Abstract
This study investigated the effect of chronic AMP-kinase (AMPK) activation with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) on white adipose tissue (WAT) metabolism and the implications for visceral (VC) and subcutaneous (SC) adiposity, whole body-energy homeostasis, and hypothalamic leptin sensitivity. Male Wistar rats received daily single intraperitoneal injections of either saline or AICAR (0.7g/kg body weight) for 4 and 8 weeks and were pair-fed throughout the study. AICAR-treated rats had reduced adiposity with increased mitochondrial density in VC and SC fat pads, which was accompanied by reduced circulating leptin and time-dependent and depot-specific regulation of AMPK phosphorylation and FA oxidation. Interestingly, the anorectic effect to exogenous leptin was more pronounced in AICAR-treated animals than controls. This corresponded to reductions in hypothalamic AMPK phosphorylation and suppressor of cytokine signaling 3 content, whereas signal transducer and activator of transcription 3 phosphorylation was either unchanged or increased at 4 and 8 weeks in AICAR-treated rats. Ambulatory activity and whole-body energy expenditure (EE) were also increased with AICAR treatment. Altogether, chronic AICAR-induced AMPK activation increased WAT oxidative machinery, whole-body EE, and hypothalamic leptin sensitivity. This led to significant reductions in VC and SC adiposity without inducing energy-sparing mechanisms that oppose long-term fat loss.
Keywords: adipocytes, beta-oxidation, fatty acid/oxidation, mitochondria, protein kinases/AMP-activated protein kinase, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
Successful treatment of obesity requires a continuous reduction in adiposity and maintenance of a healthy body weight. The conventional approaches used to achieve weight loss involve exercise and diet. However, as body fat is reduced through these approaches, energy-sparing mechanisms are activated and impose a major obstacle to long-term weight loss (1). Therefore, identifying strategies to overcome these energy-sparing mechanisms is crucial to improve the outcome of weight loss programs. One potential approach would be to remodel white adipose tissue (WAT) metabolism toward a highly metabolic brown adipose tissue (BAT) phenotype that shifts metabolism toward fat oxidation instead of storage independently of altering whole-body energy expenditure (EE) through physical activity (2).
The acquisition of a “brown-like” phenotype by white adipocytes requires a substantial increase in mitochondrial content and upregulation of the oxidative machinery in these cells. These functional changes could ultimately reduce fat storage and adipose tissue mass. In this context, one enzyme that is central to sense the energy state of the cell and regulate ATP production through FA oxidation is AMP-kinase (AMPK). In its activated state, AMPK shuts down anabolic pathways and promotes catabolism by regulating the activity of key enzymes of intermediary metabolism (3). AMPK has also been shown to block adipocyte differentiation in the early stages by inhibiting clonal expansion, which is a critical step for adipogenesis to occur (4, 5). Additionally, treatment of preadipocytes with pharmacological agents to activate AMPK prevents the expression of late adipogenic markers, fatty acid synthase, acetyl-CoA carboxylase (ACC), and transcription factors peroxisome proliferator-activated receptor (PPAR)γ1/2 and CCAAT-enhancer-binding protein (C/EBP)α which are required for the synthesis and storage of lipids in mature adipocytes (4, 5). AMPK also phosphorylates and activates PPAR-γ coactivator-1α (PGC-1α) and promotes mitochondrial biogenesis in skeletal muscle (6).
We have recently demonstrated that prolonged (15 h) AICAR-induced AMPK activation increased mRNA expression of PPAR-γ and of its coactivator PGC-1α in isolated rat epididymal adipocytes (7). These cells also had ∼4-fold higher than control expression of carnitine palmitoyl transferase-1b, which was accompanied by a 2-fold increase in palmitate oxidation and by a marked reduction in lipogenesis (7). Based on these observations, we hypothesized that chronic activation of AMPK in vivo could lead to a shift in WAT metabolism toward oxidation and lead to reduced adiposity. Additionally, the effects of chronic AMPK activation on remodeling WAT metabolism could potentially overcome the opposition to fat reduction triggered by the centrally-mediated activation of energy-sparing mechanisms as adiposity is reduced (8). Even though alterations in fat mass with chronic in vivo AICAR treatment in rodents have been previously reported (9–11), it is unknown whether these effects arise from direct structural and functional alterations in WAT or indirectly through alterations in whole-body energy homeostasis. Therefore, this study was designed to investigate the time-course effects of chronic in vivo AICAR-induced AMPK activation on visceral (VC) and subcutaneous (SC) WAT metabolism, as well as on whole-body energy balance. Previous studies have demonstrated that major depot-specific differences exist with respect to metabolic properties and plasticity under specific conditions (12). Thus, a goal of this study was also to determine whether VC and SC fat depots would elicit distinct responses to chronic AICAR-induced AMPK activation with regards to oxidative capacity. Furthermore, because alterations in fat mass also determine leptin expression and release by the WAT and this hormone exerts a major role in the regulation of whole-body EE (13), we assessed the time-course plasma profile of leptin. Phosphorylation and/or content of signal transducer and activator of transcription 3 (STAT3), AMPK, and suppressor of cytokine signaling 3 (SOCS3) were measured in the hypothalamus to assess leptin signaling in this tissue, as well as the anorectic response of AICAR-treated animals to exogenous leptin adminstration. This is the first study to provide evidence that chronic systemic pharmacological AMPK activation in rats remodels WAT metabolism by inducing mitochondrial biogenesis and promoting fat loss without inducing energy-sparing mechanisms. Importantly, this effect appears to be at least partially mediated by increased hypothalamic leptin sensitivity.
MATERIALS AND METHODS
Reagents
FA-free BSA, free glycerol determination kit, glucose oxidase kit, isoproterenol, and palmitic acid were obtained from Sigma. [1-14C]palmitic acid was from GE Healthcare Radiochemicals (Quebec City, Quebec, Canada). Leptin was measured using an ELISA from Millipore (Billerica, MA). AICAR was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Recombinant rat leptin was obtained from Dr. A. F. Parlow at the National Hormone and Peptide Program (Torrance, CA). All antibodies were from Cell Signaling Technology Inc. (Beverly, MA) unless noted otherwise. Phospho-acetyl-CoA carboxylase was obtained from Upstate (Charlottesville, VA), and uncoupling protein 1 (UCP-1) was from Abcam (Cambridge, MA). All other chemicals were of the highest grade available.
In vivo AICAR treatment and plasma analyses
Male albino rats (Wistar strain) weighing 150–200 g were maintained on a 12/12 h light/dark cycle at 22°C and fed standard laboratory chow ad libitum. Rats were given a single daily intraperitoneal (i.p.) injection of either saline or AICAR [0.7g/kg body weight (b.w.)] for 4 and 8 weeks. The dosage was chosen based on previous in vivo rat studies that used between 0.5 and 1.0g/kg b.w. for chronic AICAR injections (7, 10, 11). Saline-injected rats were pair-fed to the AICAR-treated group to control for effects of altered food intake induced by the treatment. Control animals were pair-fed according to the average amount of chow consumed by the AICAR-treated animals the previous day. Pair-fed animals received one-third of the total food in the morning (09:00) and the remaining two-thirds immediately prior to lights off at 19:00 to avoid prolonged periods of fasting. Weekly blood samples were collected prior to daily injections from the saphenous vein and the plasma was frozen for later analyses of leptin.
