Abstract
Insulin signaling in the central nervous system (CNS) is required for the inhibitory effect of insulin on glucose production. Our aim was to determine whether the CNS is also involved in the stimulatory effect of circulating insulin on the tissue-specific retention of fatty acid (FA) from plasma. In wild-type mice, hyperinsulinemic-euglycemic clamp conditions stimulated the retention of both plasma triglyceride-derived FA and plasma albumin-bound FA in the various white adipose tissues (WAT) but not in other tissues, including brown adipose tissue (BAT). Intracerebroventricular (ICV) administration of insulin induced a similar pattern of tissue-specific FA partitioning. This effect of ICV insulin administration was not associated with activation of the insulin signaling pathway in adipose tissue. ICV administration of tolbutamide, a KATP channel blocker, considerably reduced (during hyperinsulinemic-euglycemic clamp conditions) and even completely blocked (during ICV administration of insulin) WAT-specific retention of FA from plasma. This central effect of insulin was absent in CD36-deficient mice, indicating that CD36 is the predominant FA transporter in insulin-stimulated FA retention by WAT. In diet-induced insulin-resistant mice, these stimulating effects of insulin (circulating or ICV administered) on FA retention in WAT were lost. In conclusion, in insulin-sensitive mice, circulating insulin stimulates tissue-specific partitioning of plasma-derived FA in WAT in part through activation of KATP channels in the CNS. Apparently, circulating insulin stimulates fatty acid uptake in WAT but not in BAT, directly and indirectly through the CNS.
Keywords: brain, insulin resistance, lipid metabolism, lipoprotein lipase, triglycerides, brown adipose tissue
The central nervous system (CNS) is highly sensitive to insulin (1–6). Insulin in the brain is mostly derived from the circulation, and only a modest amount, if any, is produced locally (6, 7). Circulating insulin can cross the blood-brain barrier (8, 9) and exert metabolic effects in peripheral organs via the CNS. Intracerebroventricular (ICV) administration of insulin decreases food intake, resulting in reduced body weight (3, 10, 11). In addition, the central action of insulin plays a crucial role in the inhibitory effect of the hormone on hepatic glucose production (4, 12).
Recently, a novel regulatory function for the effects of insulin through the CNS with regard to adipose tissue metabolism has been proposed, suggesting that intracellular lipolysis and lipogenesis in WAT is under the neuronal control of central insulin (13, 14). Furthermore, it has been shown that central glucose lowers plasma triglyceride (TG) levels by inhibiting the secretion of TG-rich lipoproteins by the liver (15). Taken together, these observations imply an important role of the CNS in general and of central effects of insulin in the CNS in particular in the regulation of whole body TG metabolism.
The aim of the present study was to determine whether circulating insulin affects tissue-specific fatty acid (FA) partitioning from plasma through effects in the CNS. Using a dual tracer method, we report here that circulating insulin stimulates the uptake of TG-derived FA as well as albumin-bound FA specifically in WAT during hyperinsulinemic-euglycemic clamp conditions. Short-term (2.5 h) ICV administration of insulin stimulates the retention of FA in WAT in a similar fashion. This effect, which requires the presence of the long-chain FA transporter CD36, was prevented by inhibition of central ATP-dependent potassium (KATP) channels. Moreover, we show that the increase in FA retention in WAT, induced by hyperinsulinemic-euglycemic clamp conditions, is considerably reduced by inhibition of KATP channels in the CNS, demonstrating that the well-known effect of circulating insulin on insulin-mediated FA retention in WAT is mediated to a considerable extent by the effects of circulating insulin in the CNS. Finally, we show that these stimulating effects of insulin on FA retention through the CNS in WAT are lost in diet-induced obese mice.
MATERIALS AND METHODS
Animals
Male wild-type (WT) and CD36−/− mice (15 weeks old, both on C57Bl/6J background) were housed in a temperature-controlled room on a 12 h light/dark cycle and were fed a standard mouse chow diet with free access to water. In the diet-induced obesity experiment, mice were fed ad libitum high-fat diet for 12 weeks (45 energy% of fat derived from palm oil; Research Diet Services BV, Wijk bij Duurstede, The Netherlands). All animal experiments were performed in accordance with the regulations of Dutch law on animal welfare, and the Institutional Ethics Committee for Animal Procedures from the Leiden University Medical Center, Leiden, The Netherlands, approved the protocol.
Surgical procedure
For ICV cannula implantation, the mice were anesthetized with 0.5 mg/kg Medetomidine (Pfizer, Capelle a/d IJssel, The Netherlands), 5 mg/kg Midazolam (Roche, Mijdrecht, The Netherlands) and 0.05 mg/kg Fentanyl (Janssen-Cilag, Tilburg, The Netherlands) and placed in a stereotactic device (TSE Systems, Homburg, Germany). A 25 gauge guide cannula was implanted into the left lateral ventricle using the following coordinates from Bregma: 1.0 mm lateral, 0.46 mm posterior, and 2.2 mm ventral. The guide cannula was secured to the skull surface with dental cement (GC Europe N.V., Leuven, Belgium) and the anesthesia was antagonized using 2.5 mg/kg Antipamezol (Pfizer, Capelle a/d IJssel, The Netherlands), 0.5 mg/kg Flumazenil (Roche, Mijdrecht, The Netherlands) and 1.2 mg/kg Naloxon (Orpha, Purkersdorf, Austria). After a recovery period of one week, cannula placement was verified. Mice that ate more than 0.3 g in 1 h in response to ICV injection of 5 µg neuropeptide Y (NPY, Bachem, St. Helens, United Kingdom) in 1 µl of artificial cerebrospinal fluid (aCSF) (Harvard Apparatus, Natick, MA) were considered to have the cannula correctly placed and were included in the study (16).
Preparation of radiolabeled emulsion particles
Protein-free VLDL-like TG-rich emulsion particles were prepared from 100 mg total lipid at a weight ratio of triolein (Sigma, St. Louis, MA): egg yolk phosphatidylcholine (Lipoid, Ludwigshafen, Germany): lysophosphatidylcholine (Sigma): cholesteryl oleate (Janssen, Beersse, Belgium): cholesterol (Sigma) of 70: 22.7: 2.3: 3.0: 2.0 in the presence of 200 µCi of glycerol tri[9,10(n)-3H]oleate ([3H]TG) (GE Healthcare, Little Chalfont, United Kingdom), as previously described (17). Lipids were hydrated in 10 ml of 2.4 M sodium chloride, 10 mM Hepes, 1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4, and sonicated for 30 min at 10 µm output using a Soniprep 150 (MSE Scientific Instruments, United Kingdom) equipped with a water bath for temperature (54°C) maintenance. Subsequently, the emulsion particles were divided into fractions with a different average size by density gradient ultracentrifugation. Intermediate-sized (80 nm) [3H]TG-labeled particles were mixed with a trace amount of [14C]oleic acid ([14C]FA) (GE Healthcare) complexed with BSA in a 3H:14C ratio of 3:1.
Tissue-specific TG and FA partitioning
Postabsorptive (mice were fasted for 15 h with food withdrawn at 18:00 h the day prior to the study), body weight-matched male mice were anesthetized with 6.25 mg/kg Acepromazine (Alfasan, Woerden, The Netherlands), 6.25 mg/kg Midazolam (Roche, Mijdrecht, The Netherlands), and 0.31 mg/kg Fentanyl (Janssen-Cilag, Tilburg, The Netherlands). In the hyperinsulinemic-euglycemic clamp studies, insulin (Actrapid, Novo Nordisk, Bagsværd, Denmark) was administered intravenously by primed (4.5 mU), continuous (6.8 mU/h for 2.5 h) infusion to attain steady-state circulating insulin levels of ∼4 ng/ml (16, 18, 19). A variable intravenous infusion of a 12.5% D-glucose solution was used to maintain euglycemia as determined at 10 min intervals via tail bleeding (<3 µl, Accu-chek, Sensor Comfort; Roche Diagnostics, Mannheim, Germany). Insulin (0.5 mU/h, Actrapid; Novo Nordisk, Bagsværd, Denmark) or the KATP channel blocker tolbutamide (12 nmol/h; Sigma) (4, 20) dissolved in aCSF was infused intracerebroventricularly at a constant rate of 2.5 µl/h during the entire experiment using a Harvard infusion pump. The dose of ICV insulin was ascertained in a dose-finding study to ensure that plasma insulin and glucose levels did not change. Control animals received 5% DMSO in aCSF. Thirty minutes after the start of the ICV infusions or thirty minutes after the start of the hyperinsulinemic-euglycemic clamp experiments (as indicated), an intravenous infusion of the glycerol tri[3H]oleate-labeled emulsion particles (0.33 nmol/h) together with albumin-bound [14C]oleic acid (10.22 nmol/h) was started at a rate of 100 µl/h and maintained for 2 h. Blood samples were taken at baseline and 2 h after starting the intravenous infusion. Subsequently, the mice were euthanized, and organs were quickly harvested and snap frozen in liquid nitrogen.
