Abstract
Acyl CoA:monoacylglycerol acyltransferase 2 (MGAT2) is thought to be crucial for dietary fat absorption. Indeed, mice lacking the enzyme (Mogat2−/−) are resistant to obesity and other metabolic disorders induced by high-fat feeding. However, these mice absorb normal quantities of fat. To explore whether a high level of dietary fat is an essential part of the underlying mechanism(s), we examined metabolic responses of Mogat2−/− mice to diets containing varying levels of fat. Mogat2−/− mice exhibited 10−15% increases in energy expenditure compared with wild-type littermates; although high levels of dietary fat exacerbated the effect, this phenotype was expressed even on a fat-free diet. When deprived of food, Mogat2−/− mice expended energy and lost weight like wild-type controls. To determine whether MGAT2 deficiency protects against obesity in the absence of high-fat feeding, we crossed Mogat2−/− mice with genetically obese Agouti mice. MGAT2 deficiency increased energy expenditure and prevented these mice from gaining excess weight. Our results suggest that MGAT2 modulates energy expenditure through multiple mechanisms, including one independent of dietary fat; these findings also raise the prospect of inhibiting MGAT2 as a strategy for combating obesity and related metabolic disorders resulting from excessive calorie intake.
Keywords: triacylglycerol, dietary fat, neutral lipid metabolism, monoacylglycerol acyltransferase, high fat
Triacylglycerols, the bulk of dietary fat, are sources of essential FAs and condensed metabolic fuels (1, 2). They can be readily stored as body fat. Compared with other energy-yielding nutrients, triacylglycerols cost less energy for assimilation and thus exert a lower thermic effect of food (diet-induced thermogenesis) (3–5). Individuals that assimilate dietary fat efficiently to meet their needs between meals have an advantage in enduring starvation. Thus, genes conferring this trait may be selected through evolution. During times of continuous abundance, however, individuals thrifty of energy substrates and high in metabolic efficiency are prone to accumulating fuel surpluses in adipose and other tissues (6, 7). In humans, excessive fat accumulation is associated with metabolic diseases, including obesity, hepatic steatosis, and insulin resistance, which plague many industrialized parts of the world (8, 9).
The absorption of dietary fat involves the resynthesis of digested triacylglycerol in enterocytes, mainly through a pathway catalyzed by acyl CoA:monoacylglycerol acyltransferase (MGAT) (10). Three related genes have been identified that code for MGAT; among them, only Mogat2 is highly expressed in the small intestine of both mice and humans (11–15). Thus, Mogat2 probably encodes the intestinal MGAT activity, although Mogat3 is also found in the distal intestine in humans (14).
Consistent with a role of MGAT2 in promoting conservation of dietary fat, mice lacking the enzyme (Mogat2−/−) are resistant to obesity and other metabolic disorders induced by high-fat feeding (16, 17). When fed a regular low-fat chow, body weight and composition of Mogat2−/− mice do not differ from those of wild-type controls (17). In contrast, when fed diets high in fat, Mogat2−/− mice maintain their body weight, while controls gain weight rapidly and develop metabolic disorders. Fat mass accounts for the differences, and lean body mass does not differ between genotypes. Heterozygous Mogat2+/− mice show an intermediate phenotype, indicating a gene-dose effect. Interestingly, Mogat2−/− mice absorb normal quantities of dietary fat but exhibit increased energy expenditure after long-term high-fat feeding (17).
These findings raise the possibility that inhibiting MGAT2 could be a strategy for combating human metabolic disorders caused by excess fat accumulation. However, obesity in humans often results from excess caloric intake but not necessarily high levels of dietary fat. To determine whether a high level of dietary fat is required for the increases in energy expenditure induced by MGAT2 deficiency, we examined acute metabolic responses of Mogat2−/− mice to diets containing different levels of fat. In addition, to determine whether the protection against excessive weight gain is limited to obesity induced by a high-fat diet, we examined the effects of MGAT2 deficiency in hyperphagic Agouti mice (18), a genetic model of obesity.
MATERIALS AND METHODS
Mice
MGAT2-targeted (Mogat2−/−) C57Bl/6J mice were generated as reported previously (17). Mogat2+/− mice were intercrossed to produce Mogat2−/− mice and wild-type littermate controls. Agouti yellow (Ay/a) mice in the C57Bl/6J genetic background, which develop adult onset obesity even when fed a chow diet, were from the Jackson Laboratory (Bar Harbor, ME). Male Mogat2+/− mice carrying the Agouti mutation were crossed with female Mogat2+/− mice without the mutation to generate four experimental groups of mice as indicated. Age and sex of mice used in each experiment were as indicated in figure legends. Mice were housed at 22°C on a 12 h light, 12 h dark cycle. Weighing of mice and changes of diets and cages were performed between 3 and 6 PM. All animal procedures were approved by the University of Wisconsin–Madison Animal Care and Use Committee and were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Diets
A series of semi-purified (defined) diets containing 10, 45, or 60% calories from fat were used to examine the effect of replacing carbohydrate with dietary fat (D12450B, D12451, and D12492; Research Diets, New Brunswick, NJ). In these diets, protein calories (from casein) were held constant at 20%, as were micronutrient and fiber contents, while fat (lard) replaced carbohydrate (corn starch and sucrose) to increase fat calories from 10 to 45 or 60%. Each diet contained 3.8, 4.7, and 5.2 kcal/g metabolizable energy, respectively (formulas are available at www.researchdiets.com). Chow diet (#8604, Teklad; Madison, WI), on which mice were normally maintained, was a complete fixed-formula diet providing approximately 33% energy from crude protein (24.5% w/w) and 14% energy from fat (4.4% w/w). Chow diet contains 3.9 kcal/g gross energy and 3.1 kcal/g metabolizable energy. To determine whether dietary fat is required, a fat-free diet and its low-fat (10%) control diet were made in our laboratory to match protein, micronutrient, and fiber contents (maintaining the same nutrient-to-calorie ratios) of defined diet D12450B using the same ingredients. Fat was replaced by cornstarch.
