Abstract
Background
Inflammation, as measured by the circulating inflammatory marker high sensitivity C-reactive protein (hsCRP), has been associated with cardiovascular disease. However, data regarding CRP and risk of colorectal cancer have been conflicting. The Adenoma Prevention with Celecoxib (APC) trial demonstrated that the anti-inflammatory drug celecoxib prevents recurrence of colorectal adenoma but increases risk of cardiovascular events. We examined if serum hsCRP modified these results.
Methods
We measured hsCRP from serum specimens provided at study entry by patients enrolled in the APC trial. Patients were stratified according to use of low-dose aspirin, randomized to receive three years of treatment with placebo, 200-mg-bid celecoxib, or 400-mg-bid celecoxib, and underwent follow-up colonoscopies at Year 1 and 3.
Results
Among 1,680 patients, the estimated three-year cumulative incidence of adenoma was 42% for patients with hsCRP <1mg/L, compared with 43% (RR=1.02; 95% confidence interval (CI)=0.85–1.22) for hsCRP 1–3mg/L, and 41% (RR=1.1; CI=0.90–1.34) for hsCRP >3mg/L. The effect of celecoxib on adenoma recurrence did not vary among patients with high (>3mg/L) compared with low (≦3mg/L) hsCRP. However, among patients with high hsCRP, the RR of cardiovascular events compared with placebo was 2.27 (95%CI=0.72–7.14) for those randomized to celecoxib 200-mg-bid and 3.28 (CI=1.09–9.91) for 400-mg-bid. In contrast, among patients with low hsCRP, the corresponding RRs were 0.99 (CI=0.53–1.83) and 1.11 (CI=0.61–2.02).
Conclusions
HsCRP may predict risk of celecoxib-associated cardiovascular toxicity, but not adenoma recurrence or celecoxib treatment efficacy. Patients with low hsCRP may be a subgroup with a favorable risk-benefit profile for celecoxib chemoprevention.
Keywords: Adenoma, celecoxib, c-reactive protein, inflammation, chemoprevention
Aspirin and selective COX-2 inhibitors such as celecoxib reduce risk of colorectal adenoma and cancer (1–5). This effect may be mediated through abrogation of inflammation (6–8). High-sensitivity C-reactive protein (hsCRP) is a circulating inflammatory biomarker of chronic conditions, including cardiovascular disease (9, 10). However, prospective studies relating hsCRP to risk of colorectal cancer and adenoma have been equivocal (11–29).
In the Adenoma Prevention with Celecoxib (APC) trial, patients who had recently undergone colonoscopic removal of an adenoma were randomly assigned to receive placebo, 200 mg twice daily (200-mg-bid) of celecoxib, or 400 mg twice daily (400-mg-bid) of celecoxib and underwent follow-up colonoscopies at 1 and 3 years. The relative risk (RR) of the detection of one or more new adenomas by year 3 compared with placebo was 0.67 (95% confidence interval [CI] 0.59–0.77) for those receiving 200-mg-bid celecoxib and 0.55 (95% CI, 0.48–0.64) for those receiving 400-mg-bid celecoxib (4). Unfortunately, in a separate, adjudicated safety analysis, the APC trial also revealed unexpected dose-related cardiovascular toxicity (30). Because hsCRP may be related to both risk of neoplasia and cardiovascular events and celecoxib has been shown to reduce hsCRP levels (31, 32), we examined baseline hsCRP in relation to 1) risk of recurrent adenoma; 2) celecoxib-related chemopreventative efficacy; 3) celecoxib-related cardiovascular toxicity.
Materials and Methods
Study population
The APC trial was an NCI-sponsored, randomized, placebo-controlled trial which enrolled patients within 6 months of colonoscopic removal of multiple adenomas or a single adenoma >5 mm in diameter (ClinicalTrials.gov NCT00005094) (4). Beginning in November 1999, 2,457 potential participants at 91 clinical sites were entered into a 30-day placebo run-in period during which they were required to have at least 80 percent adherence to medication use. After the run-in period, 2,035 patients were subsequently randomly assigned to placebo, 200-mg-bid of celecoxib, or 400-mg-bid of celecoxib. Randomization was stratified on the basis of the use or nonuse of low-dose aspirin (325 mg or less every other day or 162.5 mg or less every day) and clinical site. For the duration of the study, patients were required to abstain from long-term use of NSAIDs. Patients were excluded if they had a history of familial adenomatous polyposis, hereditary nonpolyposis colon cancer, inflammatory bowel disease, or large-bowel resection other than appendectomy. Other exclusion criteria included a history of a renal or hepatic disorder, a clinically significant bleeding disorder, or treatment for a gastrointestinal ulcer before study entry. Study drug treatment was initially planned for three years for all participants. However, at the recommendation of the APC trial Data Safety Monitoring Board (DSMB) treatment was terminated prematurely on December 17, 2004 based on the results of an unscheduled safety analysis performed by an independent cardiovascular safety committee. At that time 1,762 patients (86.6 percent) had completed three years of treatment and 273 patients had 1 to 3 months of treatment remaining. In addition, 639 patients had begun participation in the extension study in which study medication was continued in a blinded manner for an additional 2 years. The median duration of treatment exposure in the extension study was 3.5 years (33). All patients provided written informed consent and the human subjects committee at each site approved the study. This analysis was approved by the Human Subjects Committee of Partners HealthCare.