Determination of in vivo metabolic parameters
The Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Inc., Columbus, OH) was used to perform all automated in vivo determinations as previously described (14). Briefly, the CLAMS measures oxygen consumption (VO2), carbon dioxide production (VCO2), and respiratory exchange ratio (RER). Each cage is also equipped with a system of infrared beams that detects animal movement in the X and Z axes. EE was calculated by multiplying the calorific value (CV = 3.815 + 1.232 × RER) by VO2. Measurements using the CLAMS were performed on a weekly basis throughout the 8 week period. Animals were placed in the CLAMS at 10:00 immediately following the daily saline or AICAR injections. The first hour of data collected in the CLAMS was discarded, because it is the time required for the rats to fully acclimatize to the cage environment (14). The rats were monitored for a 24 h period encompassing the light (10:00–19:00 and 07:00–10:00) and dark (19:00–07:00) cycles.
Assessment of the anorectic response to leptin
Rats received daily injections of either saline or AICAR (0.7 mg/kg b.w.) for 2 weeks. During this treatment period, control animals were pair-fed as described above. On the day of the test, food was removed from the cages at 07:00 and the animals were injected at 09:00 with either saline (control) or AICAR. Rats were fasted until the onset of the dark cycle (19:00; 12 h fast). At this point, the animals were subdivided into four groups: control, leptin, AICAR, and AICAR plus leptin and then injected i.p. with either saline (control conditions) or recombinant rat leptin (5 mg/kg b.w.). We used this dosage of leptin because it has been previously demonstrated to induce a significant reduction in food intake 4 h postinjection in rats (15). In order to measure food intake, animals were placed in the CLAMS 15 min postleptin injection for 24 h with ad libitum access to food.
Extraction of tissues and calculation of LBM
Fat depots from subcutaneous-inguinal (ING), epididymal (EPI), and retroperitoneal (RP) regions were quickly removed and weighed prior to being processed for various metabolic assays. Weights of all tissues were normalized per 100g of body weight. Lean body mass (LBM) was calculated as carcass weight with all skin, viscera, and fat depots removed (14).
Isolation of adipocytes and measurement of palmitate oxidation
After the 4 and 8 week treatment periods, fat depots from ING, EPI, and RP regions were quickly removed and adipocytes were isolated from each tissue as previously described (7, 16). For palmitate oxidation, cells (5 × 105) were incubated in Krebs Ringer Buffer with HEPES (KRBH)-3.5% containing 0.2 μCi/ml of [1-14C]palmitic acid, 200 μM nonlabeled palmitate, and 500 μM of L-carnitine. Oxidation of palmitate was determined by collection of 14CO2 (7, 16).
Morphometric and ultrastructural analyses
Rats were anesthetized and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Subsequently, WAT depots were carefully dissected and postfixed by overnight immersion in the same fixative at 4°C. For light microscopy, tissues were washed in phosphate buffer and dehydrated using an ethanol gradient, cleared in a solution of xylene, and embedded into paraffin blocks (17). Once embedded, 3 μm thick sections were mounted onto glass slides, deparaffinized/rehydrated, and stained with hemotoxylin and eosin to assess morphological changes. Adipocyte size was calculated as the mean adipocyte area of 200 random adipocytes (100 per section) from each animal using the Nikon LUCIA Image program (version 4.61; Laboratory Imaging, Prague, Czech Republic). Tissue sections were observed with a Nikon Eclipse E800 light microscope using a 20× objective lens. Digital images were captured with a Nikon DXM 1200 camera. For electron microscopy, small fragments of tissue were excised from perfused animals and placed into a solution consisting of 2% paraformaldehyde + 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, overnight at 4°C. After primary fixation, tissues were placed in a solution of 1% osmium tetraoxide and 1% potassium ferrocyanide for 1 h. Samples were then dehydrated using an acetone gradient and embedded in an Epon-Araldite mixture (17). Subsequently, ultrathin sections (50–65 nm) were obtained with an MT-X ultratome (RMC; Tucson, AZ), stained with lead citrate, and examined with a CM10 transmission electron microscope (Philips; Eindhoven, The Netherlands). Mitochondrial and cytoplasmic areas were quantified using the LUCIA program from 30 to 40 images per tissue captured at 12,500× magnification. Mitochondrial density was determined as area of mitochondria occupying the area of cytoplasm.
Determination of content and phosphorylation of proteins by Western blot
Fat depots were extracted and immediately snap-frozen in liquid nitrogen. Immediately after decapitation, the hypothalamus was dissected using the optic tracts, the thalamus, and the mammillary body as landmarks, and then quickly frozen in liquid nitrogen and stored at −80°C. The tissues were homogenized in a buffer containing 25 mM Tris-HCl and 25 mM NaCl (pH 7.4), 1 mM MgCl2, 2.7 mM KCl, 1% Triton-X, and protease and phosphatase inhibitors (0.5 mM Na3VO4, 1 mM NaF, 1 μM leupeptin, 1 μM pepstatin, and 20 mM PMSF). Homogenates were centrifuged, the infranatant collected, and an aliquot was used to measure protein by the Bradford method. Samples were diluted 1:1 (v/v) with 2× Laemmli sample buffer, heated to 95°C for 5min, and subjected to SDS-PAGE. All primary antibodies were used in a dilution of 1:1,000 except for phospho-AMPK (1:500). Equal loading was confirmed by both β-actin detection and Coomasie blue staining of gels.