Metabolic phenotyping studies
A metabolic phenotyping system (LabMaster modular animal monitoring system; TSE Systems, Chesterfield, MO) with housing and wood chip bedding similar to the home cage environment was used to continuously monitor phenotypes related to acute energy balance. As indicators of energy expenditure, oxygen consumption and carbon dioxide production were measured, and the metabolic rates were calculated from a modified Weir equation: kcal = 3.941 × O2 (L) + 1.106 × CO2 (L) (19). No adjustment for protein metabolism was included, because errors introduced by a moderate protein diet are practically negligible (19) and because the defined diets contained a constant level of protein calories. Consumption of food and drink also were monitored continuously. Male mice (2−4 months old) were acclimated to individual housing and metabolic cages for 1 week before experiments. Most mice were acclimatized in the metabolic cages within 24 h, as indicated by characteristic circadian rhythms of O2 consumption and CO2 production (data not shown).
For studies on acute metabolic responses to low- versus high-fat feeding, mice were fed indicated diets sequentially for 3 days each. For studies on metabolic responses to fat-free feeding and fasting, mice were fed 10% fat, fat-free, and again the 10% fat diet for 3 days each, followed by a 48 h fast and another 10% fat refeeding for 3 days. Indirect calorimetry measurements (VO2 and VCO2) from the same time on days 2 and 3 of each diet treatment were averaged. During the 48 h fast, measurements from both days were averaged. Body weight measurements were made at the start of each diet treatment. To examine whether there was a genotype effect on oxygen consumption, total oxygen consumption per day, calculated by area under the curve, was plotted against baseline body weight for each mouse on each diet. In addition, VO2 and metabolic rate were calculated and analyzed both with and without adjusting for body weight (20–22).
Pair feeding
To determine whether increases in energy expenditure of Mogat2−/− mice are obligatory or are in response to increased food intake, we pair-fed weight-stable Mogat2−/− mice (1 year-old males) to their wild-type littermates. Mouse body weight and food intake (chow) were measured daily during acclimation to individual housing. Pair-feeding began after body weight and food intake stabilized. Food intake of wild-type mice was determined each day; the average amount of food consumed by wild-type mice was fed to each of the Mogat2−/− mice.
Statistical analyses
All data are presented as mean ± SEM. P < 0.05 was considered statistically significant. For oxygen consumption per day, differences between genotypes were assessed by the mixed-effects model with repeated measures and adjusted for body weight within the four-diet strata using the PROC MIXED procedure in SAS (SAS, Inc.; Cary, NC) (23). Likewise, differences between diets were assessed in parallel within the two genotype strata. For weight patterns, differences between genotype were assessed by repeated measures ANOVA, followed by Tukey's multiple comparison test (Prism 5.01, GraphPad Inc.; La Jolla, CA). For all other parameters, differences between genotypes were determined by one-way ANOVA followed by the protected least significant differences technique (Prism 5.01). To identify changes in body weight after each diet treatment, we performed paired t-tests (Prism 5.01).
RESULTS
MGAT2 deficiency increases oxygen consumption, an indicator of energy expenditure, throughout all diets
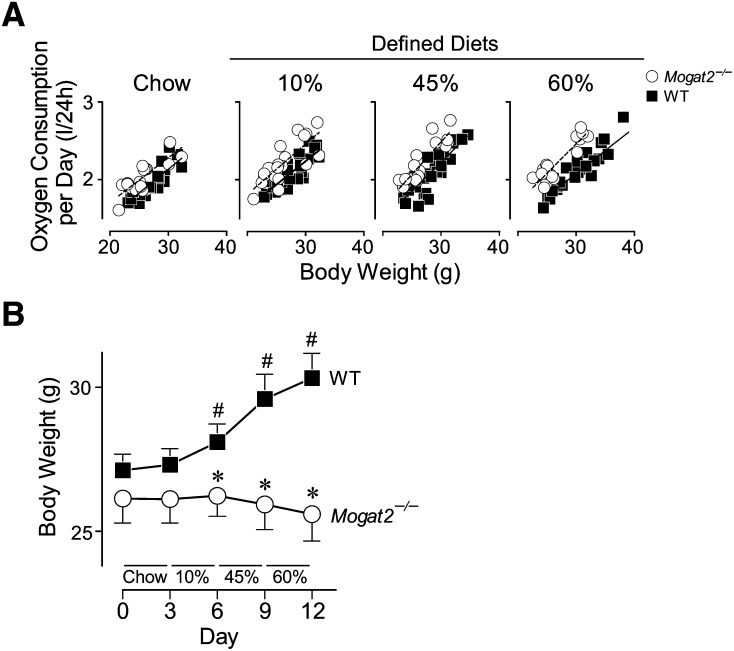
To determine if a high level of dietary fat is required for the increases in energy expenditure induced by MGAT2 deficiency, Mogat2−/− mice and their wild-type littermates were housed in the metabolic chambers and fed sequentially for 3 days on each of the four diets: a regular mixed-meal chow and a defined diet containing 10, 45, or 60% of calories from fat (referred to as chow, 10%, 45%, and 60% diet, respectively). When total oxygen consumption per day was plotted against body weight of each mouse, the regression lines of Mogat2−/− mice were significantly elevated over those of wild-type controls fed the same diets (Fig. 1A), indicating higher energy expenditure in Mogat2−/− mice than in wild-type controls. Based on the mixed-effects model within the four-diet strata, when fed chow, Mogat2−/− mice consumed approximately 8% more oxygen than did controls at any given body weight, and 10, 11, and 13% more when fed 10, 45, and 60% diets, respectively (main effect of genotype, P < 0.05). When stratified by genotype, as calories from fat increased in purified diets, wild-type, but not Mogat2−/−, mice significantly decreased their oxygen consumption (main effect of diet, P < 0.05). For example, wild-type mice when fed the 60% diet consumed approximately 5% less oxygen than when fed the 10% diet, whereas there was no difference in Mogat2−/− mice.
Fig. 1.
MGAT2 deficiency increases oxygen consumption and prevents weight gain throughout all diets. Male mice (2−4 months old) deficient in MGAT2 (Mogat2−/−; open circles) and wild-type littermates (WT; black squares) were sequentially fed chow, then semi-purified (defined) diets containing 10, 45, or 60% calories from fat for 3 days each diet. A: Regression analyses of oxygen consumption versus body weight of mice. Total oxygen consumption per mouse per day was plotted against baseline body weight of each mouse at the start of each diet. Lines represent linear regressions of each group (r2 = 0.62−0.83 for each regression). B: Body weights of mice during the metabolic study. WT, n = 23–26 mice per group; Mogat2−/−, n = 16–19 mice per group. Values for panel B are means ± SEM. #, P < 0.05 versus WT at the start of each diet treatment (paired t-tests); *, P < 0.05 versus diet-matched WT.