Plasma analysis
Blood samples were taken from the tail tip into chilled capillaries coated with paraoxon to prevent ex vivo lipolysis. The tubes were placed on ice and centrifuged at 4°C. Plasma levels of TG, free fatty acids (FFA), and glucose were determined using commercially available kits and standards according to the instructions of the manufacturer (Instruchemie, Delfzijl, The Netherlands). Plasma insulin levels were measured using a mouse-specific insulin ELISA kit (Mercodia AB, Uppsala, Sweden) and plasma leptin levels using a rat/mouse-specific ELISA kit (Millipore, St Charles, MO). Lipids were extracted from plasma according to Bligh and Dyer (21). The lipid fraction was dried under nitrogen, dissolved into chloroform/methanol (5:1 v/v) and subjected to thin layer chromatography (TLC) (LK5D gel 150; Whatman) using hexane: diethylether: acetic acid (83:16:1 v/v/v) as mobile phase. Standards for TG and FA were included during the TLC procedure to locate spots of these lipids. Spots were scraped, lipids were dissolved in hexane, and radioactivity was measured (22).
Tissue-specific FA retention analysis
Tissues were dissolved in 5 M potassium hydroxide in 50% (v/v) ethanol. After overnight saponification, protein content was determined in the various organs using a bicinchoninic acid (BCA) protein assay kit (BCA Protein Assay Kit, Thermo Scientific Pierce Protein Research Products, Rockford, IL). Retention of radioactivity in the saponified tissues was measured per milligram of protein and corrected for the corresponding plasma-specific activities of [3H]TG and [14C]FA (23, 24).
Western blot analysis
Tissues were homogenized by Ultra-Turrax (22.000 rpm; 2 × 5 s) in a 10:1 (v/w) ratio of ice-cold buffer containing 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.6), 50 mM sodium fluoride, 50 mM potassium chloride, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 5 mM β-glycerophosphate, 1 mM sodium vanadate, 1 mM dithiothreitol, 1% nonyl phenoxypolyethoxylethanol (Tergitol-type NP40), and protease inhibitors cocktail (Complete, Roche, Mijdrecht, The Netherlands). Homogenates were centrifuged (13,200 rpm; 15 min, 4°C) and the protein content of the supernatant was determined using the BCA protein assay kit. Proteins (10-30 µg) were separated by 7-10% SDS-PAGE followed by transfer to a polyvinylidene fluoride transfer membrane. Membranes were blocked for 1 h at room temperature in tris-buffered saline Tween-20 buffer with 5% nonfat dry milk followed by an overnight incubation with phospho-specific or total antibodies (all from Cell Signaling Technology, Beverly, MA). Blots were then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Bands were visualized by ECL and quantified using Image J (National Institutes of Health).
Statistical analysis
All data are presented as means ± SEM. Most data were analyzed using SPSS. A Kruskall-Wallis test for several independent samples was used, followed by a Mann-Whitney test for independent samples. P values less than 0.05 were considered statistically significant.
RESULTS
Intravenous administration of insulin increases the retention of plasma TG-derived and albumin-bound FA by WAT
To establish to what extent tissue-specific FA uptake from plasma is stimulated by circulating insulin, we compared in various organs the retention of FA derived from both glycerol tri[3H]oleate-labeled VLDL-like emulsion particles and albumin-bound [14C]oleic acid between mice during hyperinsulinemic-euglycemic clamp conditions and mice in basal conditions infused with vehicle (Fig. 1). During the clamp study, insulin levels were significantly higher compared with basal conditions (4.4 versus 0.3 ng/ml, P < 0.01), whereas average plasma glucose levels remained similar (6.1 versus 5.4 mmol/l, not significant). Plasma TG levels were similar (0.3 versus 0.4 mmol/l, not significant), whereas plasma FFA levels decreased upon insulin administration (0.3 versus 0.7 mmol/l, P < 0.05).
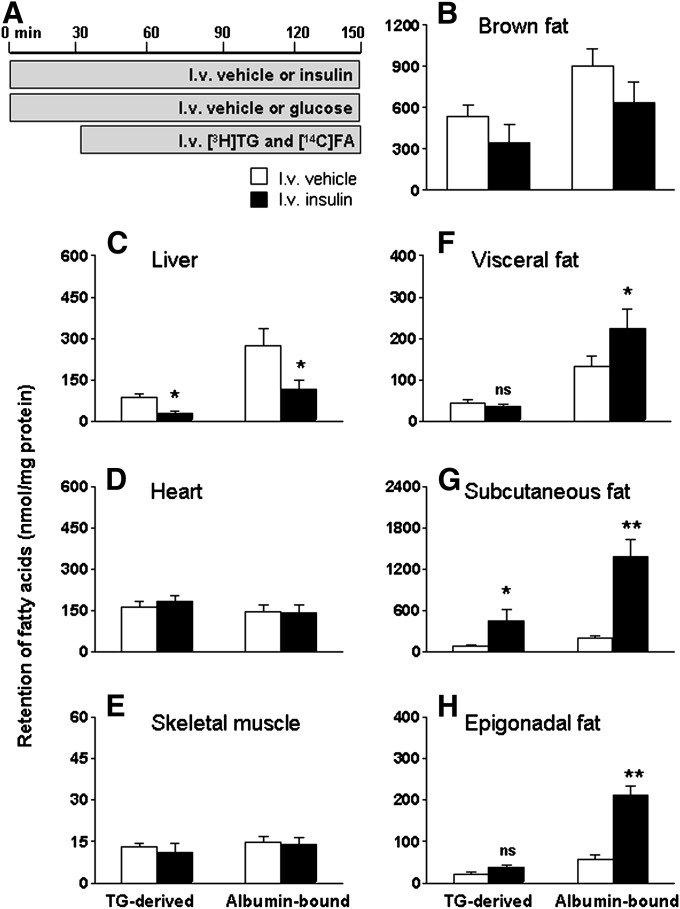
Fig. 1.
Circulating insulin stimulates FA retention by WAT while decreasing FA retention by liver. Postabsorptive, body weight-matched WT mice received continuous intravenous infusion of vehicle (white bars) or insulin/glucose (hyperinsulinemic-euglycemic clamp study, black bars) (A). Infusion of glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid was started 30 min after the start of intravenous infusion of vehicle or glucose/insulin and maintained for 2 h. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in brown fat (B), liver (C), heart (D), skeletal muscle (E), visceral fat (F), subcutaneous fat (G), and epigonadal fat (H). Values are means ± SEM for at least five mice per group. *P < 0.05 versus vehicle, **P < 0.01 versus vehicle.
As shown in Fig. 1, hyperinsulinemia decreased the retention of plasma TG-derived FA and albumin-bound FA in liver (Fig. 1C) without affecting the retention in brown adipose tissue (BAT) (Fig. 1B), heart (Fig. 1D), or skeletal muscle (Fig. 1E). Moreover, hyperinsulinemic-euglycemic clamp conditions increased albumin-bound FA retention by 68%, (P < 0.05) in visceral fat and TG-derived FA and albumin-bound FA retention by 397% (P < 0.05) and 579% (P < 0.01), respectively, in subcutaneous fat and by 68% (not significant) and 267% (P < 0.01), respectively, in epigonadal fat pads (Fig. 1F–H).