RESULTS
Food intake, body weight, fat mass, and adipocyte morphology
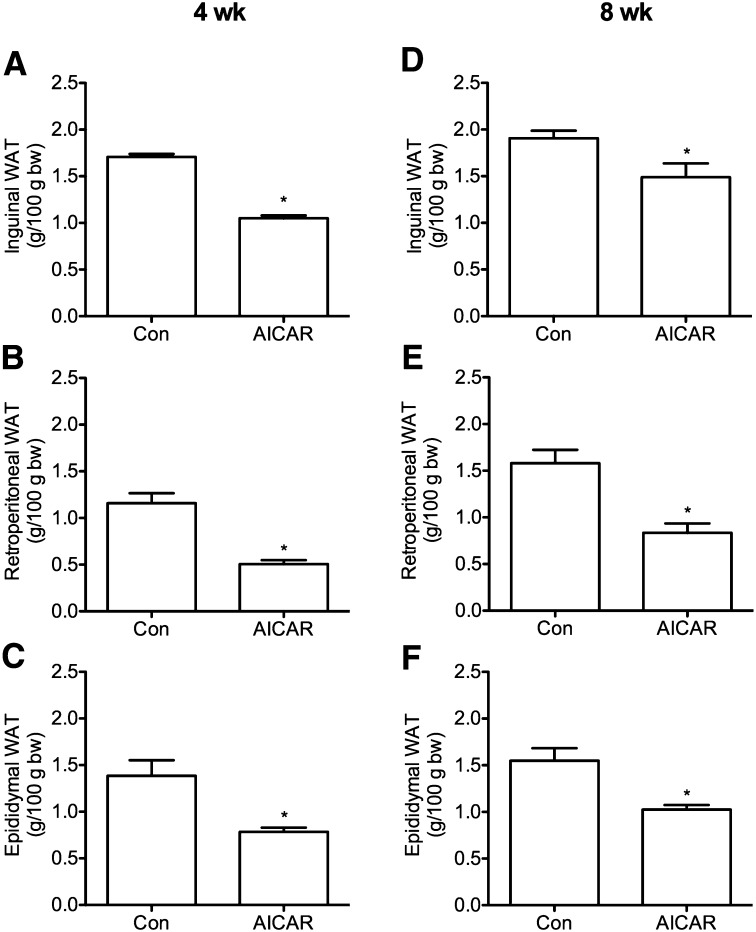
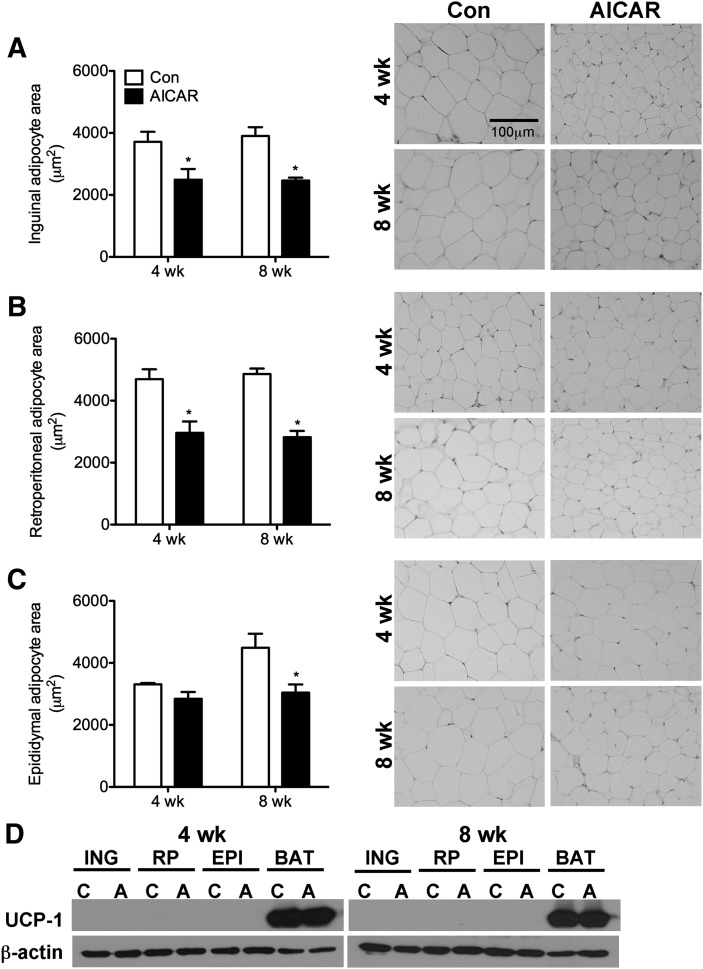
A reduction in food intake with AICAR injections has been observed in previous studies in rodents (18); therefore, food intake was measured on a daily basis in order to pair-feed control rats. Compared with ad libitum-fed rats, food intake was reduced by 10% and 3% after 4 and 8 weeks, respectively, in AICAR-treated rats. No differences in body weight or LBM were detected between control and AICAR-treated animals at any time point (data not shown). Analyses of WAT mass indicated that ING, RP, and EPI fat depots were reduced in AICAR-treated animals by 39%, 56%, and 43% after 4 weeks and by 22%, 47%, and 34% after 8 weeks, respectively (Fig. 1). In ING and RP fat depots, adipocyte area was reduced by 33% and 37% at week 4 and by 37% and 42% at week 8, respectively (Fig. 2A–C). Adipocyte area was unchanged in EPI fat pads after 4 weeks of AICAR treatment but was 33% lower than controls after 8 weeks (Fig. 2B). Western blot analyses of UCP-1 protein content (Fig. 2D) and immunohistochemistry in WAT depots (data not shown) indicated that AICAR treatment did not increase the prevalence of brown adipocytes within the WAT depots.
Fig. 1.
Fat pad mass of inguinal, retroperitoneal, and epididymal depots after 4 and 8 weeks of AICAR treatment (panels A–C and D–F, respectively). Fat pad mass was normalized to 100 g of total body weight of the animal at the time of tissue harvest. N=3–4 per group. *P < 0.05 versus control (Con).Data are expressed as mean ± SEM. Data are expressed as mean ± SEM.
Fig. 2.
Measurement of adipocyte area in 4 and 8 week animals in inguinal (A), retroperitoneal (B), and epididymal (C) fat depots. All images were taken at 20× magnification and represent the same field of view for comparative purposes. The scale bar represents 100 μm and applies to all panels. Analysis of UCP-1 protein content in inguinal (ING), retroperitoneal (RP), epididymal (EPI), and brown adipose tissue (BAT) depots at 4 and 8 weeks (D). Samples from control and AICAR-treated animals are denoted as ‘C’ and ‘A’, respectively. BAT was used as the positive control for the UCP-1 antibody and β-actin was used as a loading control. *P < 0.05 versus respective control (Con) for 4 or 8 weeks. Data are expressed as mean ± SEM.