Increased oxygen consumption is associated with resistance to weight gain
Associated with the decreased oxygen consumption, wild-type mice increased body weight after consuming the calorie-dense defined diets (Fig. 1B). In contrast, Mogat2−/− mice maintained similar levels of oxygen consumption and their body weights throughout. As a result, with chow feeding, the body weights did not differ between genotypes. After consuming each of the defined diets, wild-type mice weighed 3.6, 9.1, and 11.8% more than they did at the start of the study (P < 0.05 for all comparisons). Mogat2−/− mice, on the other hand, maintained their weights throughout the experiment and weighed 15.6% less than wild-type mice did at the end of the study (Fig. 1B).
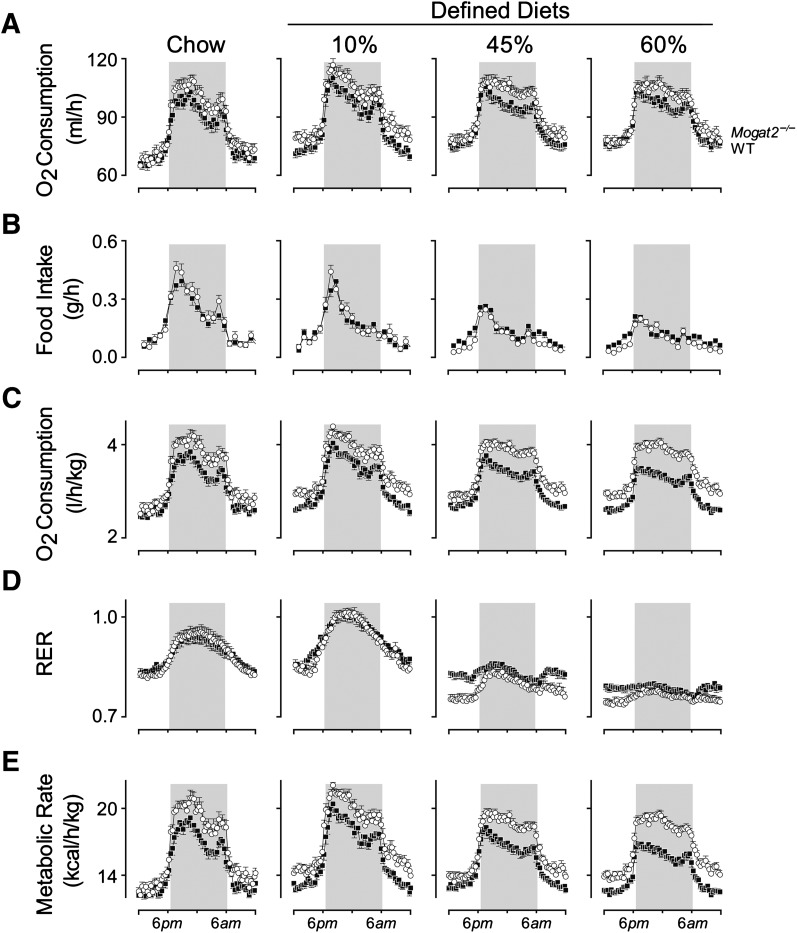
The increase in oxygen consumption is augmented during feeding periods
When rates of oxygen consumption were plotted over time, both groups of mice exhibited a clear circadian rhythm. As expected, oxygen consumption rates were higher during the dark phase of the light cycle (Fig. 2A), when mice are active and feeding (Fig. 2B). This pattern was present for wild-type and Mogat2−/− mice across each of the diets. Compared with wild-type mice, Mogat2−/− mice consumed more oxygen per mouse, as indicated by greater areas under the curves across diets (Fig. 2A; genotype main effect, P < 0.05). The differences between genotypes were most evident during the dark phase. For example, on chow, the oxygen consumption of Mogat2−/− mice was 7.4% higher than that of controls during the dark phase, but not significantly different during the light phase. Likewise, when consuming each of the defined diets, oxygen consumption of Mogat2−/− mice increased 5.4–7.0% over that of the wild-type. Although the increases when consuming 45% and 60% diets did not reach statistical significance (P = 0.09 and 0.15, respectively), the differences were remarkable because wild-type, but not Mogat2−/−, mice gained weight when fed the high-fat diets (Fig. 1B).
Fig. 2.
Metabolic phenotyping of mice fed diets with varying levels of fat. Male mice (2–4 months old) deficient in MGAT2 (Mogat2−/−; open circles) and wild-type littermates (WT; black squares) were sequentially fed chow, then semi-purified (defined) diets containing 10, 45, or 60% calories from fat for 3 days each diet. A: Oxygen consumption rates. B: Food intakes per h. C: Oxygen consumption rates adjusted for baseline body weights of each mouse at the start of each diet treatment. D: RERs calculated by dividing carbon dioxide production with oxygen consumption (VCO2/VO2). E: Metabolic rates calculated by a modified Weir equation (metabolic rate = 3.941 × VO2 + 1.106 × VCO2). Data from each mouse were pooled from the same time of the day of the same diet treatment. WT, n = 23–26 mice per group; Mogat2−/−, n = 16–19 mice per group. Graphs represent average days. Gray areas mark dark phase of the light cycle (6 PM to 6 AM). Error bars not shown are smaller than symbols. The statistical analyses were performed as described in the Materials and Methods section.
High-fat feeding exacerbates differences in oxygen consumption
Differences in oxygen consumption between wild-type and Mogat2−/− mice were accentuated when adjusted for body weight. When mice were fed purified diets, compared with chow, the differences in oxygen consumption were more pronounced; moreover, the differences increased as dietary fat content increased (Fig. 2C). When mice were fed chow, the difference between genotypes was 10.2%, whereas the differences were 11.2, 12.3, and 15.7% on the 10, 45, and 60% diets, respectively (P < 0.05 for all comparisons). The magnitude of differences cannot be explained fully by the differences in body weights at the beginning of the high-fat diet treatments.
Mogat2−/− mice oxidize more fat than do wild-type mice when consuming the defined diets
Mice consistently showed an increase in respiratory exchange ratio (RER) during the dark phase (Fig. 2D), indicating a shift from utilizing body fat stores to utilizing dietary carbohydrate.