ICV administration of insulin increases the retention of plasma TG-derived and albumin-bound FA by WAT
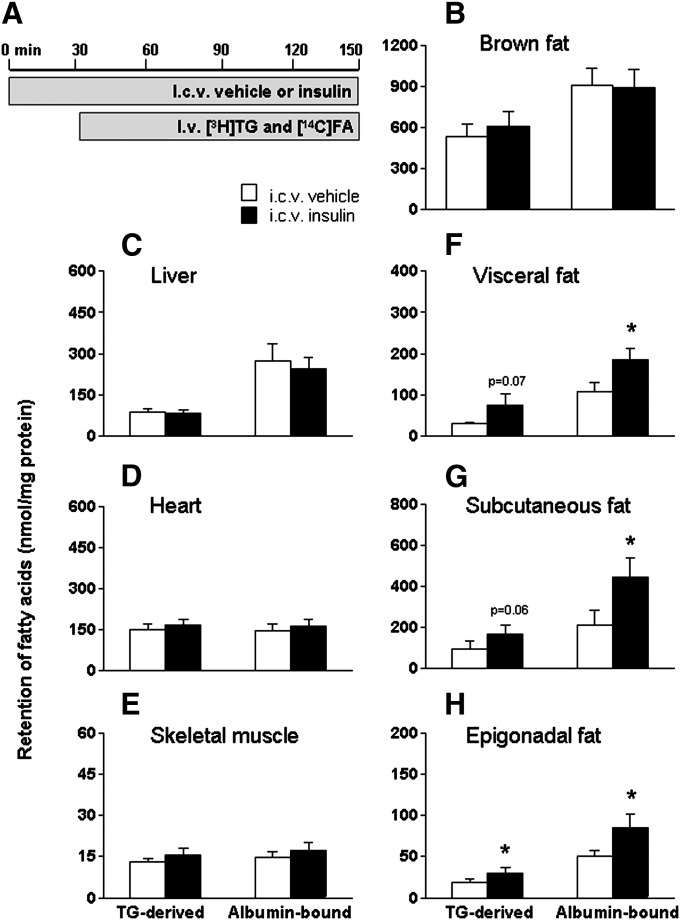
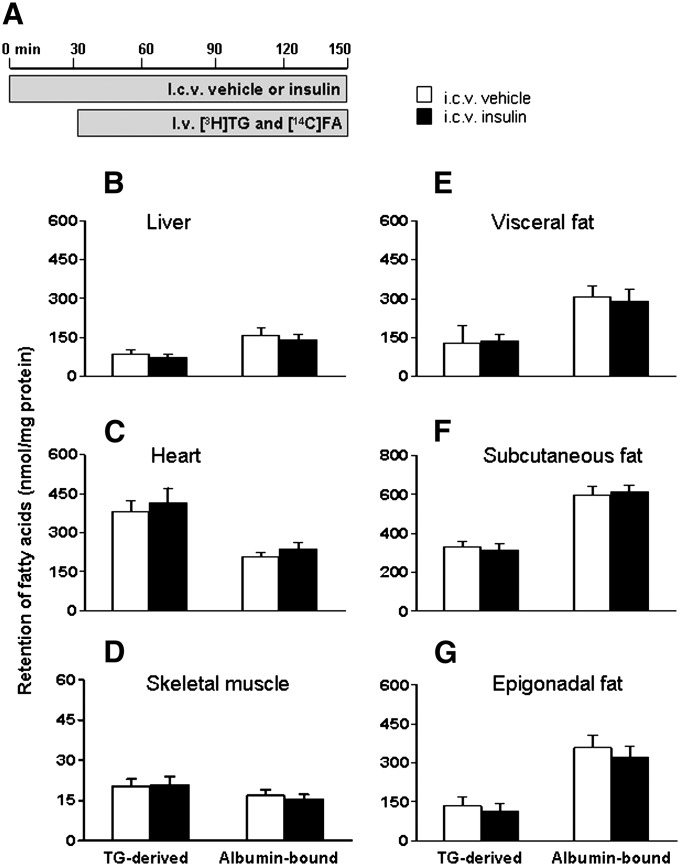
Subsequently, the dual-tracer method was used to determine tissue-specific FA retention after ICV administration of insulin (0.5 mU/h for 2.5 h) (Fig. 2). Although ICV insulin administration did not alter circulating levels of glucose, TG, FFA, insulin, or leptin, it decreased the plasma half-life of [3H]TG by 34% (P < 0.05) (Table 1). Central insulin administration did not affect the retention of TG-derived FA and albumin-bound FA in brown adipose tissue, liver, heart, or skeletal muscle (Fig. 2B–E). In contrast, ICV insulin administration significantly increased FA retention in the different fat compartments (Fig. 2F–H): ICV insulin increased the retention of TG-derived FA and albumin-bound FA by 145% (P = 0.07) and 71% (P < 0.05) in visceral fat, by 72% (P = 0.06) and 111% (P < 0.05) in subcutaneous fat, and by 58% (P < 0.05) and 70% (P < 0.05) in epigonadal fat, respectively.
Fig. 2.
ICV insulin administration stimulates FA retention by WAT. Postabsorptive, body weight-matched WT mice received continuous ICV infusion of vehicle (white bars) or insulin (black bars, 0.5 mU/h) (A). Thirty minutes after starting the ICV infusion, the mice were infused for 2 h with glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in brown fat (B), liver (C), heart (D), skeletal muscle (E), visceral fat (F), subcutaneous fat (G), and epigonadal fat (H). Values are means ± SEM for at least seven mice per group. *P < 0.05 versus vehicle.
TABLE 1.
ICV insulin administration does not affect plasma parameters, except for [3H]TG half-life
| At Baseline |
After Two Hours |
|||
| ICV Vehicle | ICV Insulin | ICV Vehicle | ICV Insulin | |
| Glucose (mmol/l) | 4.9 ± 0.2 | 5.5 ± 0.3 | 4.7 ± 0.2 | 4.8 ± 0.4 |
| TG (mmol/l) | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| FFA (mmol/l) | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Insulin (ng/ml) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Leptin (ng/ml) | 1.0 ± 0.1 | 1.3 ± 0.4 | 1.3 ± 0.1 | 1.4 ± 0.3 |
| Half-life [14C]FA (min) | – | – | 0.6 ± 0.2 | 0.6 ± 0.1 |
| Half-life [3H]TG (min) | – | – | 3.5 ± 0.1 | 2.3 ± 0.1a |
Plasma parameters of mice that received ICV infusion of vehicle or insulin obtained at baseline and 2 h after starting an intravenous infusion of glycerol tri[3H]oleate-labeled VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Values are means ± SEM for at least nine mice per group.
P < 0.05 versus vehicle.
The effect of ICV insulin administration on FA retention in WAT is independent of modulation of insulin, leptin, or cAMP-dependent signaling pathways in WAT
To investigate the molecular mechanism(s) underlying the ICV insulin-induced FA retention in WAT, we studied various signaling pathways involved in the regulation of FA metabolism in both epigonadal and visceral fat. In agreement with the absence of any effect on plasma insulin levels (Table 1), ICV insulin administration did not affect peripheral insulin signaling pathways, as neither PKB phosphorylation on Ser473 and Thr308 (supplementary Fig. I) nor FOXO1 phosphorylation on Ser256 (data not shown) was increased in WAT. In addition, ICV insulin administration did not induce any changes in STAT3 Tyr705 or CREB Ser133 phosphorylation in WAT, indicating that neither leptin nor PKA signaling pathways are modified by ICV insulin administration.