hsCRP measurements
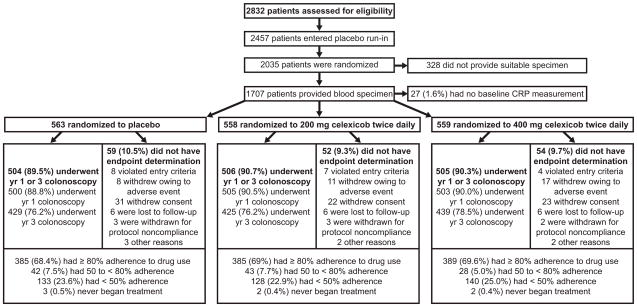
At baseline randomization, 1,707 participants provided a serum specimen at baseline randomization which was subsequently stored at −70°C. Personnel blinded to quality control and outcome data assayed for hsCRP using a high-sensitivity latex-enhanced immunoturbidimetric assay (intraassay coefficient of variation=2.9%; Denka Seiken, Tokyo, Japan). Among the 1,707 specimens, 27 could not be measured for technical reasons (Figure). Thus, this analysis included 1,680 participants, which were similar according to baseline characteristics to those whom we did not measure hsCRP (data not shown).
Figure.
Flow of patients through the study. Patients who violated study entry criteria were those for whom the presence or absence of adenoma on the baseline colonoscopy could not be confirmed. Adherence to the use of study medication was calculated as duration of use in days, divided by 1095.
Outcome Ascertainment
A study investigator performed follow-up colonoscopies with endoscopic removal of polyps at one and three years after randomization. A central study pathologist examined, in a blinded fashion, all polyps removed during these colonoscopies. Adverse events were reported by investigators and classified according to criteria from the Medical Dictionary for Regulatory Activities (MedDRA), version 8.1 (4, 33).
Statistical analysis
As in prior studies (21), we examined hsCRP levels according to cutpoints proposed in clinical guidelines (<1 mg/L, 1–3 mg/L and >3 mg/L) (34). Consistent with the intent-to-treat principle, we used the detection of an adenoma during a post-randomization colonoscopy, regardless of whether the patient adhered to the treatment regimen, as the primary endpoint. We estimated the cumulative incidence of adenoma at 3 years within different subgroups using Kaplan-Meier method. The effect of hsCRP levels on having a recurrent adenoma at a post-randomization colonoscopy was estimated by relative risks (RR) derived from Cox proportional hazards modeling with ties handled by the exact method, stratified by age (≥65 vs. <65), sex, time, baseline use of low dose aspirin (≤ 325 mg/every other day or ≤162.5 mg/day), postmenopausal hormones, and duration of statin use (≤3yr vs. >3yr) as a time-dependent variable (35). We used Cox proportional hazards models, adjusted for the same variables as the efficacy analyses, to estimate the RR of an investigator-reported adverse event after the first dose and up to 30 days after the last dose of study medication including events among patients who continued study medication in the 24 month extension study (33, 35). We used the SAS version 9.1 (SAS institute, Cary, NC) for all analyses. All significance tests were two-sided at a 5% level of significance.
Results
Among the 1,680 participants, the median age was 59 (IQR=31–88) years, 92% were white, and 68% were men, and the median hsCRP level was 1.6mg/L (IQR=0.1mg/L-2.72mg/L). Patients with elevated CRP levels (>3 mg/L) more frequently smoked, had a higher body mass index, had a prior history of cardiovascular events, hypertension, and diabetes mellitus, and used low-dose aspirin and statin drugs. Consistent with the known effect of postmenopausal hormones on CRP levels (36), the prevalence of postmenopausal hormone use was higher among women with elevated CRP. The number of adenomas (P=0.24) or adenoma burden (sum of diameter of all adenomas) (P=0.61) at the baseline qualifying examination did not vary according to hsCRP (Table 1).
Table 1.