In keeping with oxidation of more carbohydrates than fat, the average RERs over 24 h were higher when mice were switched from chow (53% carbohydrate) to the 10% diet high in refined carbohydrate (70%; from 0.89 ± 0.06 to 0.93 ± 0.06). Consistently, RERs decreased step-wise, from 0.93 to 0.83 and then 0.78, when wild-type mice were fed diets with increasing amounts of fat replacing carbohydrate, from 10% to 45% and then 60%.
Like wild-types, Mogat2−/− mice also showed the expected step-wise decreases in RER, from 0.92 to 0.79 and then 0.75, when dietary carbohydrate was replaced by fat. Interestingly, Mogat2−/− mice oxidized higher proportions of fat than did wild-type mice when fed the defined diets. Their RERs were 3−7% lower than those of controls during the light phase. The decreases reached statistical significance when mice were fed the 45% and 60% diets (Fig. 2D). However, the differences diminished after dark, when RER rose in both groups, presumably due to increased oxidation of carbohydrate coming from the diet.
Energy expenditure is elevated in Mogat2−/− mice
To determine levels of energy expenditure as metabolic rates, we used a modified Weir equation that takes both oxygen consumption and RER into account after adjusting for body weight (19). In parallel with oxygen consumption, metabolic rates of wild-type mice were higher during the dark phase as compared with during the light phase, and the rates decreased with high-fat feeding (Fig. 2E). Mogat2−/− mice exhibited significantly higher metabolic rates compared with wild-type mice on each of the four diets (12.6, 10.7, 12.3, and 16.2%, respectively; P < 0.05). These increases were associated with food intake and remained high during the postprandial state. The magnitudes of differences were similar, but not identical, to those of oxygen consumption described above, consistent with differences in substrate utilization as indicated by RERs.
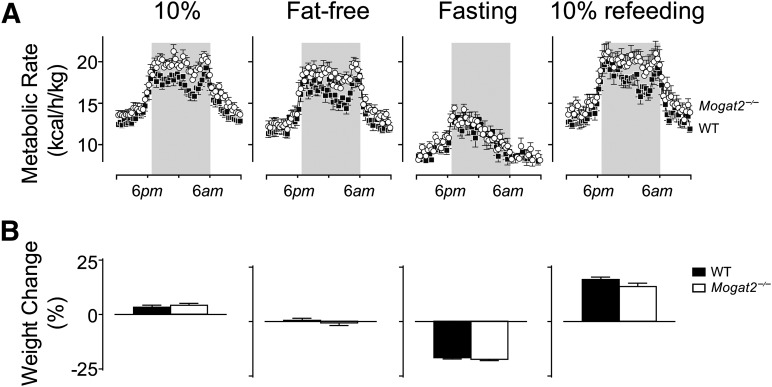
Dietary fat is not required for MGAT2 deficiency-induced increases in energy expenditure
Because Mogat2−/− mice exhibited increased metabolic rates after feeding on a diet containing as low as 10% fat, we next determined whether dietary fat is essential for the underlying mechanisms. We did so by examining the metabolic responses of Mogat2−/− mice to a fat-free diet and to fasting. When switched to a defined fat-free diet, Mogat2−/− mice maintained their increased energy expenditure and food intake to degrees similar to those seen when they were fed a comparable 10% fat diet (Fig. 3A). During the dark phase, when mice were most active and feeding, metabolic rate in Mogat2−/− mice was elevated 10.9% over that of wild-type mice on a fat-free diet, as compared with a 10.5% elevation when they were fed the 10% diet.
Fig. 3.
Increased energy expenditure in Mogat2−/− mice does not require fat intake, but does require feeding. A: Metabolic rates of adult male mice (4−9 months old) consuming low-fat (10%) or fat-free diet, or under fasting or refeeding conditions. Metabolic rates of Mogat2−/− mice (open circles; n = 10) and wild-type littermates (WT; black squares; n = 11) were calculated by a modified Weir equation (metabolic rate = 3.941 × VO2 + 1.106 × VCO2). B: Body weight changes of Mogat2−/− mice (white bars) and their wild-type littermates (black bars) during each diet treatment. Mice had similar body weights at the start of the study (28.5 ± 1.0 vs. 28.6 ± 0.6 g). Values are means ± SEM.
Even though dietary fat is not essential for expression of the phenotype, the presence of some dietary component is. When fasted, Mogat2−/− mice reduced metabolic rate (Fig. 3A) to the same levels as their wild-type controls, both before and after adjustment for body weight. Consistent with their similar levels of energy expenditure, Mogat2−/− mice and their wild-type controls lost a similar percent of their body weights during the 2 day fast (17.4 ± 0.6 vs. 16.6 ± 0.6%, P = 0.365; Fig. 3B).
Mogat2−/− mice maintain energy balance despite increased energy expenditure
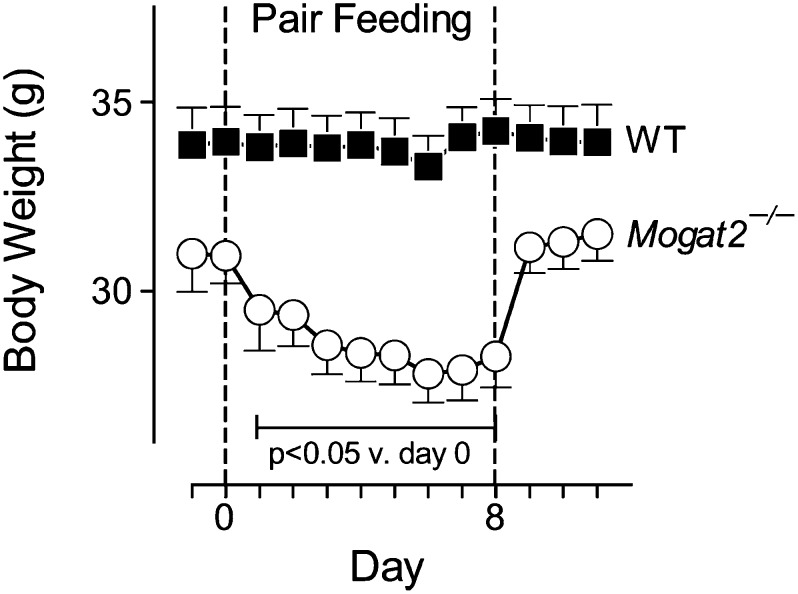
When Mogat2−/− mice were fed chow, their weight curves were not significantly different from those of wild-type littermates for at least 1 year [(17); data not shown]. Thus, the increases in energy expenditure of Mogat2−/− mice in the absence of high-fat feeding were not expected. Because body weight is determined by a balance of energy intake and energy expenditure, we found that when fed chow, Mogat2−/− mice consumed 8.4% more per day than did wild-type mice (4.64 ± 0.15 vs. 4.28 ± 0.11 g/day; Fig. 2B). By pair-feeding Mogat2−/− mice to wild-type controls, we next tested whether MGAT2 deficiency induces an obligatory increase in metabolic rate or if increased metabolic rate is secondary to increased food intake. When their chow intake was restricted to the level of controls, Mogat2−/− mice lost a significant 4.5% of their weight within a day and the negative energy balance continued until they reached a new equilibrium after 6 days of pair-feeding (Fig. 4). This finding suggests that increased energy expenditure of Mogat2−/− mice consuming a chow diet is obligatory and is normally compensated for by increases in food intake to maintain energy homeostasis.