The effect of ICV insulin administration on FA retention by WAT is dependent on the activation of central KATP channels
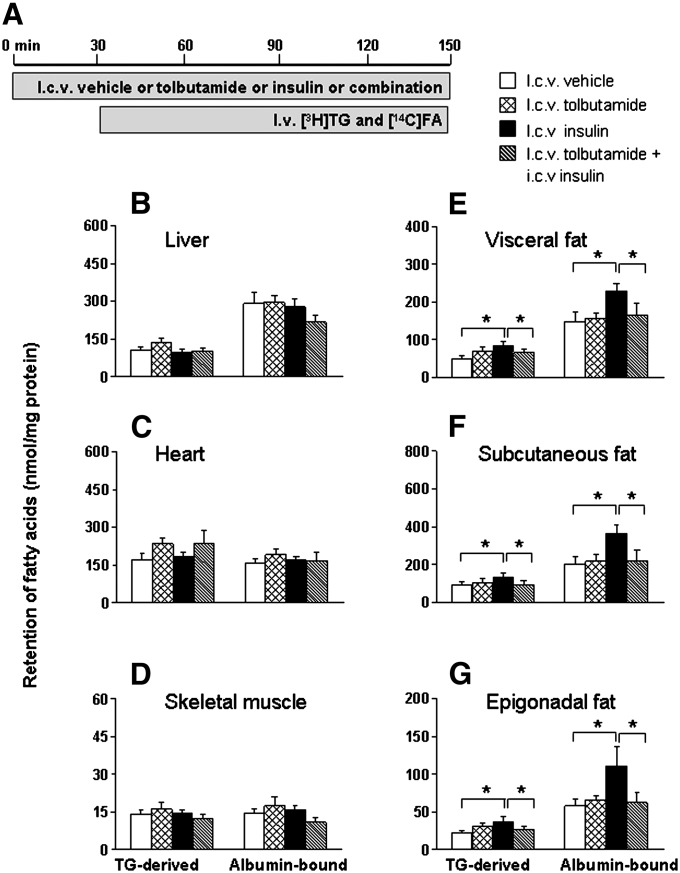
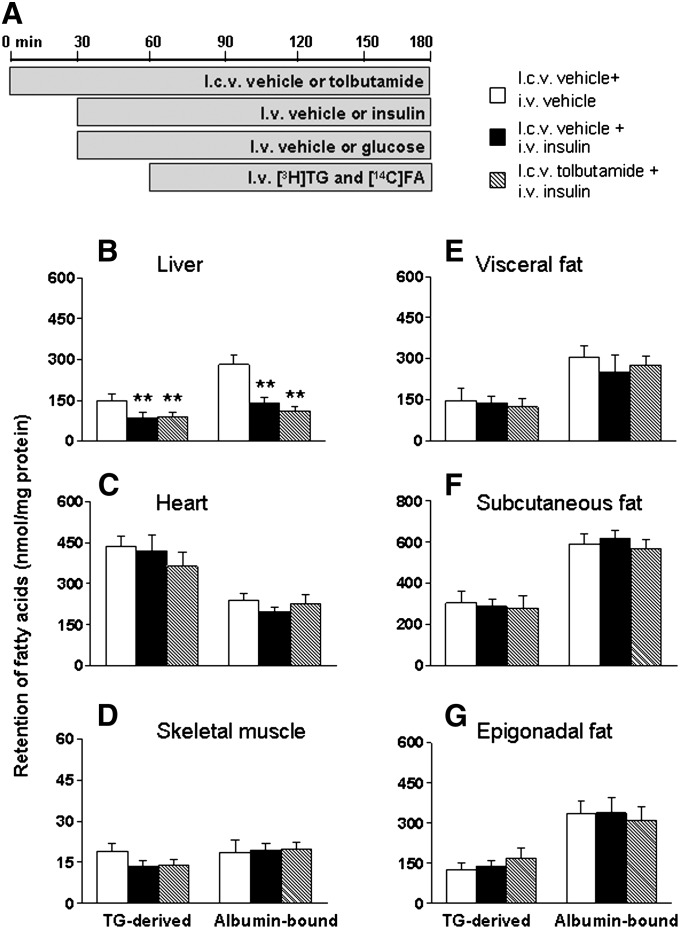
Part of the central effects of insulin on the regulation of food intake and hepatic glucose production are dependent on activation of hypothalamic KATP channels (25). Therefore, we investigated whether the stimulatory effect of ICV insulin administration on FA retention in WAT could be blocked by ICV coadministration of the KATP channel blocker tolbutamide (Fig. 3). ICV administration of insulin (0.5 mU/h) did not affect plasma levels of glucose, TG, FFA, or insulin, irrespective of coadministration of tolbutamide (12 nmol/h) (supplementary Table I). In accordance with the previous experiments, the plasma half-life of [3H]TG decreased by 29% (P < 0.05) upon ICV insulin administration compared with vehicle. However ICV insulin administered concurrently with ICV tolbutamide did not alter plasma TG and FA kinetics. ICV administration of tolbutamide alone did not affect tissue-specific FA partitioning. Comparable to the previous study, FA retention in liver, heart, and skeletal muscles was unaltered upon ICV insulin administration and remained unaltered upon coadministration of tolbutamide (Fig. 3B–D). Interestingly, tolbutamide completely blocked the stimulation of both plasma TG-derived FA and albumin-bound FA retention in WAT induced by ICV insulin administration (Fig. 3E–G).
Fig. 3.
ICV coadministration of the KATP channel blocker tolbutamide blocks the stimulation of FA retention in WAT by ICV insulin. Postabsorptive, body weight-matched WT mice received continuous ICV infusion of vehicle (white bars), tolbutamide (cross-hatched bars, 12 nmol/h), insulin (black bars), or insulin in combination with tolbutamide (hatched bars) (A). Thirty minutes after starting the ICV infusion, the mice were infused for 2 h with glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in liver (B), heart (C), skeletal muscle (D), visceral fat (E), subcutaneous fat (F), and epigonadal fat (G). Values are means ± SEM for at least eight mice per group. *P < 0.05 versus vehicle.
The effect of circulating insulin on FA retention by WAT is mediated through KATP channel activation in the CNS
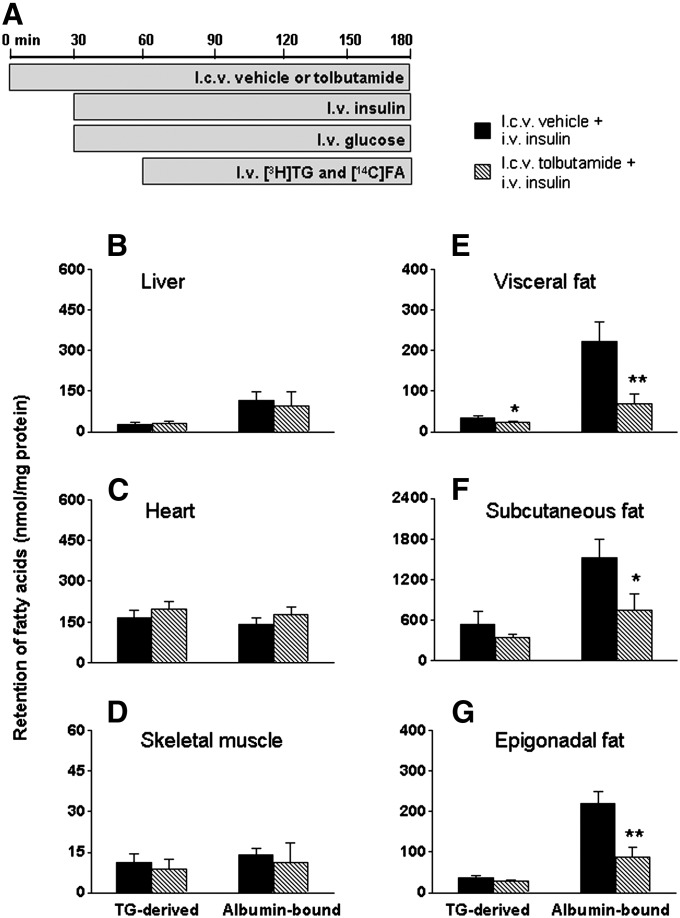
To investigate the contribution of the CNS to the effect of circulating insulin on FA retention in WAT, we examined whether ICV administration of the KATP channel blocker tolbutamide inhibits the insulin-stimulated FA retention in WAT during hyperinsulinemic-euglycemic clamp conditions (Fig. 4). In steady-state clamp conditions, plasma glucose, TG, and insulin concentrations were similar in both groups, as shown in Table 2. Hyperinsulinemia suppressed FFA levels to a similar extent in ICV tolbutamide-infused and ICV vehicle-infused mice. The glucose infusion rates required to maintain euglycemia, however, were 22% lower in mice that received ICV tolbutamide compared with ICV vehicle (P < 0.05). This observation is consistent with previous findings showing that ICV administration of the KATP channel blocker tolbutamide impairs the inhibition of hepatic glucose production in response to hyperinsulinemia (4, 26). The plasma half-life of [3H]TG decreased by 60% (P < 0.05) during hyperinsulinemic conditions but was partly restored by ICV tolbutamide (Table 2). FA retention in liver, heart, and skeletal muscles was unaffected by ICV tolbutamide (Fig. 4B–D). Remarkably, ICV tolbutamide considerably decreased the stimulatory effect of circulating insulin during clamp conditions on FA retention in WAT (Fig. 4E–G).