Baseline Characteristics of the Patients According to Serum HsCRP Levels*
| Characteristics | hS CRP Level | ||||
|---|---|---|---|---|---|
| All (n = 1680) | < 1 mg/L (n = 563) | 1–3 mg/L (n = 603) | > 3 mg/L (n = 514) | Pvalue† | |
| CRP, median mg/L, (range) | 1.6 (0.1–272) | 0.6 (0.1–0.99) | 1.7 (1–2.99) | 5.5 (3–272) | -- |
| Age, median y, (range) | 59 (31, 88) | 58 (31–82) | 59 (35–88) | 59.5 (35–87) | 0.004 |
| Women, No. (%) | 535 (32) | 146 (26) | 154 (26) | 235 (46) | <0.001 |
| Race or ethnic group, No. (%)‡ | 0.04 | ||||
| Non-hispanic White | 1549 (92) | 518 (92) | 569 (94) | 462 (90) | |
| Non-hispanic Black | 88 (5) | 28 (5) | 21 (3) | 39 (7) | |
| Hispanic | 29 (2) | 11 (2) | 9 (2) | 9 (2) | |
| Asian/Pacific Islander/Other | 14 (1) | 2 (0.3) | 4 (0.8) | 8 (1.4) | |
| Current cigarette smoker, No. (%) | 277 (16) | 54 (10) | 115 (19) | 108 (21) | <0.001 |
| Body-mass index§ | 28 (15–58) | 26 (15–43) | 28 (17–43) | 31 (19–58) | <0.001 |
| Men | 28 (15–49) | 27 (15–43) | 29 (17–43) | 30 (20–49) | <0.001 |
| Women | 28 (18–58) | 24 (18–41) | 28 (18–43) | 30 (19–58) | <0.001 |
| Colorectal cancer in a parent, No. (%) | 355 (21) | 122 (22) | 136 (23) | 97 (19) | 0.3 |
| Findings at baseline colonoscopy | |||||
| No. of adenomas | 2 (1–17) | 2 (1–11) | 2 (1–17) | 2 (1–10) | 0.09 |
| At least one adenoma ≥ 1cm, No. (%) | 719 (43) | 241 (43) | 245 (41) | 233 (45) | 0.29 |
| Multiple adenomas, No. (%) | 932 (55) | 310 (55) | 341 (57) | 281 (55) | 0.80 |
| Adenoma burden, cm.** | 1.1 (0.08–12.4) | 1 (0.15–12.4) | 1.1 (0.15–11.9) | 1.2 (0.08–10.4) | 0.22 |
| History of cardiovascular events, No. (%)†† | 237 (14) | 59 (10) | 102 (17) | 76 (15) | 0.006 |
| History of hypertension, No. (%) | 677 (40) | 175 (31) | 261 (43) | 241 (47) | <0.001 |
| History of diabetes, No. (%) | 156 (9) | 37 (7) | 55 (9) | 64 (12) | 0.004 |
| Use of low-dose aspirin, No. (%)‡‡ | 539 (32) | 177 (31) | 219 (36) | 143 (28) | 0.009 |
| Use of postmenopausal hormones, No. (%)§§ | 211 (39) | 31 (21) | 56 (36) | 124 (53) | <0.001 |
| Use of statins, No. (%) | 421 (25) | 147 (21) | 166 (28) | 108 (21) | 0.03 |
| Randomized to placebo, No. (%) | 563 (34) | 192 (34) | 209 (35) | 162 (32) | 0.05 |
| Randomized to celecoxib, 200-mg-bid, No. (%) | 558 (33) | 175 (31) | 212 (35) | 171 (33) | 0.06 |
| Randomized to celecoxib, 400-mg-bid, No. (%) | 559 (34) | 196 (35) | 182 (30) | 181 (35) | 0.74 |
Data are expressed as mean (SD) unless otherwise indicated.
Test of difference between hsCRP groups was calculated by analysis of variance for continuous variables, χ2 for categorical variables.
Race or ethnic group was determined by the investigator.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
The adenoma burden was defined as the sum of the diameter of all adenomas reported during colonoscopy at baseline.
Cardiovascular events were defined as myocardial infarction, cerebrovascular disease, congestive heart failure, angina, and atherosclerotic heart disease.
Low-dose aspirin was defined as 325 mg or less every other day or 162.5 mg or less every day.
Percentage using postmenopausal hormones was calculated among women only.
Baseline hsCRP was not statistically significantly associated with risk of recurrent colorectal adenoma. The RR of adenoma through year 3 associated with a 1 standard deviation increase in log hsCRP was 0.96(CI=0.89–1.03). The estimated three-year cumulative incidence of adenoma was 42% for patients with hsCRP < 1mg/L, compared with 43% (RR, 1.02; CI=0.85–1.22) for hsCRP 1–3mg/L, and 41% (RR, 1.1; CI=0.90–1.34) for hsCRP >3mg/L. These results did not vary according to strata defined by celecoxib assignment, aspirin use, sex, use of postmenopausal hormones, body-mass index, or use of statin drugs (Table 2). When we analyzed data according to quartile cutpoints of the distribution of hsCRP, our results were essentially unchanged (data not shown). We also examined hsCRP in relation to risk of advanced adenoma. The estimated three-year cumulative incidence of advanced adenoma was 8.2% for patients with hsCRP < 1mg/L, compared with 7.5% (RR, 0.90; CI=0.59–1.38) for hsCRP 1–3mg/L, and 5.6% (RR, 0.72; CI=0.44–1.17) for hsCRP >3mg/L.
Table 2.
