: 10.1007/s00213-007-0701-7. [DOI] [PubMed] [Google Scholar]
- Feola TW, De Wit H, Richards JB. Effects of D-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2001;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S, Visker K, Maisonneuve I. An oral self-administration model of nicotine preference in rats: effects of mecamylamine. Psychopharmacology. 1996;128:426–431. doi: 10.1007/s002130050153. [DOI] [PubMed] [Google Scholar]
- Horger B, Shelton K, Schenk S. Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav. 1990;37:707–711. doi: 10.1016/0091-3057(90)90552-s. [DOI] [PubMed] [Google Scholar]
- Jackson K, Kota D, Martin B, Damaj M. The role of various nicotinic receptor subunits and factors influencing nicotine conditioned place aversion. Neuropharm. 2009;56:970–974. doi: 10.1016/j.neuropharm.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Brown S, Hughes D, Doughty A. DRL schedules and nicotine administration: a systematic evaluation of dose and schedule requirement. Behav Pharmacol. 2008;19:683–697. doi: 10.1097/FBP.0b013e328315ecbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AP, Johnson MW, Schwarz SL, Jackson EJ. Response disinhibition evoked by nicotine and nicotine-associated contextual cues. Drug Alcohol Depend. 2009;105:97–108. doi: 10.1016/j.drugalcdep.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action. A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. San Diego, CA: Academic; 1994. pp. 189–236. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Collins AC, Paylor R, Marks MJ. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology. 2006;184:314–327. doi: 10.1007/s00213-005-0076-6. [DOI] [PubMed] [Google Scholar]
- McClure GYH, McMillan DE. Effects of drugs on response duration differentiation. VI: differential effects under differential reinforcement of low rates of responding schedules. J Pharmacol Exp Ther. 1997;281:1368–1380. [PubMed] [Google Scholar]
- Morrison CF. A comparison of the effects of nicotine and amphetamine on DRL performance in the rat. Psychopharmacologia. 1968;12:176–180. doi: 10.1007/BF00401548. [DOI] [PubMed] [Google Scholar]
- O’Dell L, Koob G. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearle RG, Sieden LS. The existence of tolerance to and cross-tolerance between D-amphetamine and methylphenidate for their effects on milk consumption and on differential-reinforcement-of-low-rate performance in the rat. J Pharmacol Exp Ther. 1976;198:635–647. [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not ‘either/or’: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popke JE, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav. 2000;65:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with ADHD. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Pradhan SN, Dutta SN. Comparative effects of nicotine and amphetamine on timing behavior in rats. Neuropharmacology. 1970;9:9–15. doi: 10.1016/0028-3908(70)90043-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Berger DF. An animal model of attention deficit disorder: the female shows more behavioral problems and is more impulsive than the male. Eur Psychologist. 1996;1:113–122. [Google Scholar]
- Sanabria F, Killeen P. Evidence for impulsivity in the spontaneously hypertensive rat drawn from complementary response-withholding tasks. Behav Brain Funct. 2008;84:7. doi: 10.1186/1744-9081-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger D. Effects of D-amphetamine on temporal and spatial discrimination in rats. Psychopharmacology. 1978;58:185–188. doi: 10.1007/BF00426905. [DOI] [PubMed] [Google Scholar]
- Saulsgiver K, McClure E, Wynne C. Effects of amphetamine on differential reinforcement of low rates of responding. Behav Pharmacol. 2007;18:119–133. doi: 10.1097/FBP.0b013e3280ae6caa. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav. 1997;57:543–550. doi: 10.1016/s0091-3057(96)00447-9. [DOI] [PubMed] [Google Scholar]
- Schenk S, Davidson E. Stimulant preexposure sensitizes rats and humans to the rewarding effects of cocaine. NIDA Res Monogra. 1998;169:56–82. [PubMed] [Google Scholar]
- Schuster CR, Zimmerman J. Timing behavior during prolonged treatment with dl-amphetamine. J Exp Anal Behav. 1961;4:327–330. doi: 10.1901/jeab.1961.4-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E. Effects of dl-amphetamine under concurrent VI DRL reinforcement. J Exp Anal Behav. 1962;51:105–112. doi: 10.1901/jeab.1962.5-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR. Mecamylamine (inversine): an old hypertensive with new research directions. J Hum Hypertension. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- Sokolowski JD, Seiden LS. The behavioral effects of sertraline, fluoxetine, and paroxetine differ on the differential-reinforcement-of-low-rate 72-second operant schedule in the rat. Psychopharmacology. 1999;147:153–161. doi: 10.1007/s002130051155. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D. Effects of methylphenidate on inhibitory control in hyperactive children. Journal of Abnormal Child Psychology: An official publication of the International Society for Research in Child and Adolescent Psychopathology. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Robinson T. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice-mediation by enhanced incentive motivation? Eur J Neurosci. 2006;24:2345–2354. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- Varanda W, Aracava Y, Sherby S, VanMeter W, Eldefrawi M, Albuquerque E. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol. 1985;28:128–137. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J, Compton A, Golden K. Separation of drug effects on timing and behavioral inhibition by increased stimulus control. Exp Clin Psychopharmacol. 2000;8:451–461. doi: 10.1037//1064-1297.8.4.451. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J, Schuster CR. Spaced responding on multiple DRL schedules. J Exp Anal Behav. 1962;5:497–504. doi: 10.1901/jeab.1962.5-497. [DOI] [PMC free article] [PubMed] [Google Scholar]