Fig. 4.
Mogat2−/− mice increase chow consumption to maintain body weight. Body weights and food intakes of male mice (1 year old) deficient in MGAT2 (Mogat2−/−; open circles; n = 7) and wild-type littermates (WT; black squares; n = 11) were measured daily. Average food intake for WT mice was calculated daily, and the same amount was fed to each Mogat2−/− mouse during the pair-feeding period (between dotted lines). Values are means ± SEM. Bracket includes data points that differ from body weight at the start of pair-feeding in Mogat2−/− mice.
When fed the defined 10% diet, the 5% increase in food consumption of Mogat2−/− mice as compared with wild-type littermates (3.84 ± 0.13 vs. 3.65 ± 0.10 g/day) did not reach statistical significance. Mogat2−/− mice maintained energy balance, whereas wild-type mice gained 0.8 ± 0.1 g in 3 days when switched to the calorie-dense diet rich in refined carbohydrates (Fig. 1B). In an independent longer-term study, wild-type mice gained a significant amount of weight after 1 week on the 10% diet and continued to gain weight throughout the nearly 2 month experiment, resulting in a 14.5% weight gain (Fig. 5). Mogat2−/− mice, on the other hand, maintained their baseline body weights. At the end of the study, wild-type mice weighed 20% more than did Mogat2−/− mice.
Fig. 5.
MGAT2 deficiency prevents weight gain on a low-fat diet rich in refined carbohydrate. Body weights of male mice (14–15 months old) deficient in MGAT2 (Mogat2−/−; open circles; n = 7) and wild-type littermates (WT; black squares; n = 12) were measured weekly after switching from chow to 10% diet. Values are means ± SEM. Bracket includes data points that differ from body weight at the start of 10% feeding in wild-type mice. *, P < 0.05 versus diet-matched WT.
When switched to 45% and 60% diets, Mogat2−/− mice also were protected from weight gains seen in controls (Fig. 1B). Interestingly, Mogat2−/− mice transiently decreased caloric intake when fed high-fat diets, compared with when they were fed the 10% diet; whereas wild-type mice slightly increased their caloric intake over the first 3 days of high-fat feeding (data not shown). The effects of MGAT2 deficiency on intake of high-fat diets were transient, inasmuch as food intakes between genotypes were not significantly different after 3 days of high-fat feeding (data not shown). In contrast, the effects on energy expenditure persisted after mice were fed the high-fat diets for 10 weeks (data not shown), suggesting that the reported protective effects upon chronic high-fat feeding (17) are mainly conferred by the increases in energy expenditure.
MGAT2 deficiency increases energy expenditure in genetically obese Agouti mice
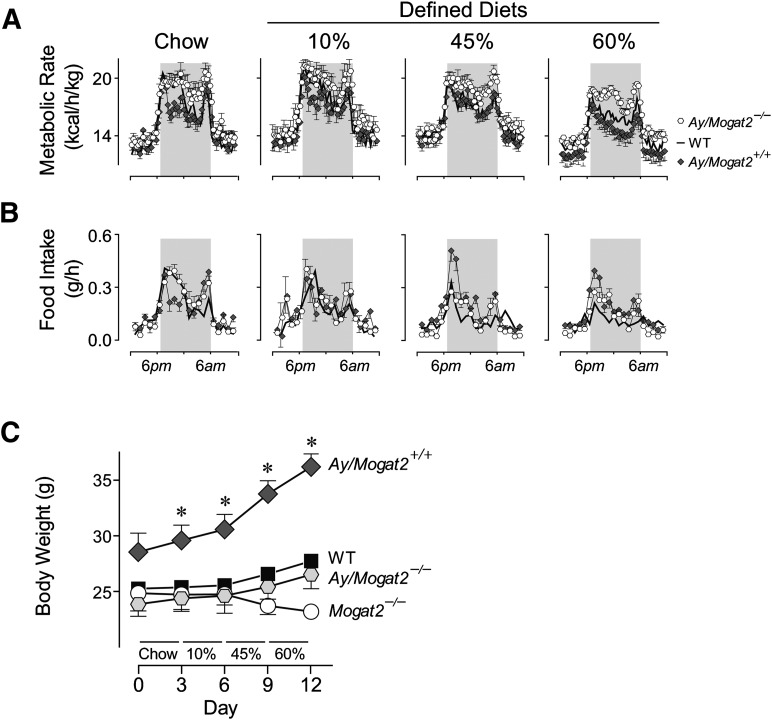
To rigorously test the biological significance of MGAT2 deficiency on energy balance without high-fat feeding, we introduced the Mogat2-targeted allele into genetically obese Agouti mice (Ay/a). We first examined their metabolic responses to the same four diets. On chow and 10% diet, Ay/a, Mogat2−/− mice exhibited 10.6% and 11.7% increases, respectively, in metabolic rates, compared with Agouti mice (Fig. 6A). When first exposed to the 45% high-fat diet, however, Ay/a, Mogat2−/− mice showed only an 8.8% increase in metabolic rate. The increase was probably subdued by a 30% lower food intake of Ay/a, Mogat2−/− mice than that of Agouti mice (Fig. 6B; 2.96 ± 0.28 vs. 4.17 ± 0.14 g/day). On 60% diet, these mice had 16.6% higher metabolic rates than Agouti mice despite 32% lower food intake (2.49 ± 0.18 vs. 3.64 ± 0.19 g/day). Like mice without the Agouti mutation, Ay/a, Mogat2−/− mice decreased their food intakes when fed the more-calorie-dense diets; in contrast, Agouti mice had elevated food intakes and consumed 32, 54, and 50% more calories when fed 10, 45, and 60% diets, respectively, than when fed chow. Accordingly, during the metabolic phenotyping studies, Agouti mice gained weight rapidly, especially when fed the defined diets, whereas MGAT2 deficiency strongly attenuated the acute increases in body weight to the levels of wild-type mice without the Agouti mutation (Fig. 6C).