Risk of Adenoma According to Serum HsCRP Levels
| hsCRP < 1 mg/L | hsCRP 1–3 mg/L | hsCRP > 3 mg/L | |
|---|---|---|---|
| All patients* | |||
| No. at risk | 533 | 529 | 453 |
| Cumulative incidence, 3 yrs, % ± SE | 42 ± 2 | 43 ± 2 | 41 ± 2 |
| RR (95% CI) | 1(ref) | 1.02 (0.85–1.22) | 1.1 (0.90–1.34) |
| P value | 0.86 | 0.36 | |
|
| |||
| By celecoxib treatment | |||
| Patients randomized to placebo, No. at risk | 179 | 177 | 148 |
| Cumulative incidence, 3 yrs, % ± SE | 54 ± 4 | 53 ± 4 | 57 ± 4 |
| RR (95% CI) | 1(ref) | 0.98 (0.74–1.3) | 1.22 (0.9–1.64) |
| P value | 0.91 | 0.20 | |
| Patients randomized to celecoxib 200-mg-bid, No. at risk | 163 | 190 | 153 |
| Cumulative incidence, 3 yrs, % ± SE | 36 ± 4 | 41 ± 4 | 37 ± 4 |
| RR (95% CI) | 1(ref) | 1.18 (0.84–1.64) | 1.13 (0.79–1.62) |
| P value | 0.34 | 0.50 | |
| Patients randomized to celecoxib 400-mg-bid, No. at risk | 191 | 162 | 152 |
| Cumulative incidence, 3 yrs, % ± SE | 35 ± 3 | 34 ± 4 | 30 ± 4 |
| RR (95% CI) | 1(ref) | 0.89 (0.63–1.27) | 0.94 (0.64–1.38) |
| P value | 0.52 | 0.76 | |
| P value for interaction | 0.53 | 0.58 | |
|
| |||
| By low dose aspirin strata† | |||
| Patients taking aspirin, No. at risk | 171 | 183 | 129 |
| Cumulative incidence, 3 yrs, % ± SE | 40 ± 4 | 49 ± 4 | 41 ± 4 |
| RR (95% CI) | 1(ref) | 1.34 (0.98– 1.82) | 1.24 (0.87–1.78) |
| P value | 0.07 | 0.23 | |
| Patients not taking aspirin, No. at risk | 362 | 346 | 324 |
| Cumulative incidence, 3 yrs, % ± SE | 43 ± 3 | 40 ± 3 | 41 ± 4 |
| RR (95% CI) | 1(ref) | 0.89 (0.71–1.11) | 1.04 (0.82–1.31) |
| P value | 0.29 | 0.77 | |
| P value for interaction | 0.05 | 0.80 | |
|
| |||
| By sex | |||
| Men, No. at risk | 395 | 391 | 249 |
| Cumulative incidence, 3 yrs, % ± SE | 45 ± 2 | 46 ± 2 | 48 ± 3 |
| RR (95% CI) | 1(ref) | 1.01 (0.82–1.23) | 1.11 (0.88–1.40) |
| P value | 0.95 | 0.37 | |
| Women, No. at risk | 138 | 138 | 204 |
| Cumulative incidence, 3 yrs, % ± SE | 34 ± 4 | 34 ± 4 | 33 ± 3 |
| RR (95% CI) | 1(ref) | 1.05 (0.70–1.57) | 1.03 (0.71–1.50) |
| P value | 0.81 | 0.86 | |
| P value for interaction | 0.89 | 0.81 | |
|
| |||
| By postmenopausal hormone use‡ | |||
| Patients taking hormones, No. at risk | 29 | 49 | 110 |
| Cumulative incidence, 3 yrs, % ± SE | 30 ± 8 | 29 ± 6 | 35 ± 4 |
| RR (95% CI) | 1(ref) | 1.02 (0.44–2.37) | 1.25 (0.60–2.59) |
| P value | 0.97 | 0.55 | |
| Patients not taking hormones,No. at risk | 109 | 89 | 94 |
| Cumulative incidence, 3 yrs, % ± SE | 35 ± 5 | 37 ± 5 | 30 ± 5 |
| RR (95% CI) | 1(ref) | 1.12 (0.70–1.79) | 0.95 (0.59–1.54) |
| P Value | 0.63 | 0.83 | |
| P value for interaction | 0.74 | 0.56 | |
|
| |||
| By body mass index§ | |||
| Patients with high BMI, No. at risk | 175 | 279 | 297 |
| Cumulative incidence, 3 yrs, % ± SE | 48 ± 4 | 41 ± 3 | 41 ± 3 |
| RR (95% CI) | 1(ref) | 0.90 (0.68–1.19) | 0.98 (0.74–1.30) |
| P value | 0.44 | 0.91 | |
| Patients with low BMI, No. at risk | 358 | 250 | 156 |
| Cumulative incidence, 3 yrs, % ± SE | 39 ± 3 | 45 ± 3 | 41 ± 4 |
| RR (95% CI) | 1(ref) | 1.12 (0.87–1.43) | 1.16 (0.86–1.56) |
| P value | 0.37 | 0.33 | |
| P value for interaction | 0.24 | 0.49 | |
|
| |||
| By statin use | |||
| Patients taking statins, No. at risk | 142 | 143 | 98 |
| Cumulative incidence, 3 yrs, % ± SE | 46 ± 4 | 42 ± 4 | 51 ± 5 |
| RR (95% CI) | 1(ref) | 0.96 (0.68–1.36) | 1.22 (0.84–1.78) |
| P value | 0.81 | 0.30 | |
| Patients not taking statins, No. at risk | 391 | 386 | 355 |
| Cumulative incidence, 3 yrs, % ± SE | 41 ± 2 | 43 ± 2 | 38 ± 3 |
| RR (95% CI) | 1(ref) | 1.05 (0.85–1.3) | 1.05 (0.83–1.32) |
| P value | 0.66 | 0.68 | |
| P value for interaction | 0.67 | 0.52 | |
No. at risk includes patients who underwent a follow-up colonoscopy at year 1 and/or year 3. A patient with a colonoscopy at year 3 but with no colonoscopy at year 1 was included in the analysis through year 1, with the assumption that the patient had no adenoma at year 1, and was then included in the analysis through year 3 according to the findings of the colonoscopy at year 3. The analyses at year 3 excluded patients with an adenoma at year 1 colonoscopy and patients with no adenoma at year 1 and no colonoscopy at year 3 (4). P values for interaction were assessed by using cross-product terms for each celecoxib treatment group and each risk factor strata.