Fig. 4.
ICV administration of tolbutamide impairs the stimulation of FA retention in WAT induced by hyperinsulinemic-euglycemic clamp conditions. Postabsorptive, body weight-matched mice received continuous ICV infusion of vehicle (black bars) or tolbutamide (12 nmol/h, hatched bars) 30 min before the start of hyperinsulinemic-euglycemic clamp study (A). Infusion of glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid was started 30 min after the start of the hyperinsulinemic-euglycemic clamp study and maintained for 2 h. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in liver (B), heart (C), skeletal muscle (D), visceral fat (E), subcutaneous fat (F), and epigonadal fat (G). Values are means ± SEM for at least five mice per group. *P < 0.05 versus vehicle, **P < 0.01 versus vehicle.
TABLE 2.
ICV administration of tolbutamide decreases glucose infusion rate during hyperinsulinemic-euglycemic clamp conditions
| At Baseline |
During Clamp Conditions |
|||
| ICV Vehicle | ICV Tolbutamide | ICV Vehicle | ICV Tolbutamide | |
| Glucose (mmol/l) | 7.3 ± 1.3 | 7.3 ± 1.0 | 6.1 ± 0.4 | 6.7 ± 1.3 |
| TG (mmol/l) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| FFA (mmol/l) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Insulin (ng/ml) | 0.3 ± 0.1 | 0.2 ± 0.1 | 4.4 ± 1.5 | 4.1 ± 0.8 |
| GIR (μmol/kg.min) | – | – | 114 ± 5.7 | 89 ± 3.2a |
| Hematocrit (%) | 43 ± 2 | 42 ± 2 | 42 ± 2 | 41 ± 2 |
| Half-life [14C]FA (min) | – | – | 0.5 ± 0.1 | 0.7 ± 0.1 |
| Half-life [3H]TG (min) | – | – | 1.4 ± 0.1 | 1.7 ± 0.1 |
Plasma parameters of mice that received ICV infusion of vehicle or tolbutamide at baseline and during hyperinsulinemic conditions. Values are means ± SEM for at least nine mice per group. GIR, glucose infusion rate.
aP < 0.05 versus vehicle.
The effect of ICV insulin administration on FA retention in WAT requires the presence of CD36
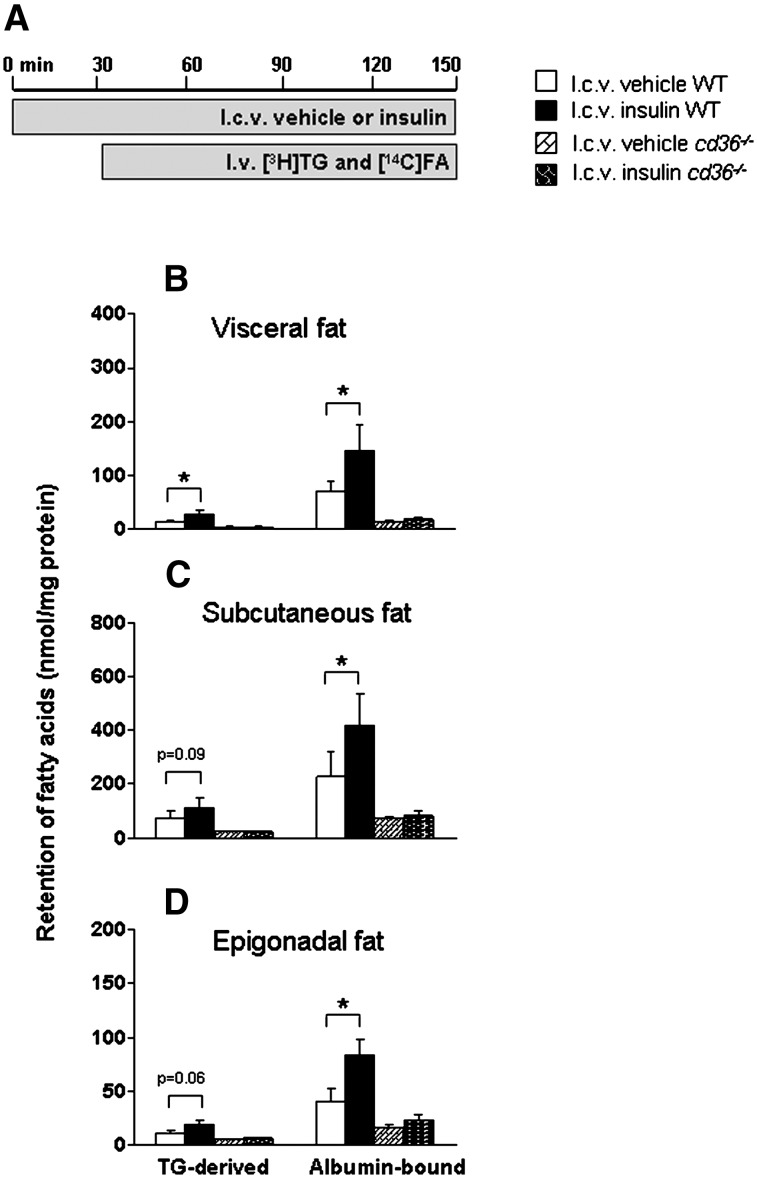
As CD36 is one of the main long-chain FA transporters and insulin can upregulate translocation and protein expression of CD36 (27, 28), we studied to what extent CD36 is involved in the ICV insulin-stimulated FA retention by WAT. Therefore, we investigated the effects of ICV insulin on tissue-specific FA retention in CD36−/− versus WT mice (Fig. 5). In accordance with previous observations (29), basal plasma TG and FFA levels were increased in CD36−/− mice compared with WT mice by 28% and 26%, respectively (P < 0.05), but they remained unaltered, as did circulating glucose and insulin levels, following ICV administration of insulin (data not shown). ICV insulin decreased the half-lives of plasma [3H]TG and [14C]FA in WT mice but not in CD36−/− mice (data not shown). Again, we confirmed that in WT mice, ICV insulin stimulated the retention of TG-derived FA and albumin-bound FA by 100% (P < 0.05) and 107% (P < 0.05) in visceral fat, by 53% (P = 0.09) and 85% (P < 0.05) in subcutaneous fat, and by 77% (P = 0.06) and 104% (P < 0.05) in epigonadal fat pads, respectively. In basal conditions (i.e., ICV administration of vehicle), the retention of both FA by WAT was dramatically decreased in CD36−/− mice compared with wild-type mice (Fig. 5B–D). More importantly, ICV insulin administration did not stimulate FA retention from plasma by WAT in CD36−/− mice. ICV insulin administration did not affect FA retention in other organs of CD36−/− mice, in accordance with the observations made in WT mice (see above). Since ICV insulin administration was unable to stimulate FA retention by WAT in CD36−/− mice, these data suggest that CD36 is the predominant FA transporter mediating ICV insulin-stimulated FA retention by WAT.
Fig. 5.
The stimulation of FA retention in WAT by ICV insulin administration is abrogated in CD36-deficient mice. Postabsorptive, body weight-matched WT and CD36-deficient mice received continuous ICV infusion of vehicle (white and hatched bars) or insulin (black and cross-hatched bars) (A). Thirty minutes after starting the ICV infusion, the mice were infused for 2 h with glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in visceral fat (B), subcutaneous fat (C), and epigonadal fat (D). Values are means ± SEM for at least six mice per group. *P < 0.05 versus vehicle.
The effect of ICV insulin on FA retention in WAT is lost in diet-induced obesity
Subsequently, we examined the effect of ICV insulin administration on FA retention in high-fat fed mice (Fig. 6). Body weight of the diet-induced obese mice was 40% higher compared with chow fed mice (P < 0.01). ICV insulin administration did not alter glucose, TG, FFA, or insulin levels (Table 3). In contrast to chow fed mice, ICV administration of insulin did not decrease the plasma half-life of [3H]TG in these diet-induced obese mice. ICV insulin administration did not alter FA retention in liver, heart, skeletal muscles, or surprisingly, WAT (Fig. 6B–G).