Mitochondrial density and morphology
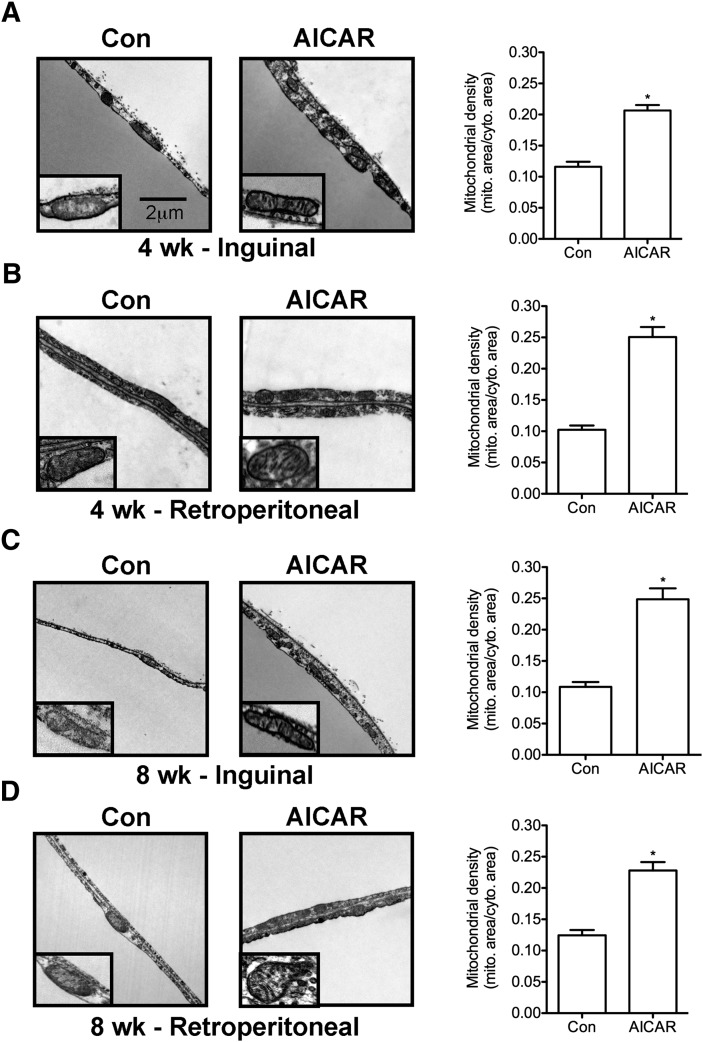
For assessment of mitochondrial density and ultrastructural changes, we analyzed ING and RP fat pads as representative depots for SC and VC WAT, respectively. After 4 and 8 weeks of AICAR treatment, mitochondrial density in both ING and RP WAT was increased by 1.78-fold and 2.45-fold and by 2.28-fold and 1.83-fold, respectively (Fig. 3A–D). Additionally, electron microscopy analysis revealed that adipocytes from rats treated with AICAR for 4 or 8 weeks had mitochondria with more defined cristae when compared with adipocytes from control animals (Fig. 3A–D, insets).
Fig. 3.
Mitochondrial density and morphology in inguinal (A, C) and retroperitoneal (B, D) fat depots at 4 and 8 weeks. Lipid droplet (LD). Arrows point to cristae within mitochondria (inset). All images were taken at 12,500× magnification and insets are magnified. Scale bar represents 2 μm and applies to all panels. N=3 per group. Unpaired t-tests. *P < 0.05 versus control (Con). Data are expressed as mean ± SEM.
Effects of AICAR on AMPK and ACC phosphorylation and palmitate oxidation
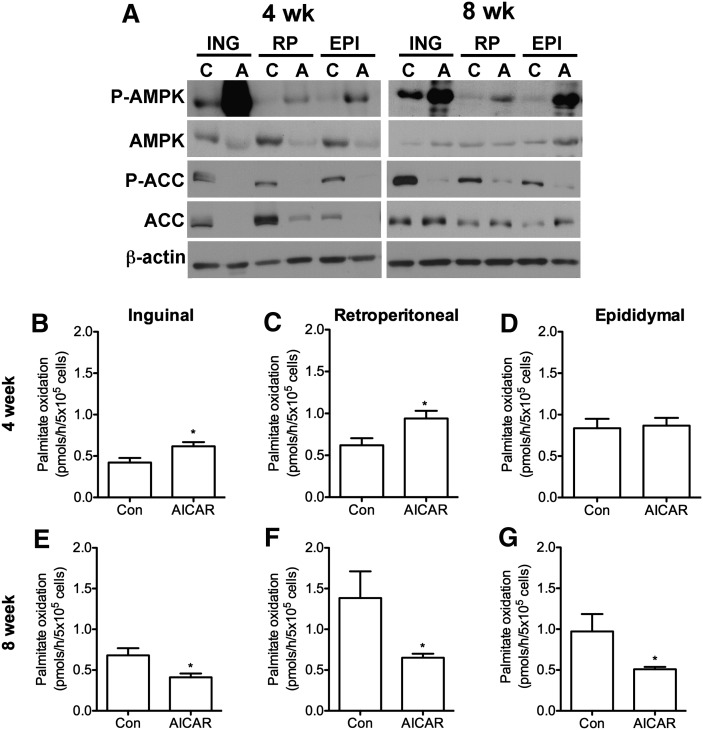
As expected, AMPK phosphorylation (Thr172) increased in ING, RP, and EPI fat depots after 4 and 8 weeks of AICAR treatment. The most pronounced increase in AMPK phosphorylation was in the ING fat pads (Fig. 4A). AMPK content in all fat depots was reduced in the AICAR group at week 4, an effect no longer present at week 8. ACC phosphorylation and protein content were markedly reduced at week 4 in ING, RP, and EPI fat pads; however the suppressive effect of AICAR on this variable was reversed at the 8 week time point with increased ACC content (Fig. 4A). Palmitate oxidation increased in ING and RP adipocytes by ∼1.46- and 1.84-fold, respectively, after 4 weeks of AICAR treatment, with no change observed in EPI cells (Fig. 4B–D). Conversely, after 8 weeks of AICAR treatment, intra-adipocyte palmitate oxidation reduced by 40%, 53%, and 48% in ING, RP, and EPI adipocytes, respectively (Fig. 4E–). Quantification of all WAT blots from 4 and 8 week animals are available in supplementary Figs. I and II, respectively.
Fig. 4.
Phosphorylation and content of AMPK and ACC in inguinal (ING), retroperitoneal (RP), and epididymal (EPI) fat pads at 4 and 8 weeks (A). Samples from control and AICAR-treated animals are denoted as ‘C’ and ‘A’, respectively. Blots are representative from N=3–4 per group. Palmitate oxidation in isolated adipocytes from ING (B, E), RP (C, F), and EPI (D, G) fat depots. Data are compiled from four independent experiments with N=4 per group, with triplicates in each assay performed. Unpaired t-tests. *P < 0.05 versus control (Con). Data are expressed as mean ± SEM.