At the time of randomization, patients were stratified according to use of low dose aspirin, defined as ≤ 325 mg/every other day or ≤ 162.5 mg/day.
Analyses were restricted to women.
High body mass index (BMI) defined as ≥ median [28 mg/kg2] and low BMI defined as < median.
The overall reduction in cumulative incidence of adenoma associated with celecoxib did not appear to vary among patients with high (>3 mg/L) compared with low (≦ 3 mg/L) hsCRP (Table 3). However, among patients with high hsCRP, the RR of cardiovascular events compared with placebo was 2.27 (CI=0.72–7.14) for those randomized to celecoxib 200-mg-bid and 3.28 (CI=1.09–9.91) for 400-mg-bid. In contrast, among patients with low hsCRP, the corresponding RRs were 0.99 (CI= 0.53–1.83) and 1.11 (CI=0.61–2.02) (Table 4). A formal test of interaction between high hsCRP and 400-mg-bid celecoxib approached statistical significance(P=0.11). We considered the possibility that concurrent aspirin use may modify the interaction between high hsCRP levels and celecoxib-associated cardiovascular events. Among patients with hsCRP > 3mg/L and were not taking low-dose aspirin, compared to those randomized to placebo, the RR for a cardiovascular event was 2.74 (CI=0.54–13.70) for those randomized to celecoxib 200-mg-bid and 4.98 (CI=1.10–22.59) for those randomized to celecoxib 400-mg-bid. In contrast, among patients with hsCRP > 3mg/L and were taking low-dose aspirin, compared to those randomized to placebo, the RR for a cardiovascular event was 1.72 (CI=0.33–8.96) for those randomized to celecoxib 200-mg-bid, and 1.65 (CI=0.30–9.0) for those randomized to celecoxib 400-mg-bid. Although these results do suggest a possible attenuation of cardiovascular risk associated with low-dose aspirin, a formal test of interaction between celecoxib treatment and use of low dose aspirin was not statistically significance(P=0.57). Moreover, because there were limited numbers of events within each aspirin strata (19 events in the group not taking aspirin and 12 events in the group taking low-dose aspirin), these results should be considered exploratory. Finally, among all patients, there did not appear to be an overall dose-related increase in either renal and hypertensive disorders or gastrointestinal ulceration and hemorrhage, consistent with the overall results of the trial (4).
Table 3.
Risk of Adenoma According to Celecoxib Treatment, Stratified by HsCRP Level*
| Placebo | Celecoxib, 200mg Twice Daily |
Celecoxib, 400mg Twice Daily |
|
|---|---|---|---|
| All patients, No. at risk | 563 | 558 | 559 |
| Cumulative incidence, 3 yrs, % ± SE | 55 ± 2 | 30 ± 2 | 33 ± 2 |
| RR (95% CI) | 1 (ref) | 0.60 (0.50 – 0.72) | 0.49 (0.40 – 0.59) |
| P value | <0.001 | <0.001 | |
| Patients with hsCRP <3, No. at risk | 401 | 387 | 378 |
| Cumulative incidence, 3 yrs, % ± SE | 53 ± 3 | 39 ± 3 | 35 ± 3 |
| RR (95% CI) | 1 (ref) | 0.62 (0.52 – 0.75) | 0.54 (0.44 – 0.65) |
| P value | <0.001 | <0.001 | |
| Patients with hsCRP ≥3, No. at risk | 162 | 171 | 181 |
| Cumulative incidence, 3 yrs, % ± SE | 57 ± 4 | 37 ± 4 | 30 ± 4 |
| RR (95% CI) | 1 (ref) | 0.48 (0.36 – 0.65) | 0.41 (0.30 – 0.55) |
| P value | <0.001 | <0.001 | |
| P value for interaction | 0.50 | 0.58 |
No. at risk includes patients who underwent a follow-up colonoscopy at year 1 and/or year 3. A patient with a colonoscopy at year 3 but with no colonoscopy at year 1 was included in the analysis through year 1, with the assumption that the patient had no adenoma at year 1, and was then included in the analysis through year 3 according to the findings of the colonoscopy at year 3. The analyses at year 3 excluded patients with an adenoma at year 1 colonoscopy and patients with no adenoma at year 1 and no colonoscopy at year 3 (4). P values for interaction were assessed by using cross-product terms for each celecoxib treatment group and each hsCRP strata.