Fig. 6.
ICV insulin administration in diet-induced obese mice does not stimulate FA retention by WAT. Postabsorptive, body weight-matched WT mice received continuous ICV infusion of vehicle (white bars) or insulin (black bars, 0.5 mU/h) (A). Thirty minutes after starting the ICV infusion, the mice were infused for 2 h with glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in liver (B), heart (C), skeletal muscle (D), visceral fat (E), subcutaneous fat (F), and epigonadal fat (G). Values are means ± SEM for at least nine mice per group.
TABLE 3.
ICV insulin administration in diet-induced obese mice does not affect plasma parameters
| At Baseline |
After Two Hours |
|||
| ICV Vechile | ICV Insulin | ICV Vehicle | ICV Insulin | |
| Bodyweight (g) | 32.5 ± 0.6 | 32.0 ± 0.4 | 32.5 ± 0.6 | 32.0 ± 0.4 |
| Glucose (mmol/l) | 7.7 ± 0.3 | 7.7 ± 0.2 | 6.0 ± 0.3 | 5.6 ± 0.2 |
| TG (mmol/l) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| FFA (mmol/l) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Insulin (ng/ml) | 3.8 ± 0.3 | 4.0 ± 0.4 | 4.0 ± 0.4 | 3.8 ± 0.3 |
| Half-life [14C]FA (min) | – | – | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Half-life [3H]TG (min) | – | – | 9.2 ± 1.1 | 9.4 ± 1.6 |
Parameters of diet-induced obese mice that received ICV infusion of vehicle or insulin obtained at baseline and 2 h after starting an intravenous infusion of glycerol tri[3H]oleate-labeled VLDL-like emulsion particles and albumin-bound [14C]oleic acid. Values are means ± SEM for at least nine mice per group.
KATP channel blockage does not affect circulating insulin-stimulated FA retention by WAT in diet-induced obese mice
Finally, we examined whether ICV administration of the KATP channel blocker tolbutamide would still inhibit the insulin-stimulated FA retention in WAT during hyperinsulinemic-euglycemic clamp conditions in high-fat fed mice (Fig. 7). Therefore, we determined FA retention between mice during hyperinsulinemic-euglycemic clamp conditions infused intracerebroventricularly either with tolbutamide or vehicle and mice in basal conditions infused intracerebroventricularly with vehicle. Body weight of the high-fat fed mice was 65% higher compared with chow fed mice (P < 0.01). In steady-state clamp conditions, plasma glucose and TG concentrations were similar between tolbutamide- and vehicle-treated mice as shown in Table 4. During hyperinsulinemic-euglycemic clamp conditions, circulating insulin levels were 4-fold higher in both groups compared with basal conditions, resulting in a decrease of ∼50% in FFA levels. The rate of glucose infusion necessary to maintain euglycemia was not different between tolbutamide- and vehicle-treated animals. The plasma half-life of [3H]TG was similar in basal and hyperinsulinemic conditions, irrespective of ICV tolbutamide administration. Similar to chow fed mice, hyperinsulinemia decreased the retention of plasma TG-derived FA and albumin-bound FA in liver, which was unaltered by ICV tolbutamide administration (Fig. 7B). FA retention in liver, heart, and skeletal muscles was similar in all groups (Fig. 7B–D). Unlike in chow fed mice, hyperinsulinemia did not stimulate FA retention in WAT, and ICV tolbutamide did not decrease FA retention in WAT (Fig. 7E–G).
Fig. 7.
Intravenous insulin administration in diet-induced obese mice does not stimulate FA retention by WAT. Postabsorptive, body weight-matched mice received continuous ICV infusion of vehicle (white and black bars) or tolbutamide (12 nmol/h, hatched bars) in basal state (intravenous infusion of vehicle, white bars) or hyperinsulinemic-euglycemic state (black and hatched bars) (A). Infusion of glycerol tri[3H]oleate within VLDL-like emulsion particles and albumin-bound [14C]oleic acid was started 30 min after initiating the intravenous infusion of vehicle or insulin/glucose, and maintained for 2 h. Subsequently, the mice were euthanized, and the retention of TG-derived FA and albumin-bound FA was determined in liver (B), heart (C), skeletal muscle (D), visceral fat (E), subcutaneous fat (F), and epigonadal fat (G). Values are means ± SEM for at least five mice per group. **P < 0.01 versus vehicle.
TABLE 4.
ICV administration of tolbutamide in diet-induced obese mice during hyperinsulinemic-euglycemic clamp conditions does not affect plasma parameters
| At Baseline |
During Basal Infusion |
During Clamp Conditions |
|||
| ICV Vehicle | ICV Tolbutamide | ICV Vehicle | ICV Vehicle | ICV Tolbutamide | |
| Bodyweight (g) | 38.6 ± 0.8 | 38.6 ± 0.4 | 38.6 ± 0.8 | 39.6 ± 0.3 | 38.6 ± 0.4 |
| Glucose (mmol/l) | 6.6 ± 0.2 | 6.0 ± 0.2 | 5.7 ± 0.2 | 7.0 ± 0.2 | 7.5 ± 0.2 |
| TG (mmol/l) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.1 |
| FFA (mmol/l) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Insulin (ng/ml) | 1.8 ± 0.3 | 1.4 ± 0.2 | 2.2 ± 0.3 | 7.7 ± 0.4 | 6.9 ± 0.1 |
| GIR (μmol/kg.min) | – | – | – | 52.4 ± 1.5 | 53.3 ± 2.7 |
| Hematocrit (%) | 44 ± 2 | 44 ± 1 | 42 ± 2 | 41 ± 2 | 41 ± 2 |
| Half-life [14C]FA (min) | – | – | 1.2 ± 0.1 | 1.5 ± 0.2 | 1.2 ± 0.1 |
| Half-life [3H]TG (min) | – | – | 14.6 ± 1.5 | 16.1 ± 0.5 | 17.0 ± 1.2 |
Parameters of diet-induced obese mice that received ICV infusion of vehicle or tolbutamide at baseline and during basal infusions or hyperinsulinemic conditions. Values are means ± SEM for at least five mice per group. GIR, glucose infusion rate.
DISCUSSION
This study addressed the effects of circulating insulin on tissue-specific TG-derived FA and albumin-bound FA retention, and the role of central insulin action in these effects. Circulating insulin, which activated insulin signaling in the brain, stimulated retention of both TG-derived FA and albumin-bound FA in WAT. Centrally administered insulin stimulated retention of both FA sources in a similar manner but without activating insulin signaling in WAT. Tolbutamide, a KATP channel blocker, decreased this insulin-stimulated FA partitioning to WAT during peripheral insulin infusion, and it even abolished this effect during ICV coadministration with insulin. Taken together, we show that the central effects of circulating insulin contribute on average ∼30% to TG-derived FA uptake in WAT and ∼66% to albumin-derived FA uptake in WAT to the total effects of circulating insulin during hyperinsulinemic clamp conditions. In contrast, in diet-induced obese mice, centrally administered insulin was unable to stimulate FA retention in WAT. Furthermore, inhibition of central action of ICV administered insulin or circulating insulin by tolbutamide did not affect FA retention in WAT. Collectively, these data indicate that circulating insulin stimulates FA partitioning from plasma to WAT to a considerable extent through indirect effects on central neural pathways and that insulin-stimulated FA partitioning through these central neural pathways is absent in mice with insulin resistance after 12 weeks of high-fat feeding.
First, we determined tissue-specific FA uptake during hyperinsulinemic-euglycemic clamp conditions compared with basal conditions. In agreement with the rise in plasma insulin levels, hypothalamic insulin signaling was activated during hyperinsulinemic-euglycemic clamp conditions as phosphorylation of PKB on Thr308 (1.24 ± 0.05 versus 1.00 ± 0.10, P = 0.08) and its downstream target PRAS40 on Thr246 (1.51 ± 0.11 versus 1.00 ± 0.06, P < 0.05), which were increased compared with basal conditions (unpublished data). We observed that peripheral as well as ICV insulin administration promotes FA storage specifically in WAT. By employing the dual tracer methods described by Teusink et al. (23), we made a distinction between FA derived from plasma TG and FA derived from plasma albumin. Both peripheral and ICV administration of insulin stimulated the retention of both sources of plasma FA in WAT. This insulin-stimulated FA retention by WAT was accompanied by a decreased half-life of TG, reflecting increased turnover of plasma TG. Interestingly, the observation that these acute effects of ICV insulin administration on plasma TG and FA flux toward WAT, together with recent data showing that brain insulin suppresses intracellular lipolysis and stimulates lipogenesis in adipocytes (14), provides an explanation for the finding that chronic ICV insulin administration increases fat mass (13).