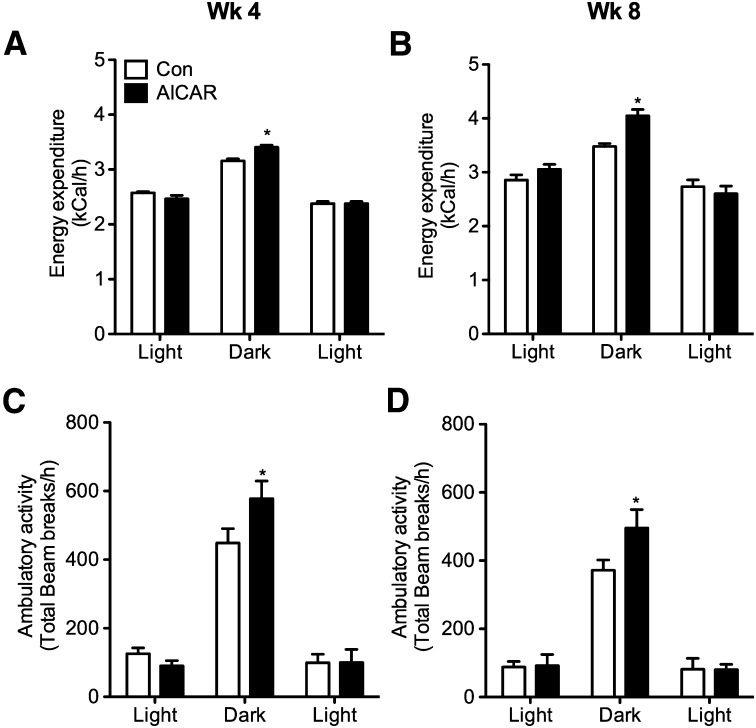
Whole-body EE, spontaneous ambulatory activity, and RER
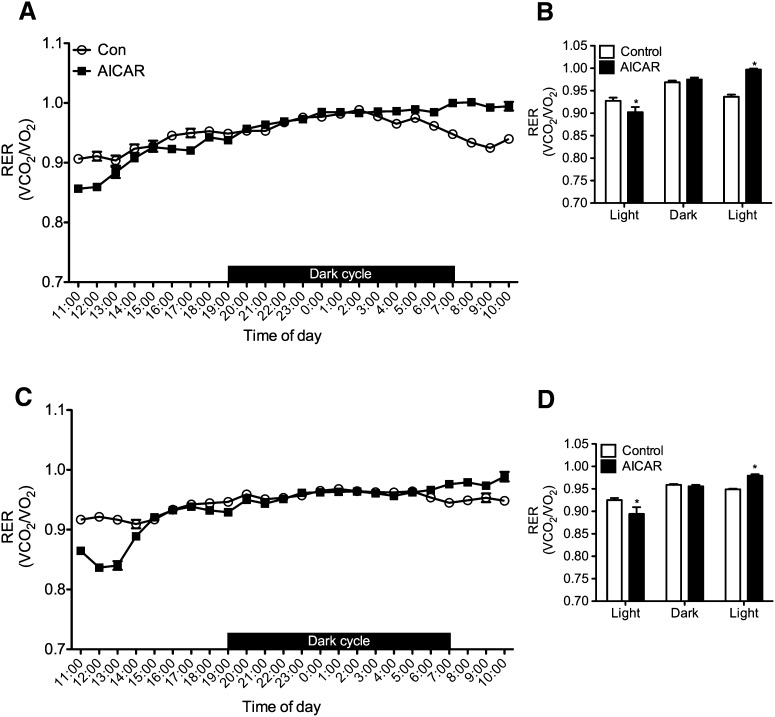
Baseline values were collected prior to commencing the study and no differences existed between the two groups with respect to EE and ambulatory activity (data not shown). Time-course analysis revealed that EE increased by 8% at week 4 and then by 16% at week 8 during the dark cycle in AICAR-treated rats (Fig. 5A, B). This was also accompanied by 29 and 33% increases in dark cycle ambulatory activity as compared with controls in weeks 4 and 8, respectively (Fig. 5C, D). RER was lower during the first light cycle (11:00 to 19:00) in AICAR-treated versus control rats (0.928 ± 0.007 vs. 0.902 ± 0.011 and 0.925 ± 0.005 vs. 0.0.894 ± 0.015) ∼8 h subsequent to AICAR injection after 4 (Fig. 6A, B) and 8 weeks (Fig. 6C, D) respectively, indicating a shift toward fat oxidation in AICAR-treated animals. No differences in RER were detected between control and AICAR-treated rats during the dark cycle. However, during the second light cycle that preceded the daily AICAR injection (07:00 to 10:00), RER was lower in control than AICAR-treated animals (0.937 ± 0.005 vs. 0.997 ± 0.002 and 0.949 ± 0.002 vs. 0.979 ± 0.003) after 4 and 8 weeks, respectively (Fig. 6). This resulted from the pair-feeding procedure in which the control animals consumed their allotment of food before the end of the dark cycle leading to a reduction in RER.
Fig. 5.
In vivo measurements of energy expenditure (A, B) and ambulatory activity (C, D). Rats were placed into the CLAMS for 24 h after 4 and 8 weeks of treatment. Values from all animals were averaged with N=4 per group. Data were analyzed based on light and dark cycles. The first light cycle occurred from 11:00 to 19:00, where the animals were placed into the CLAMS immediately after their daily saline or AICAR injections. The dark cycle occurred from 19:00 to 07:00, and the second light cycle is from 07:00 to 10:00 in order to complete a full 24 h cycle in the metabolic cages. Two-way ANOVA with Bonferroni post hoc tests. *P < 0.05 versus control (Con) for each particular light/dark cycle. Data are expressed as mean ± SEM.
Fig. 6.
Respiratory exchange ratio (RER) after 4 (A, B) and 8 weeks (C, D) of AICAR treatment. Rats were placed into the CLAMS for 24 h after 4 and 8 weeks of treatment. Panels B and D represent the average values for RER during the light and dark cycles and were analyzed using two-way ANOVA with Bonferroni post hoc tests. *P < 0.05 versus control (Con) for each particular light/dark cycle. Data are expressed as mean ± SEM.
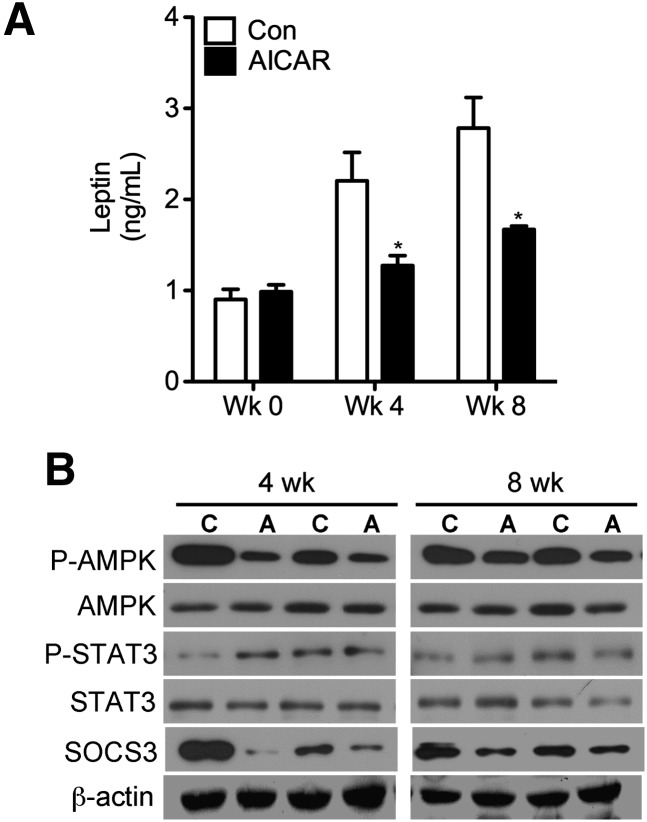
Effects of AICAR on plasma measurements and hypothalamic leptin signaling
At weeks 4 and 8 of treatment, plasma leptin in AICAR-injected animals was lower than controls by 42% and 48%, respectively (Fig. 7A). In the hypothalamus, STAT3 phosphorylation at tyrosine 705 was either unchanged or increased and AMPK phosphorylation was consistently reduced after 4 and 8 weeks of AICAR treatment, although the content of these proteins remained unchanged (Fig. 7B). Hypothalamic SOCS3 content was also markedly reduced in the AICAR group at 4 and 8 weeks. Quantification of all hypothalamic blots from 4 and 8 week animals are available in supplementary Fig. III.