Table 4.
Risk of Adverse Events According to Celecoxib Treatment, Stratified by HsCRP Level*
| Placebo | Celecoxib, 200mg Twice Daily |
Celecoxib, 400mg Twice Daily |
|
|---|---|---|---|
| Risk of Cardiovascular Disorders† | |||
| All patients | |||
| No. with event/No. at risk | 23/563 | 34/558 | 43/559 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 1 | 8 ± 1 | 9 ± 1 |
| RR (95% CI) | 1(ref) | 1.19 (0.69–2.03) | 1.50 (0.90–2.50) |
| P value | 0.53 | 0.12 | |
| Patients with hsCRP <3 | |||
| No. with event/No. at risk | 19/401 | 22/387 | 28/378 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 2 | 7 ± 2 | 8 ± 2 |
| RR (95% CI) | 1 (ref) | 0.99 (0.53–1.83) | 1.11 (0.61–2.02) |
| P value | 0.96 | 0.74 | |
| Patients with hsCRP ≥3 | |||
| No. with event/No. at risk | 4/162 | 12/171 | 15/181 |
| Cumulative incidence, 3 yrs, % ± SE | 5 ± 2 | 8 ± 2 | 11 ± 3 |
| RR (95% CI) | 1 (ref) | 2.27 (0.72–7.14) | 3.28 (1.09–9.91) |
| P value | 0.16 | 0.03 | |
| P value for interaction | 0.29 | 0.11 | |
|
| |||
| Risk of Renal and Hypertensive Disorders‡ | |||
| All patients | |||
| No. with event/No. at risk | 95/563 | 125/558 | 100/559 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 1 | 8 ± 1 | 9 ± 1 |
| RR (95% CI) | 1 (ref) | 1.24 (0.94–1.62) | 0.87 (0.66–1.16) |
| P value | 0.12 | 0.35 | |
| Patients with hsCRP <3 | |||
| No. with event/No. at risk | 63/401 | 82/387 | 60/378 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 2 | 7 ± 2 | 8 ± 2 |
| RR (95% CI) | 1 (ref) | 1.26 (0.90–1.75) | 0.78 (0.55–1.12) |
| P value | 0.18 | 0.18 | |
| Patients with hsCRP ≥3 | |||
| No. with event/No. at risk | 32/162 | 43/171 | 40/181 |
| Cumulative incidence, 3 yrs, % ± SE | 5 ± 2 | 8 ± 2 | 11 ± 3 |
| RR (95% CI) | 1 (ref) | 1.13 (0.71–1.80) | 1.02 (0.63–1.63) |
| P value | 0.60 | 0.95 | |
| P value for interaction | 0.68 | 0.46 | |
|
| |||
| Gastrointestinal Ulceration/Hemorrhage§ | |||
| All patients | |||
| No. with event/No. at risk | 57/563 | 58/558 | 54/559 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 1 | 8 ± 1 | 9 ± 1 |
| RR (95% CI) | 1 (ref) | 0.87 (0.60–1.25) | 0.82 (0.56–1.19) |
| P value | 0.45 | 0.29 | |
| Patients with hsCRP <3 | |||
| No. with event/No. at risk | 36/401 | 44/387 | 36/378 |
| Cumulative incidence, 3 yrs, % ± SE | 6 ± 2 | 7 ± 2 | 8 ± 2 |
| RR (95% CI) | 1 (ref) | 1.06 (0.68–1.65) | 0.79 (0.50–1.27) |
| P value | 0.80 | 0.33 | |
| Patients with hsCRP ≥3 | |||
| No. with event/No. at risk | 21/162 | 14/171 | 18/181 |
| Cumulative incidence, 3 yrs, % ± SE | 5 ± 2 | 8 ± 2 | 11 ± 3 |
| RR (95% CI) | 1 (ref) | 0.52 (0.26–1.04) | 0.80 (0.42–1.52) |
| P value | 0.06 | 0.49 | |
| P value for interaction | 0.07 | 0.83 | |
No. at risk includes patients randomized regardless of whether they had a follow-up colonoscopy. P values for interaction were assessed by using cross-product terms for each celecoxib treatment group and each hsCRP strata.
Includes investigator-reported cardiovascular disorders, which was a prespecified category encompassing cardiovascular death or circulatory collapse, stroke, myocardial infarction or angina, congestive heart failure, venous thrombosis or thromboembolism, cardiovascular therapeutic procedures, vascular therapeutic procedures, cerebrovascular disease, and vascular disease.