In addition to stimulating FA retention by WAT, peripheral insulin infusion decreased FA storage by the liver. However, ICV administration of insulin or blockage of central KATP channels did not affect FA retention by the liver. This finding suggests that the effect of peripheral insulin on liver FA retention is not mediated by the CNS but, rather, seems to be a direct effect on the liver caused by an associated reduction in the availability of plasma FFAs.
Recently, Bartelt et al. described the fundamental role of BAT for TG and FA clearance (30). Exposure of mice to cold accelerated TG uptake by BAT in a lipoprotein lipase (LPL)- and CD36-dependent manner. In the current study, we also determined the effects of insulin on FA retention by BAT. However, neither peripheral nor central insulin administration increased FA retention by BAT, indicating that insulin, in contrast to cold exposure, is not a major stimulator of TG and FA uptake by BAT.
The question arises how insulin exerts its indirect effects through the central nervous system on FA uptake in WAT. We used an ICV dose of insulin, which was ascertained in a dose-finding study, that did not affect plasma insulin and glucose levels. Nonetheless, this ICV dose of insulin stimulated FA uptake by WAT. Therefore, the stimulatory effect of ICV insulin on retention of FA by WAT is not caused by ICV insulin-induced changes in plasma levels of glucose and insulin. We cannot exclude the possibility of other neuroendocrine, insulin-potentiating effects induced by the effects of insulin on the central nervous system, like a decrease in plasma levels of the insulin antagonist epinephrine or corticosterone. Furthermore, it is possible that the indirect effects of insulin through the CNS involve alterations in the activity of the autonomic nerves projecting toward WAT [i.e., in (para)sympathetic activity]. In accordance with this concept, we documented in a model of suprarenal fat pads in rats that selective vagotomy (i.e., parasympathetic denervation of the suprarenal fat pads) reduced FA uptake in WAT by 36% during hyperinsulinemic clamp conditions (31). An increase in parasympathetic activity toward WAT might be involved in the indirect effects of circulating insulin through the CNS, although the existence of parasympathetic innervation of other fat compartments is at present uncertain. Alternatively, it might be hypothesized that a change in the activity of the sympathetic activity toward WAT may be involved in the indirect effects of insulin on FA uptake in WAT in vivo, as it has been shown that insulin administered in the mediabasal hypothalamus dampens the sympathetic activity (14).
We determined to what extent the stimulatory effects of circulating insulin on FA retention in WAT were mediated through the CNS by ICV administration of tolbutamide. Tolbutamide, which belongs to the sulfonylurea family, inhibits KATP channels in the hypothalamus when administered intracerebroventricularly (4). Sulfonylureas bind to the KATP channels on the cell membrane of certain hypothalamic neurons, where they inhibit the hyperpolarizing efflux of potassium (32). Insulin acts in central neurons by opening/activating KATP channels, so consequently, ICV administration of tolbutamide blocks central KATP channel-mediated insulin signaling (4). Our study shows that the rapid stimulatory effect of ICV insulin administration on FA uptake in WAT is blocked by ICV coadministration of tolbutamide. This indicates that tissue-specific stimulation of FA uptake by WAT induced by ICV insulin administration is mediated by activation of KATP channels in the brain. By blocking central insulin signaling by ICV administration of tolbutamide during hyperinsulinemic-euglycemic clamp conditions, we showed that insulin stimulates FA retention in WAT to a considerable extent via action in the CNS.
We also assessed the effects of high-fat diet on the central effects of circulating insulin on FA retention in WAT. The high-fat diet abolished the stimulating effect of ICV administration of insulin on FA retention in WAT. Furthermore, peripherally administered insulin did not result in FA retention in WAT, and blocking central insulin signaling by ICV administration of tolbutamide did not affect the FA retention in WAT. The absence of insulin effects on FA retention in these diet-induced obese mice is probably the result of both high-fat diet-induced insulin resistance of WAT and central insulin resistance associated with blunted activation of hypothalamic KATP channels (25, 33–36). The present study indicates that in high-fat fed conditions, FA partitioning is no longer influenced by (central acting) insulin.
The present results showed that insulin stimulates FA retention in WAT to a considerable extent through action in the CNS. Because insulin stimulates FA transport by upregulating and translocating the long-chain FA transporter CD36 in isolated skeletal muscle and cardiac myocytes (27, 28), we hypothesized that the stimulating effect of centrally acting insulin on FA retention in WAT could be mediated by CD36 translocation. When administered in CD36-deficient mice, ICV insulin was unable to stimulate FA retention in WAT, suggesting a role of this FA transporter in ICV insulin-stimulated FA retention in WAT. Accordingly, AMP-activated protein kinase (AMPK), which can stimulate FA retention by promoting translocation of CD36 from intracellular pool to the plasma membrane (37), was activated in WAT upon ICV insulin administration (supplementary Fig. II). These preliminary data suggest that central insulin action can lead to CD36 translocation following AMPK activation, thereby resulting in FA retention in WAT.
Recent observations have challenged the traditional notion that adipose tissue acquires FA from TG-rich lipoproteins mediated by the LPL system. Shadid et al. documented direct uptake of plasma FFA by adipose tissue in humans in the postabsorptive state (38). In accordance, we previously demonstrated considerable uptake of plasma FFA in adipose tissue in both fed and fasted mice (23). In older experiments, the uptake of FA in adipose tissue from plasma FFA and plasma TG was assessed by quantification of the uptake as a percentage of the total administered radioactive dose (39). In the present study, we assessed tissue FA uptake from plasma FFA and plasma TG per milligram of protein after correction for the corresponding plasma-specific activities of [14C]FA and [3H]TG. This correction for precursor pool enrichment enabled us to assess the absolute rate of FA uptake from the two plasma sources compared with the assessment of the relative uptake of FA radio-isotopes in relation to the total administered radioactive doses in the older experiments. In addition, there are other methodological differences, including labeling procedures of plasma TG and the use of the postabsorptive state versus hyperinsulinemic-euglycemic clamp procedure, that limit a simple comparison of our results and those of previous experiments.
ICV administration of insulin did not stimulate FA retention by WAT to the same extent as peripherally administered insulin. However, the ICV dose of insulin used in the ICV experiments and the intravenous dose used in the hyperinsulinemic euglycemic clamp experiments cannot be compared easily. The ICV dose of insulin used in the current study was ascertained in a dose-finding study to exclude hormone-sensitive lipase inactivation by ICV insulin shown recently by Scherer et al. (14). Therefore, the change in FA retention by WAT upon ICV insulin is the result of a net influx of FA, whereas FA retention upon peripheral insulin administration is the result of suppression of lipolysis and FA uptake, explaining the difference in the amount of FA retention by WAT between centrally and peripherally administered insulin.
In conclusion, we show that circulating insulin stimulates tissue-specific FA retention by WAT to a considerable extent by indirect pathways, involving activation of KATP channels in the brain, which is lost in diet-induced obese mice. These observations highlight a paradigm that circulating hormones can act on target tissues directly as well as indirectly through the CNS and indicate that such a central pathway is disturbed in insulin resistance of WAT in diet-induced obesity.
Supplementary Material
Acknowledgments
The authors thank Ms. M. den Boer for performing pilot experiments.
Footnotes
Abbreviations:
- aCSF
- artificial cerebrospinal fluid
- BAT
- brown adipose tissue
- CNS
- central nervous system
- ICV
- intracerebroventricular
- KATP
- ATP-dependent potassium
- TG
- triglyceride
- WAT
- white adipose tissue
- WT
- wild-type
This work was supported by grants from TI Pharma (TIP project T2-105 to J.A.R. and L.M.H.), the Netherlands Heart Foundation (NHS project 2007B81 to P.C.N.R. and J.A.R.), and the Dutch Diabetes Research Foundation (DFN project 2007.00.010 to P.C.N.R. and J.A.R.). P.C.N. Rensen is an Established Investigator of the Netherlands Heart Foundation (2009T038).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figure.and one table.