Fig. 7.
Levels of circulating leptin at weeks 0, 4, and 8 in control (Con) and AICAR-treated animals (A). Assessment of phosphorylation and content of AMPK and SOCS3 in the hypothalami of rats at 4 and 8 weeks (B). Control and AICAR-treated animals are denoted as ‘C’ and ‘A’, respectively. Two-way ANOVA with Bonferroni post hoc tests. *P < 0.05 versus all other conditions; #P < 0.05 versus all other conditions. Data are expressed as mean ± SEM.
Effects of AICAR on the anorectic response to leptin
We found that in the absence of leptin administration (saline injected in lieu of leptin), AICAR animals showed a 30% reduction in food intake after 4 h compared with control rats (Table 1). This confirmed our previous observations that this drug caused an anorectic effect in these animals. When injected with leptin, control rats ate 35% less after 4 h compared with saline-injected controls, indicating a robust response to leptin administration. Interestingly, when injected with both AICAR and leptin, animals exhibited a further significant reduction in food intake of 23% compared with animals treated with AICAR alone. This equated to a profound ∼46% reduction in food intake when compared with control animals injected with saline. No differences in food intake were detected between all groups over a 24 h period (Table 1).
TABLE 1.
Food intake in control and AICAR-treated rats injected without or with leptin
| Food intake (g/4 h) | Food intake (g/24 h) | |||
| Saline | Leptin | Saline | Leptin | |
| Control | 12.50 ± 1.54 | 8.19 ± 1.28a | 29.40 ± 0.90 | 27.92 ± 0.87 |
| AICAR | 8.74 ± 0.20a | 6.76 ± 0.34b | 26.86 ± 2.00 | 28.80 ± 0.65 |
Animals were injected on a daily basis with either saline (vehicle) or AICAR (0.7 mg/kg b.w.) for 2 weeks. Subsequently, rats were fasted for 12 h prior to injection with either saline or leptin and refed ad libitum. Food intake was monitored 4 and 24 h postleptin injection as a measure of sensitivity to the anorectic effects of leptin. N=4 for each condition. Data expressed as mean ± SEM.
P < 0.05 compared with control-saline.
P < 0.05 compared with control-saline and AICAR-saline conditions.
DISCUSSION
Here, we report novel findings that chronic AICAR-induced AMPK activation promotes alterations in adipose tissue metabolism that leads to reduced VC and SC adiposity in rats. These effects were characterized by increased mitochondrial density and by the presence of mitochondria with more defined cristae in the WAT of AICAR-treated animals. This was also accompanied by time-dependent upregulation of FA oxidation in ING and RP but not in EPI adipocytes, indicating that not all VC fat depots change their metabolic profile in response to chronic AMPK activation. Our observations are in line with previous reports that EPI fat is more “resistant” to morphological and metabolic changes compared with ING and RP fat depots, which have greater plasticity and ability to change metabolic function upon stimulation (12). Interestingly, although AICAR treatment reduced food intake and increased EE, these animals still gained fat mass between the 4 and 8 week period similar to control animals. From an energy balance perspective, this could be due to the attenuated anorectic effect of AICAR at the 8 week time point (∼3% reduction in food intake) compared with 4 weeks (∼10% reduction in food intake). Additionally, the suppression of intra-adipocyte oxidation observed at 8 weeks would also prevent depletion of lipid content from the WAT. Therefore, the increase in EE must have been counteracted by the above factors and could help explain why AICAR-treated animals still gained fat mass between the 4 and 8 week time period.
A surprising finding of this study was that the ability of adipocytes to oxidize FAs was increased and reduced after 4 and 8 weeks of AICAR treatment, respectively. This occurred despite the fact that mitochondrial density was augmented at both time points, indicating that the activity of the oxidative machinery was regulated differently during the course of treatment. The precise mechanisms underlying these differences are not clear. It may be at least partially explained by the fact that the initial adaptive responses to AMPK activation in WAT were driven to shift toward oxidation of FAs. This is supported by our previous observations that oxidation of endogenous and exogenous FAs was increased whereas the costly processes of FA esterification and lipogenesis were potently suppressed in isolated adipocytes under prolonged AICAR exposure (7). Increased AMPK phosphorylation combined with undetectable levels of ACC protein is compatible with upregulation of FA oxidation seen after 4 weeks of AICAR treatment. However, FA oxidation was reduced at week 8 despite increased mitochondrial content in VC and SC adipocytes from AICAR-treated rats. The large reduction in intra-adipocyte FA oxidation could represent an adaptive response to accommodate the increased demand for the supply of FA to peripheral tissues, particularly skeletal muscles. In fact, skeletal muscles have been previously demonstrated to significantly increase their ability to oxidize fat upon chronic AICAR-induced AMPK activation (19, 20). Therefore, the reduction in intra-adipocyte FA oxidation seems compatible with the increased systemic demand for FA in AICAR-treated rats.
As expected with AICAR treatment, P-AMPK was increased in all fat depots at both 4 and 8 weeks regardless of alterations in protein content. Surprisingly, AICAR treatment did not induce phosphorylation of ACC as expected, as this enzyme is a well-known downstream target of AMPK. However, the profound decrease in total ACC content at 4 weeks in AICAR-treated animals would serve as a limiting factor for phosphorylation of this protein. In fact, it has been demonstrated in 3T3-L1 adipocytes that the content and/or activity of ACC are decreased in cells treated with various AMPK agonists, along with other adipogenic markers required for differentiation of preadipocytes into mature fat cells (4, 5). In our study, animals begin AICAR treatment at ∼6 to 7 weeks in age, which may have affected adipogenesis in preadipocytes present in the WAT. Interestingly, at 8 weeks, there was a rebound in ACC protein content with AICAR treatment although phosphorylation of this enzyme still remained suppressed in all fat depots. It is possible that the reemergence of ACC after 8 weeks of treatment was part of the adaptive metabolic response involved in the time-dependent downregulation of FA oxidation under conditions of reduced adiposity. Importantly, we also observed that ACC phosphorylation was not induced despite marked increases in AMPK phosphorylation at week 8 of AICAR treatment. This suggests that AMPK-independent mechanisms regulated ACC activity as adipocyte lipid content reduced with AICAR treatment. Work in our laboratory with adipocytes has demonstrated that certain metabolic effects of AICAR in WAT, such as FA oxidation and glucose uptake, are indeed mediated by AMPK (21, 22). However, because our treatment is in vivo and delivered systemically, we must acknowledge that AMPK-independent effects may be taking place resulting in changes to parameters affecting whole-body metabolism.