Fig. 6.
MGAT2 deficiency increases energy expenditure and protects against excess weight gain in Agouti mice. Male (2–5 month old) wild-type mice (WT; black squares), wild-type mice with the Agouti mutation (Ay/a; dark diamonds), Mogat2−/− mice (Mogat2−/−; open circles), and Mogat2−/− mice with the Agouti mutation (Ay/a, Mogat2−/−; open hexagons) were fed chow, then semi-purified (defined) diets containing 10, 45, or 60% calories from fat for 3 days each diet. A: Metabolic rates calculated by a modified Weir equation (metabolic rate = 3.941 × VO2 + 1.106 × VCO2). Gray areas mark dark phase of the light cycle (6 PM to 6 AM). Black line represents WT (symbols of WT and curves for Mogat2−/− omitted for clarity). Graphs represent average days on each diet. B: Food intake and (C) body weights of mice during the metabolic study. n = 8–11 mice per group. Values are means ± SEM. *, P < 0.05 versus diet-matched WT mice.
Agouti mice deficient in MGAT2 are protected from excessive weight gain
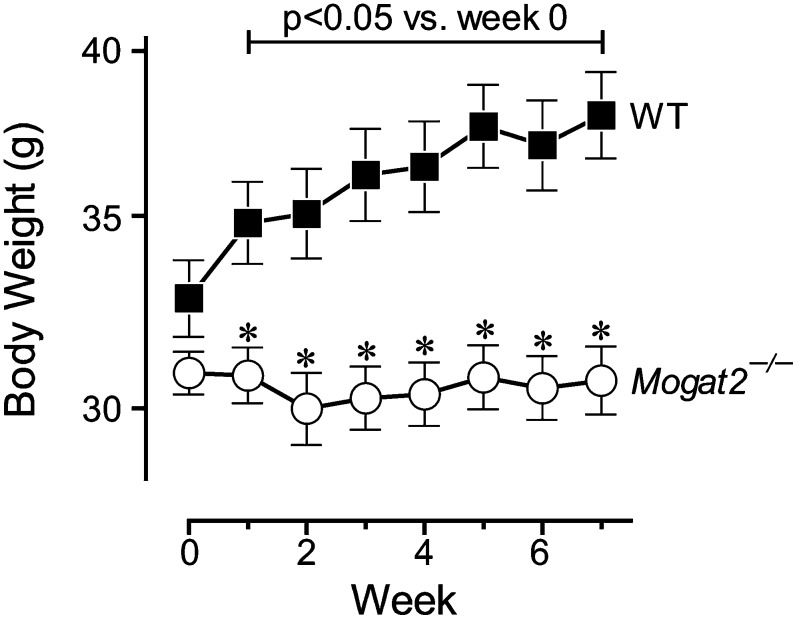
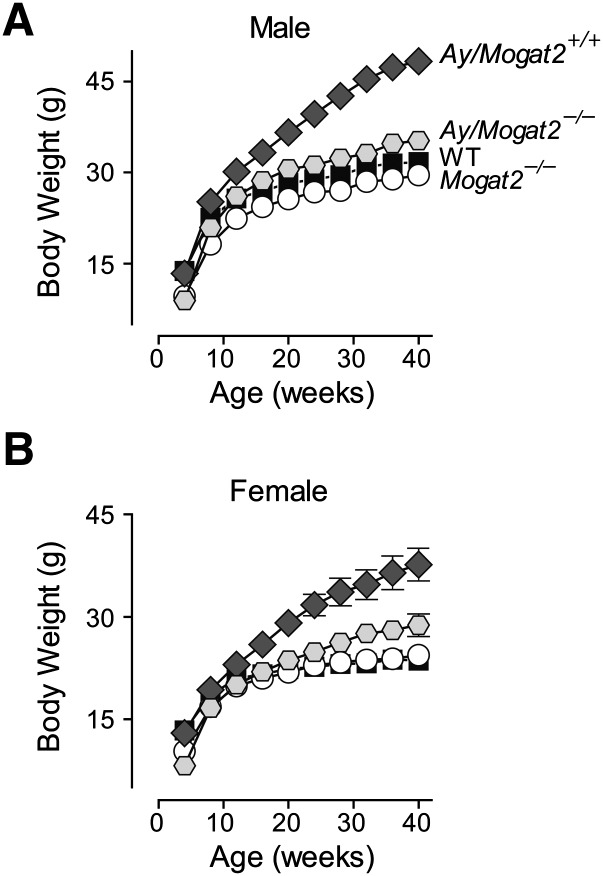
To determine the long-term effect of MGAT2 deficiency on weight gain induced by the Agouti mutation, we fed Ay/a, Mogat2−/− mice and their littermate controls chow diet for 40 weeks. In the presence of MGAT2, male Agouti mice (Ay/a, Mogat2+/+) weighed significantly more than did their wild-type littermates (a/a, Mogat2+/+) by 8 weeks of age (25.2 ± 0.6 vs. 22.5 ± 0.1 g; Fig. 7A). In the absence of MGAT2, Agouti mice (Ay/a, Mogat2−/−) were protected from excessive weight gain at all time points monitored. After 40 weeks of chow feeding, Ay/a, Mogat2+/+ mice weighed 52% more than did wild-type littermates, whereas Ay/a, Mogat2−/− mice had body weights similar to wild-type controls without the Agouti mutation throughout the study (Fig. 7A). As seen in obesity induced by a high-fat diet, heterozygous (Ay/a, Mogat2+/−) mice exhibited an intermediate phenotype, and fat mass accounted for most of the differences in weights (data not shown). Similar results were observed in females. After chow feeding for 40 weeks, Agouti mice (Ay/a, Mogat2+/+) weighed 59% more than wild-type controls. Ay/a, Mogat2−/− female mice weighed significantly less than Agouti mice at all time points, resulting in 23% lower body weight after 40 weeks (28.8 ± 1.6 vs. 37.6 ± 2.4 g; Fig. 7B).