Includes investigator-reported renal and hypertensive disorders, which was a prespecified category encompassing elevated creatinine, fluid retention or edema, hypertension, proteinuria, and renal failure.
Includes investigator-reported gastrointestinal ulceration and hemorrhage, which was a prespecified category encompassing anemia, gastrointestinal bleeding, gastritis/duodenitis, upper or lower gastrointestinal ulceration, and other hemorrhage.
Discussion
The divergent results of previous studies relating hsCRP to colorectal adenoma or cancer may be related to the timing at which hsCRP was measured (11–29). Specifically, positive studies may reflect hsCRP elevations associated with occult disease, including adenoma (11, 12, 14, 22). In support of this explanation, cross-sectional data measuring hsCRP at the time of colonoscopy have shown a modest association with prevalent adenoma (24, 25), whereas a prospective study of incident adenoma and a cross-sectional study measuring hsCRP several years before diagnosis of adenoma showed inverse or no association (26, 28). In our study, because we examined hsCRP levels among individuals within 6 months of a clearing colonoscopy and endpoints were ascertained at protocol-defined surveillance colonoscopies, our results more closely reflect the association between hsCRP and incident neoplasia. Our findings are largely consistent with those of the similarly-designed Aspirin Polyp Prevention Study (PPS) (37). However, the results from both the APC and Aspirin PPS trials do not exclude a potential association between hsCRP and the initial development of adenoma rather than recurrence. A limitation of our study is that we only had a single baseline measure of hsCRP and we could not correlate change in hsCRP with celecoxib treatment. However, celecoxib treatment reduced adenoma recurrence irrespective of baseline levels of hsCRP. Taken together with data from the Aspirin PPS which did measure hsCRP levels at baseline and at year 3, our findings support the conclusion that hsCRP does not mediate the chemopreventive effect of aspirin or celecoxib (37).
Among individuals with high hsCRP levels (>3 mg/L), celecoxib treatment was associated with a 3-fold higher risk of an adverse cardiovascular event; in contrast, among those with low hsCRP (≦ 3 mg/L), celecoxib treatment was not associated with higher risk. Although a formal test for interaction was not significant, this is likely due to the limited number of adverse cardiovascular events. These findings are consistent with emerging data that individuals can be stratified for celecoxib cardiovascular toxicity according to baseline cardiovascular risk. A prior pooled analysis of 6 placebo-controlled trials of celecoxib observed that patients with high baseline cardiovascular risk scores had the greatest risk of celecoxib-related adverse events (38). Similarly, a five-year safety analysis of an extension of the APC trial showed a significant interaction between a baseline history of atherosclerotic heart disease and risk of celecoxib-associated cardiovascular events (33). Further studies are needed to determine if baseline hsCRP alone can be used to identify patients for whom long-term use of celecoxib is relatively safe.
In the APC trial, patients were randomized to either 200-mg-bid or 400-mg-bid doses of celecoxib. Thus, it is unclear if baseline hsCRP may also predict risk of cardiovascular events among patients who take celecoxib once daily. In a parallel randomized placebo-controlled trial, treatment with 400-mg of daily celecoxib was associated with an overall non-significant increase in risk of cardiovascular events (RR 1.30; 95% CI, 0.65–2.62) (5). Thus, it is possible that use of a once daily dosing of celecoxib among patients with low hsCRP may be a particularly effective strategy to minimize cardiovascular risk.
Lastly, in our analysis, we did observe a non-significant increase in risk of renal and hypertensive events in the subgroup of patients with low hsCRP randomized to 200-mg-bid. In contrast, there was no association among those randomized to 400-mg-bid. Based on this lack of a dose-response it is unlikely that there is a unique biological interaction between celecoxib and low baseline inflammatory state and renal or hypertensive events. Nonetheless, larger studies with a greater number of such endpoints are needed to make definitive conclusions.
In this large, randomized, placebo-controlled trial, baseline hsCRP was not associated with overall risk of adenoma recurrence or celecoxib chemopreventative benefit after three years of treatment. However, individuals with high hsCRP appeared to have the greatest risk of celecoxib-related cardiovascular toxicity. Further studies are needed to determine the role of hsCRP in relation to other markers of cardiovascular risk to evaluate the risk-benefit profile of celecoxib treatment for a range of clinical indications.
Acknowledgments
The authors would like to acknowledge Dr. Nader Rifai and Mr. Gary Bradwin for technical assistance.
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R01 CA137178 to ATC and N01 CN95015 to MMB). Dr. Chan is a Damon Runyon Cancer Research Foundation Clinical Investigator.
Footnotes
Financial Disclosures: Dr. Chan has served as a consultant to Bayer HealthCare. Dr. Bertagnolli is the recipients of research funding from Pfizer Inc. Dr. Hawk has served as a consultant for Pozen Pharmaceutical Development Company. Dr. Ridker is listed as a co-inventor on patents held by Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to Siemens and AstraZeneca. Dr. Sima has no conflicts of interest. The statistical analysis of the entire data sets pertaining to efficacy and safety has been independently confirmed by Dr. Zauber, who is not employed by any corporate entity. The corresponding author had full access to all of the data and takes full responsibility for the veracity of the data and analysis.