REFERENCES
- 1.Banks W. A. 2004. The source of cerebral insulin. Eur. J. Pharmacol. 490: 5–12. [DOI] [PubMed] [Google Scholar]
- 2.Banks W. A. 2006. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol. Behav. 89: 472–476. [DOI] [PubMed] [Google Scholar]
- 3.McGowan M. K., Andrews K. M., Grossman S. P. 1992. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol. Behav. 51: 753–766. [DOI] [PubMed] [Google Scholar]
- 4.Obici S., Zhang B. B., Karkanias G., Rossetti L. 2002. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 8: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 5.Urayama A., Banks W. A. 2008. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 149: 3592–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz M. W., Figlewicz D. P., Baskin D. G., Woods S. C., Porte D., Jr 1992. Insulin in the brain: a hormonal regulator of energy balance. Endocr. Rev. 13: 387–414. [DOI] [PubMed] [Google Scholar]
- 7.Baura G. D., Foster D. M., Porte D., Jr, Kahn S. E., Bergman R. N., Cobelli C., Schwartz M. W. 1993. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J. Clin. Invest. 92: 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz M. W., Sipols A., Kahn S. E., Lattemann D. F., Taborsky G. J., Jr, Bergman R. N., Woods S. C., Porte D., Jr 1990. Kinetics and specificity of insulin uptake from plasma into cerebrospinal fluid. Am. J. Physiol. 259: E378–E383. [DOI] [PubMed] [Google Scholar]
- 9.Margolis R. U., Altszuler N. 1967. Insulin in the cerebrospinal fluid. Nature. 215: 1375–1376. [DOI] [PubMed] [Google Scholar]
- 10.Woods S. C., Lotter E. C., McKay L. D., Porte D., Jr 1979. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 282: 503–505. [DOI] [PubMed] [Google Scholar]
- 11.Porte D., Jr, Woods S. C. 1981. Regulation of food intake and body weight in insulin. Diabetologia. 20(Suppl): 274–280. [PubMed] [Google Scholar]
- 12.Könner A. C., Janoschek R., Plum L., Jordan S. D., Rother E., Ma X., Xu C., Enriori P., Hampel B., Barsh G. S., et al. 2007. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5: 438–449. [DOI] [PubMed] [Google Scholar]
- 13.Koch L., Wunderlich F. T., Seibler J., Könner A. C., Hampel B., Irlenbusch S., Brabant G., Kahn C. R., Schwenk F., Bruning J. C. 2008. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Invest. 118: 2132–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherer T., O'Hare J., Diggs-Andrews K., Schweiger M., Cheng B., Lindtner C., Zielinski E., Vempati P., Su K., Dighe S., et al. 2011. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam T. K., Gutierrez-Juarez R., Pocai A., Bhanot S., Tso P., Schwartz G. J., Rossetti L. 2007. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat. Med. 13: 171–180. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoek A. M., Voshol P. J., Karnekamp B. N., Buijs R. M., Romijn J. A., Havekes L. M., Pijl H. 2004. Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes. 53: 2529–2534. [DOI] [PubMed] [Google Scholar]
- 17.Rensen P. C., Herijgers N., Netscher M. H., Meskers S. C., van Eck M., van Berkel T. J. 1997. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J. Lipid Res. 38: 1070–1084. [PubMed] [Google Scholar]
- 18.Heijboer A. C., van den Hoek A. M., Pijl H., Voshol P. J., Havekes L. M., Romijn J. A., Corssmit E. P. 2005. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 48: 1621–1626. [DOI] [PubMed] [Google Scholar]
- 19.Parlevliet E. T., Heijboer A. C., Schroder-van der Elst J. P., Havekes L. M., Romijn J. A., Pijl H., Corssmit E. P. 2008. Oxyntomodulin ameliorates glucose intolerance in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 294: E142–E147. [DOI] [PubMed] [Google Scholar]
- 20.Plum L., Ma X., Hampel B., Balthasar N., Coppari R., Munzberg H., Shanabrough M., Burdakov D., Rother E., Janoschek R., et al. 2006. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J. Clin. Invest. 116: 1886–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 22.Silberkang M., Havel C. M., Friend D. S., McCarthy B. J., Watson J. A. 1983. Isoprene synthesis in isolated embryonic Drosophila cells. I. Sterol-deficient eukaryotic cells. J. Biol. Chem. 258: 8503–8511. [PubMed] [Google Scholar]
- 23.Teusink B., Voshol P. J., Dahlmans V. E., Rensen P. C., Pijl H., Romijn J. A., Havekes L. M. 2003. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 52: 614–620. [DOI] [PubMed] [Google Scholar]
- 24.Klieverik L. P., Coomans C. P., Endert E., Sauerwein H. P., Havekes L. M., Voshol P. J., Rensen P. C., Romijn J. A., Kalsbeek A., Fliers E. 2009. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 150: 5639–5648. [DOI] [PubMed] [Google Scholar]
- 25.Spanswick D., Smith M. A., Mirshamsi S., Routh V. H., Ashford M. L. 2000. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 3: 757–758. [DOI] [PubMed] [Google Scholar]
- 26.Pocai A., Lam T. K., Gutierrez-Juarez R., Obici S., Schwartz G. J., Bryan J., Aguilar-Bryan L., Rossetti L. 2005. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 434: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 27.Corpeleijn E., Pelsers M. M., Soenen S., Mensink M., Bouwman F. G., Kooi M. E., Saris W. H., Glatz J. F., Blaak E. E. 2008. Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. J. Physiol. Pharmacol. 59: 77–83. [PubMed] [Google Scholar]
- 28.Chabowski A., Coort S. L., Calles-Escandon J., Tandon N. N., Glatz J. F., Luiken J. J., Bonen A. 2004. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am. J. Physiol. Endocrinol. Metab. 287: E781–E789. [DOI] [PubMed] [Google Scholar]
- 29.Goudriaan J. R., Dahlmans V. E., Teusink B., Ouwens D. M., Febbraio M., Maassen J. A., Romijn J. A., Havekes L. M., Voshol P. J. 2003. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J. Lipid Res. 44: 2270–2277. [DOI] [PubMed] [Google Scholar]
- 30.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 31.Kreier F., Fliers E., Voshol P. J., Van Eden C. G., Havekes L. M., Kalsbeek A., Van Heijningen C. L., Sluiter A. A., Mettenleiter T. C., Romijn J. A., et al. 2002. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J. Clin. Invest. 110: 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Top M., Lyons D. J., Lee K., Coderre E., Renaud L. P., Spanswick D. 2007. Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neuroscience. 144: 815–824. [DOI] [PubMed] [Google Scholar]
- 33.Arase K., Fisler J. S., Shargill N. S., York D. A., Bray G. A. 1988. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am. J. Physiol. 255: R974–R981. [DOI] [PubMed] [Google Scholar]
- 34.Woods S. C., D'Alessio D. A., Tso P., Rushing P. A., Clegg D. J., Benoit S. C., Gotoh K., Liu M., Seeley R. J. 2004. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol. Behav. 83: 573–578. [DOI] [PubMed] [Google Scholar]
- 35.Ono H., Pocai A., Wang Y., Sakoda H., Asano T., Backer J. M., Schwartz G. J., Rossetti L. 2008. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J. Clin. Invest. 118: 2959–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clegg D. J., Gotoh K., Kemp C., Wortman M. D., Benoit S. C., Brown L. M., D'Alessio D., Tso P., Seeley R. J., Woods S. C. 2011. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 103: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habets D. D., Coumans W. A., Voshol P. J., den Boer M. A., Febbraio M., Bonen A., Glatz J. F., Luiken J. J. 2007. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem. Biophys. Res. Commun. 355: 204–210. [DOI] [PubMed] [Google Scholar]
- 38.Shadid S., Koutsari C., Jensen M. D. 2007. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 56: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 39.Bragdon J. H., Gordon R. S., Jr 1958. Tissue distribution of C14 after the intravenous injection of labeled chylomicrons and unesterified fatty acids in the rat. J. Clin. Invest. 37: 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.