Fig. 7.
MGAT2 deficiency protects genetically obese Agouti mice from adult-onset obesity. Body weight of (A) male and (B) female wild-type mice (WT; black squares), wild-type mice with the Agouti mutation (Ay/a; dark diamonds), Mogat2−/− mice (Mogat2−/−; open circles), and Mogat2−/− mice with the Agouti mutation (Ay/a, Mogat2−/−; gray hexagons) were fed chow after weaning through 40 weeks. n = 5–9 mice /per group. Values are means ± SEM. Error bars not shown are smaller than symbols.
DISCUSSION
We have previously reported that Mogat2−/− mice exhibit increased energy expenditure and are protected from obesity and other metabolic disorders induced by high-fat feeding (17). In the present study, we show that deficiency of MGAT2 in mice also increases energy expenditure, decreases the propensity to gain weight, and protects against obesity in the absence of high-fat feeding. In acute feeding experiments, we found that Mogat2−/− mice had 10−15% higher metabolic rates than did their wild-type littermates when they were fed diets containing 10−60% calories from fat. Unexpectedly, when fed a diet without fat, Mogat2−/− mice still exhibited increases in metabolic rates to a similar degree as when mice were fed 10% fat diet. This effect was associated with dietary intake, and it disappeared during fasting, implicating an essential role of MGAT2 in efficient assimilation of nutrients and in regulating an obligatory component of diet-induced thermogenesis. The functional significance of these increases in energy expenditure was illustrated by the lower propensity of Mogat2−/− mice to gain weight, as compared with wild-type littermates, when fed a low-fat diet rich in refined carbohydrate. Furthermore, MGAT2 deficiency prevents excessive weight gain in a genetic model of obesity without high-fat feeding.
On the basis of the established role of intestinal MGAT in fat absorption, we expected that the effects of MGAT2 deficiency would require high levels of fat intake. Indeed, the body weights of Mogat2−/− mice did not differ from those of wild-type littermates during chow feeding; and in our previous report, the increases in energy expenditure, measured using a different metabolic phenotyping system, did not reach statistical significance until mice were fed a 60% fat diet (17). In this study, using a new metabolic phenotyping system that resembles their home cage environment and allows for bedding, we found that Mogat2−/− mice exhibited increases in energy expenditure on all tested diets containing a wide range (0−60%) of fat. The increases in energy expenditure between genotypes are evidenced by increases in oxygen consumption as well as the calculated metabolic rate after considering substrate utilization. Increases in oxygen consumption were identified within the four diet strata using the mixed-effects model as well as using a ratio method adjusting for body weight (Figs. 1, 2). Mogat2−/− mice consistently consumed higher absolute amounts of oxygen than did wild-type littermates. These increases were remarkable, inasmuch as Mogat2−/− mice tended to weigh less.
The differences in energy expenditure between Mogat2−/− mice and wild-type littermates were most pronounced during the feeding period, suggesting that MGAT2 modulates diet-induced thermogenesis. Also known as the thermic effect of food, this increase in metabolic rate after a meal is estimated to account for 10% of total energy expenditure in adult humans, in addition to physical activity and basal metabolism (24, 25). Diet-induced thermogenesis is considered an obligatory energy cost for digestion, absorption, transport, and other metabolic activity needed for the assimilation of nutrients. It has also been proposed as a facultative part of adaptive thermogenesis responding to excess food intake, like nonshivering thermogensis responding to cold, that involves the sympathetic nervous system and brown adipose tissues (26–30).
Dietary fat induces the least thermogenesis (estimated at 0−5%), whereas protein induces the most (20−30%) among the energy-yielding nutrients (31). The lower thermic effect of fat compared with carbohydrate may explain the weight gains upon high-fat feeding (4, 5). In our study, wild-type, but not Mogat2−/−, mice have decreased postprandial metabolic rates when fed high-fat diets, suggesting that MGAT2 reduces the energy cost of fat assimilation. Because wild-type mice were gaining weight on high-fat diets, the decreases in weight-adjusted metabolic rate may also reflect the increases in fat mass, which expends less energy than lean mass.
Mogat2−/− mice exhibited increased energy expenditure in response to a meal, even in the absence of dietary fat, indicating that at least one underlying mechanism is independent of fat intake. These mice showed a similar 10% higher metabolic rate over wild-type littermates in response to a fat-free or a 10% fat diet (Fig. 3). Increasing dietary fat from 10% to 60% exacerbated the differences only by an additional 5% (Fig. 2E), suggesting the fat-independent mechanism has a dominating impact. Fasting eliminated this effect. In mice, food consumption and physical activity are closely correlated. We postulate that the increase in energy expenditure of Mogat2−/− mice reflects a response to meals, rather than a change in physical activity, because there was no difference detected in ambulatory activity (17), energy expenditure during fasting (Fig. 3A), or weight loss after a 48 h fast (Fig. 3B). These mice can still respond to food deprivation properly by reducing metabolic rate and slowing the rate of weight loss to the same extent as wild-type mice. During fasting, Mogat2−/− mice presumably remained active in food-seeking behaviors, like wild-type mice, as indicated by the elevation in metabolic rate during the dark phase, as compared with the light phase. The differences in energy expenditure between genotypes were seen again upon refeeding (Fig. 3).
At the systems level, MGAT2 modulates energy balance most likely through its role in the intestine, because MGAT2 is highly expressed only in the intestine in mice (11) and because the effects of MGAT2 deficiency on energy expenditure require food intake (Fig. 3). Intestinal MGAT2 is probably an integral part of the processes that enhance metabolic efficiency by reducing the cost of nutrient assimilation and suppressing thermogenesis. In contrast to the well-established role of the intestine in regulating food intake and nutrient absorption, the phenotype of Mogat2−/− mice suggests a less-recognized role of the intestine in modulating energy expenditure. This conjecture is supported also by findings from mice deficient in diacylglycerol acyltransferase 1 (DGAT1), another triacylglycerol synthesis enzyme highly expressed in the small intestine (2, 32). Mice deficient in DGAT1 exhibit increased energy expenditure and are protected from diet-induced obesity (33). Reintroducing DGAT1 specifically in the intestine is sufficient to reverse the weight protection phenotype (34). These findings suggest that MGAT2- and DGAT1-mediated lipid metabolism in the intestine plays a pivotal role in coordinating systemic responses to maximize metabolic efficiency and propensity to gain weight.