Portions of this data were previously presented in abstract form at Digestive Disease Week in Chicago, IL on May 30-June 4, 2009.
References
- 1.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–42. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 2.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 3.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101(4):256–66. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 5.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 6.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56(2):69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 8.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Huntingt) 2002;16(2):217–26. 229. discussion 230–2. [PubMed] [Google Scholar]
- 9.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. Jama. 2004;291(5):585–90. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 12.Il’yasova D, Colbert LH, Harris TB, et al. Circulating Levels of Inflammatory Markers and Cancer Risk in the Health Aging and Body Composition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Suzuki K, Tamakoshi K, et al. Colorectal cancer and serum C-reactive protein levels: a case-control study nested in the JACC Study. J Epidemiol. 2005;15 (Suppl 2):S185–9. doi: 10.2188/jea.15.S185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66(4):2483–7. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 15.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15(4):690–5. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 16.Trichopoulos D, Psaltopoulou T, Orfanos P, Trichopoulou A, Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev. 2006;15(2):381–4. doi: 10.1158/1055-9965.EPI-05-0626. [DOI] [PubMed] [Google Scholar]
- 17.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24(33):5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 18.Heikkila K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 19.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27(13):2217–24. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 20.Allin KH, Nordestgaard BG, Zacho J, Tybjaerg-Hansen A, Bojesen SE. C-reactive protein and the risk of cancer: a mendelian randomization study. J Natl Cancer Inst. 2010;102(3):202–6. doi: 10.1093/jnci/djp459. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SM, Buring JE, Lee IM, Cook NR, Ridker PM. C-reactive protein levels are not associated with increased risk for colorectal cancer in women. Ann Intern Med. 2005;142(6):425–32. doi: 10.7326/0003-4819-142-6-200503150-00008. [DOI] [PubMed] [Google Scholar]
- 22.Aleksandrova K, Jenab M, Boeing H, et al. Circulating C-reactive protein concentrations and risks of colon and rectal cancer: a nested case-control study within the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172(4):407–18. doi: 10.1093/aje/kwq135. [DOI] [PubMed] [Google Scholar]
- 23.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory Markers are Associated with Risk of Colorectal Cancer and Chemopreventative Response to Anti-Inflammatory Drugs. Gastroenterology. 2011;140(3) doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otake T, Uezono K, Takahashi R, et al. C-reactive protein and colorectal adenomas: Self Defense Forces Health Study. Cancer Sci. 2009;100(4):709–14. doi: 10.1111/j.1349-7006.2009.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68(1):323–8. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsilidis KK, Erlinger TP, Rifai N, et al. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes Control. 2008;19(6):559–67. doi: 10.1007/s10552-008-9117-x. [DOI] [PubMed] [Google Scholar]
- 27.Ognjanovic S, Yamamoto J, Saltzman B, et al. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer Causes Control. 2010;21(7):1131–8. doi: 10.1007/s10552-010-9540-7. [DOI] [PubMed] [Google Scholar]
- 28.Gunter MJ, Cross AJ, Huang WY, et al. A Prospective Evaluation of C-reactive Protein Levels and Colorectal Adenoma Development. Cancer Epidemiol Biomarkers Prev. 2011 doi: 10.1158/1055-9965.EPI-10-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of inflammatory markers with colorectal cancer incidence in the atherosclerosis risk in communities study. Cancer Epidemiol Biomarkers Prev. 2011;20(2):297–307. doi: 10.1158/1055-9965.EPI-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N Engl J Med. 2005;352(11):1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 31.Chenevard R, Hurlimann D, Bechir M, et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation. 2003;107(3):405–9. doi: 10.1161/01.cir.0000051361.69808.3a. [DOI] [PubMed] [Google Scholar]
- 32.Bogaty P, Brophy JM, Noel M, et al. Impact of prolonged cyclooxygenase-2 inhibition on inflammatory markers and endothelial function in patients with ischemic heart disease and raised C-reactive protein: a randomized placebo-controlled study. Circulation. 2004;110(8):934–9. doi: 10.1161/01.CIR.0000139338.12464.5F. [DOI] [PubMed] [Google Scholar]
- 33.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res (Phila Pa) 2009;2(4):310–21. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 35.Chan AT, Zauber AG, Hsu M, et al. Cytochrome P450 2C9 variants influence response to celecoxib for prevention of colorectal adenoma. Gastroenterology. 2009;136(7):2127–2136. e1. doi: 10.1053/j.gastro.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100(7):713–6. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- 37.Ho GY, Xue X, Cushman M, et al. Antagonistic Effects of Aspirin and Folic Acid on Inflammation Markers and Subsequent Risk of Recurrent Colorectal Adenomas. J Natl Cancer Inst. 2009;101(23):1650–54. doi: 10.1093/jnci/djp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]