Our immunohistochemistry (data not shown) and Western blot analyses for the presence of UCP-1 in VC and SC fat depots demonstrated that brown adipogenesis by either transdifferentiation of white into brown adipocytes or recruitment of brown adipocyte precursors present within WAT was not induced with chronic AICAR-induced AMPK activation. Moreover, histological analysis excluded the development of UCP-1 negative multilocular adipocytes. Considering that AMPK is activated under conditions of ATP depletion and acts to restore intracellular energy levels (3), it is compatible that UCP-1 content and activity was not upregulated. This would allow substrate oxidation to be diverted toward ATP synthesis instead of nonshivering thermogenesis, ultimately serving to restore cellular energy levels. Therefore, our data provide evidence that the increased oxidative machinery of adipocytes exposed to chronic AICAR-induced AMPK activation did not result from white adipocytes converting to brown adipocytes. The absence of AMPK-induced BAT within WAT depots in our study is at odds with recent studies that have demonstrated this effect in mice exposed to chronic AICAR treatment (18). Species differences (mice vs. rats), duration of AICAR treatment (2 weeks vs. 4 and 8 weeks), dosage (0.5 mg/g b.w. vs. 0.7 mg/g b.w.) and frequency of injections (3 vs. 7 days a week) could explain the different responses to chronic AICAR treatment between these studies.
The observed elevation in whole-body EE after 4 (8%) and 8 weeks (16%) of AICAR treatment seems to be driven mainly by increased dark cycle locomotor activity. In fact, this variable was significantly increased by 29% and 33% after 4 and 8 weeks of AICAR treatment, respectively, which is in line with studies where AICAR-injected mice elicited enhanced exercise capacity (10). Increased substrate oxidation by itself in skeletal muscle and liver did not seem to have contributed to increased EE in AICAR-treated rats. This is based on the fact that EE was only increased in AICAR-treated rats during the dark cycle when RER data indicated that there was no difference in substrate oxidation between the pair-fed controls and AICAR-treated rats. Importantly, others have also shown that the increases in mitochondrial FA oxidation do not necessarily lead to elevated whole-body EE (2).
It has been demonstrated repeatedly in rodents and humans that a reduction in fat mass is almost invariably followed by activation of energy-sparing mechanisms, which causes resistance to continuous long-term reduction in adiposity (13, 23, 24). At the whole-body level, these energy-sparing mechanisms could counteract and offset potential fat-reducing effects induced by remodeling white adipocyte metabolism toward a more oxidative phenotype. The activation of energy-sparing mechanisms under conditions of reduced adiposity has been attributed to a drop in circulating leptin because exogenous replacement of this hormone to preweight-loss values reverses this adaptive response (24). In order to exert its effects on energy balance (25), leptin must bind to its receptor (LepR), permitting STAT3 to dock onto the LepR. STAT3 is then phosphorylated, homodimerizes, and enters the nucleus to promote the expression of genes that inhibit food intake and increase EE. It also promotes the expression of SOCS3, which serves as a negative feedback regulator of leptin signaling. Therefore, although activation of AMPK in the hypothalamus induces food intake and favors weight gain, inhibition of this kinase by leptin blocks these centrally-mediated effects on energy balance (26, 27). Based on these observations, we hypothesized that energy-sparing mechanisms would be activated in AICAR-treated rats. This could occur by AICAR directly increasing hypothalamic AMPK activity, as previous studies have demonstrated that this drug can cross the blood-brain barrier with limited permeability (28). Alternatively, AICAR could indirectly lead to activation of energy-sparing mechanisms by reducing circulating leptin as a consequence of fat loss induced by prolonged AMPK activation in SC and VC fat depots. Time-course analyses revealed that circulating leptin indeed dropped ∼40% in AICAR-treated rats when compared with pair-fed controls, which was compatible with the differences in adiposity between the groups. However, phosphorylation of hypothalamic AMPK was reduced, whereas STAT3 phosphorylation was either the same or increased in AICAR-injected animals when compared with controls after 4 and 8 weeks. Furthermore, these effects were accompanied by marked reductions in the content of SOCS3 in the hypothalamus. These findings indicated that leptin sensitivity in this tissue was actually increased with systemic AICAR-induced AMPK activation. In line with this hypothesis, our data suggest that with respect to the anorectic effects of leptin, AICAR-treated animals showed a greater sensitivity to this hormone compared with their control counterparts. This is corroborated by our hypothalamic signaling data indicating that P-AMPK was reduced in AICAR-treated rats. Interestingly, leptin has been demonstrated to play a major role in relaying to the hypothalamus information regarding the amount of energy stored in the organism, allowing the central nervous system to regulate nonexercise activity thermogenesis accordingly (23, 29, 30). Therefore, the increases in ambulatory activity and EE observed in this study could also be attributed to enhanced central nervous system leptin sensitivity. Further studies are required to investigate possible direct actions of AICAR on specific hypothalamic sites involved in the control of food intake and energy homeostasis.
Altogether, our novel findings provide evidence that significant reductions in adiposity may be achieved through systemic pharmacological AMPK activation. Based on our data, this approach causes neither WAT to BAT transdifferentiation nor recruitment of brown adipocyte precursors within WAT. Rather, it increases the oxidative machinery in white adipocytes and induces remodeling of WAT metabolism toward a catabolic state. Importantly, the adipose-reducing effects of chronic AICAR-induced AMPK activation increased hypothalamic leptin sensitivity, and thus did not trigger the typical centrally-mediated adaptive responses that oppose long-term weight loss.
Supplementary Material
Acknowledgments
The authors thank Cristina Maria Zingaretti from the University of Ancona for her technical expertise in acquiring transmission electron microscope images.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMPK
- AMP-kinase
- BAT
- brown adipose tissue
- b.w.
- body weight
- CLAMS
- Comprehensive Laboratory Animal Monitoring System
- EE
- energy expenditure
- EPI
- epididymal
- ING
- inguinal
- i.p.
- intraperitoneal
- LBM
- lean body mass
- PGC-1α
- peroxisome proliferator-activated receptor coactivator-1α
- PPAR
- peroxisome proliferator-activated receptor
- RER
- respiratory exchange ratio
- RP
- retroperitoneal
- SC
- subcutaneous
- SOCS3
- suppressor of cytokine signaling 3
- STAT3
- signal transducer and activator of transcription 3
- UCP-1
- uncoupling protein 1
- VC
- visceral
- WAT
- white adipose tissue
This research was funded by an operating grant from the Canadian Institute of Health Research (CIHR MOP-86538) and by infrastructure grants from the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF) awarded to R.B.C. R.B.C. is also a recipient of the CIHR New Investigator Award and the Early Research Award from the Ontario Ministry of Research and Innovation. The morphometry and imaging portions of the study were funded by a Scientific Research Grant (RSA) of Università Politecnica delle Marche to S.C. M.P.G. was supported in part by the CIHR Michael Smith Foreign Study Supplement and is currently supported by the Canadian Diabetes Association (CDA) Doctoral Student Research Award. A.F. is supported by an Italian foundation for Research and Development (Fondazione Cariverona). The authors state no conflict or duality of interest in regards to this work.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Rosenbaum M., Leibel R. L. 1998. The physiology of body weight regulation: relevance to the etiology of obesity in children. Pediatrics. 101: 525–539. [PubMed] [Google Scholar]
- 2.Hoehn K. L., Turner N., Swarbrick M. M., Wilks D., Preston E., Phua Y., Joshi H., Furler S. M., Larance M., Hegarty B. D., et al. 2010. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 11: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie D. G. 2007. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 8: 774–785. [DOI] [PubMed] [Google Scholar]
- 4.Habinowski S. A., Witters L. A. 2001. The effects of AICAR on adipocyte differentiation of 3T3–L1 cells. Biochem. Biophys. Res. Commun. 286: 852–856. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., Wang D., Zhu Q., Gao X., Yang S., Xu A., Wu D. 2009. Inhibitory effects of A-769662, a novel activator of AMP-activated protein kinase, on 3T3–L1 adipogenesis. Biol. Pharm. Bull. 32: 993–998. [DOI] [PubMed] [Google Scholar]
- 6.Canto C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. 2009. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 458: 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaidhu M. P., Fediuc S., Anthony N. M., So M., Mirpourian M., Perry R. L., Ceddia R. B. 2009. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 50: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaidhu M. P., Ceddia R. B. 2011. The role of adenosine monophosphate kinase in remodeling white adipose tissue metabolism. Exerc. Sport Sci. Rev. 39: 102–108. [DOI] [PubMed] [Google Scholar]
- 9.Buhl E. S., Jessen N., Pold R., Ledet T., Flyvbjerg A., Pedersen S. B., Pedersen O., Schmitz O., Lund S. 2002. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 51: 2199–2206. [DOI] [PubMed] [Google Scholar]
- 10.Narkar V. A., Downes M., Yu R. T., Embler E., Wang Y. X., Banayo E., Mihaylova M. M., Nelson M. C., Zou Y., Juguilon H., et al. 2008. AMPK and PPARdelta agonists are exercise mimetics. Cell. 134: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winder W. W., Holmes B. F., Rubink D. S., Jensen E. B., Chen M., Holloszy J. O. 2000. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88: 2219–2226. [DOI] [PubMed] [Google Scholar]
- 12.Frontini A., Cinti S. 2010. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 11: 253–256. [DOI] [PubMed] [Google Scholar]
- 13.Leibel R. L. 2008. Energy in, energy out, and the effects of obesity-related genes. N. Engl. J. Med. 359: 2603–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo R. L., Andrade B. M., Padron A. S., Gaidhu M. P., Perry R. L., Carvalho D. P., Ceddia R. B. 2010. High-fat diet increases thyrotropin and oxygen consumption without altering circulating 3,5,3′-triiodothyronine (T3) and thyroxine in rats: the role of iodothyronine deiodinases, reverse T3 production, and whole-body fat oxidation. Endocrinology. 151: 3460–3469. [DOI] [PubMed] [Google Scholar]
- 15.Patterson C. M., Bouret S. G., Dunn-Meynell A. A., Levin B. E. 2009. Three weeks of postweaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296: R537–R548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidhu M. P., Anthony N. M., Patel P., Hawke T. J., Ceddia R. B. 2010. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 298: C961–C971. [DOI] [PubMed] [Google Scholar]
- 17.Cinti S., Zingaretti M. C., Cancello R., Ceresi E., Ferrara P. 2001. Morphologic techniques for the study of brown adipose tissue and white adipose tissue. Methods Mol. Biol. 155: 21–51. [DOI] [PubMed] [Google Scholar]
- 18.Vila-Bedmar R., Lorenzo M., Fernandez-Veledo S. 2010. Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology. 151: 980–992. [DOI] [PubMed] [Google Scholar]
- 19.Smith A. C., Bruce C. R., Dyck D. J. 2005. AMP kinase activation with AICAR simultaneously increases fatty acid and glucose oxidation in resting rat soleus muscle. J. Physiol. 565: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrill G. F., Kurth E. J., Hardie D. G., Winder W. W. 1997. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 273: E1107–E1112. [DOI] [PubMed] [Google Scholar]
- 21.Gaidhu M. P., Fediuc S., Ceddia R. B. 2006. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J. Biol. Chem. 281: 25956–25964. [DOI] [PubMed] [Google Scholar]
- 22.Gaidhu M. P., Perry R. L., Noor F., Ceddia R. B. 2010. Disruption of AMPKalpha1 signaling prevents AICAR-induced inhibition of AS160/TBC1D4 phosphorylation and glucose uptake in primary rat adipocytes. Mol. Endocrinol. 24: 1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., Gallagher D., Mayer L., Murphy E., Leibel R. L. 2005. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 115: 3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum M., Murphy E. M., Heymsfield S. B., Matthews D. E., Leibel R. L. 2002. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 87: 2391–2394. [DOI] [PubMed] [Google Scholar]
- 25.Bates S. H., Stearns W. H., Dundon T. A., Schubert M., Tso A. W., Wang Y., Banks A. S., Lavery H. J., Haq A. K., Maratos-Flier E., et al. 2003. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 421: 856–859. [DOI] [PubMed] [Google Scholar]
- 26.Gao S., Kinzig K. P., Aja S., Scott K. A., Keung W., Kelly S., Strynadka K., Chohnan S., Smith W. W., Tamashiro K. L., et al. 2007. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc. Natl. Acad. Sci. USA. 104: 17358–17363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferre P., Birnbaum M. J., et al. 2004. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 428: 569–574. [DOI] [PubMed] [Google Scholar]
- 28.Marangos P. J., Loftus T., Wiesner J., Lowe T., Rossi E., Browne C. E., Gruber H. E. 1990. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia. 31: 239–246. [DOI] [PubMed] [Google Scholar]
- 29.Novak C. M., Levine J. A. 2007. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J. Neuroendocrinol. 19: 923–940. [DOI] [PubMed] [Google Scholar]
- 30.Nogueiras R., Lopez M., Lage R., Perez-Tilve D., Pfluger P., Mendieta-Zeron H., Sakkou M., Wiedmer P., Benoit S. C., Datta R., et al. 2008. Bsx, a novel hypothalamic factor linking feeding with locomotor activity, is regulated by energy availability. Endocrinology. 149: 3009–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.