At the cellular level, the absorptive enterocytes (where MGAT2 is highly expressed) are most likely responsible through their impacts on delivery of lipid substrates to peripheral tissue and on secretion of gut hormones from the enteroendocrine cells (16, 17). Levels of several postprandial hormones differ between Mogat2−/− and wild-type mice (17, 35). Because both its substrate monoacylglycerols and its product diacylglycerols can serve as signaling molecules, MGAT2 may directly modulate the secretion of enteroendocrine cells in response to luminal nutrients. It remains to be explored whether MGAT2 is expressed in enteroendocrine cells in vivo. Additionally, a few tissues, such as brown adipose tissue, express low levels of MGAT2 (less than 0.1% of that found in the small intestine). At this time, the possibility cannot be excluded that these tissues might modulate energy balance in a cell-autonomous fashion through mechanisms such as substrate cycling between de novo lipogenesis and FA oxidation.
The effects of MGAT2 on food intake depend on dietary composition. Mogat2−/− mice compensated for the increased energy expenditure by consuming more chow to maintain their weight. When chow intake levels were restricted to those of wild-types, they lost weight (Fig. 4), confirming that the constant increase in food intake is an adaptive response to an obligatory increase in energy expenditure. The transient suppression of food intake, seen in these mice when first exposed to a high-fat diet, may reflect a change in factors that are involved in short-term intake regulation, such as cholecystokinin, peptide YY, and glucagon-like peptide-1 (17, 36). These factors may be overridden by factors that regulate long-term energy balance (37), inasmuch as intake levels of Mogat2−/− mice returned to normal within 3 days (data not shown). Unlike wild-type mice, Mogat2−/− mice are protected from obesity after prolonged high-fat feeding (17). This protective effect is probably due to the increases in energy expenditure, which persisted over 2 months, whereas the decreases in food intake were transient (data not shown). The levels of fat absorption and the amounts and energy content of feces do not differ between genotypes (17).
The protective effect of MGAT2 deficiency is broader than high-fat-diet-induced obesity, because inactivating MGAT2 also protects against weight gains induced by a diet rich in refined carbohydrate and by the Agouti mutation. In addition to increased food intake, Agouti mice are more metabolically efficient, allowing them to gain more weight than controls when fed chow, a high-fat, or a high-sucrose diet (38). Consistent with their higher metabolic efficiency, we found that the metabolic rates in Agouti mice trended lower than in wild-type littermates without the Agouti mutation, although the difference did not reach statistical significance during the metabolic phenotyping study. Nonetheless, they were hyperphagic and gained weight much faster than did their wild-type littermates, especially when fed each of the defined diets (Fig. 6B). In contrast, inactivation of MGAT2 in Agouti mice increased energy expenditure, attenuated hyperphagia, and minimized weight gain. These differences were most pronounced during acute high-fat feeding (Fig. 6). In the long-term chow feeding study, Agouti mice deficient in MGAT2 were protected from the adult-onset obesity seen in Agouti mice (Fig. 7). The underlying mechanisms remain to be determined.
In conclusion, the results of our study indicate that MGAT2 normally modulates diet-induced thermogenesis, promotes metabolic efficiency, and favors positive energy balance. Deficiency of MGAT2 enhances energy expenditure after meals. Although the differences are exacerbated by high-fat feeding, the underlying mechanisms are dominated by one independent of dietary fat. The protective effect of MGAT2 deficiency against excessive weight gain is not limited to obesity induced by excessive fat intake. Mice deficient in MGAT2 also were protected from weight gain induced by a low-fat diet rich in refined carbohydrate and from genetic obesity induced by the Agouti mutation. Because MGAT2 is highly expressed in human intestine (11, 39), these findings also raise the prospect of inhibiting MGAT2 as a strategy for combating obesity and related metabolic disorders resulting from overconsumption of calories in humans.
Acknowledgments
The authors thank Drs. R. Eisenstein and R. Sunde for comments on the manuscript and Drs. Zhumin Zhang and HuiChuan Lai for help with statistical analyses.
Footnotes
Abbreviations:
- DGAT
- diacylglycerol acyltransferase
- MGAT
- monoacylglycerol acyltransferase
- RER
- respiratory exchange ratio
This work was supported by funding from the University of Wisconsin-Madison, the American Heart Association (Grant 0630104N), the United States Department of Agriculture (Grant WIS01313), and the National Institutes of Health (Grants R01DK-088210 and T32DK-007665). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Coleman R. A., Lewin T. M., Muoio D. M. 2000. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu. Rev. Nutr. 20: 77–103. [DOI] [PubMed] [Google Scholar]
- 2.Yen C. L., Stone S. J., Koliwad S., Harris C., Farese R. V., Jr 2008. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flatt J. P. 1992. The biochemistry of energy expenditure. Björntorp Obesity. P., Brodoff B. N., J.B. Lippincott, New York: 100–116. [Google Scholar]
- 4.Maffeis C., Schutz Y., Grezzani A., Provera S., Piacentini G., Tato L. 2001. Meal-induced thermogenesis and obesity: is a fat meal a risk factor for fat gain in children? J. Clin. Endocrinol. Metab. 86: 214–219. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan R., King R. F., Holmfield J., Siwek R. A., Baker M., Wales J. K. 1985. Thermic effect of feeding carbohydrate, fat, protein and mixed meal in lean and obese subjects. Am. J. Clin. Nutr. 42: 177–181. [DOI] [PubMed] [Google Scholar]
- 6.Neel J. V. 1962. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am. J. Hum. Genet. 14: 353–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Neel J. V., Weder A. B., Julius S. 1998. Type II diabetes, essential hypertension, and obesity as “syndromes of impaired genetic homeostasis”: the “thrifty genotype” hypothesis enters the 21st century. Perspect. Biol. Med. 42: 44–74. [DOI] [PubMed] [Google Scholar]
- 8.Muoio D. M., Newgard C. B. 2006. Obesity-related derangements in metabolic regulation. Annu. Rev. Biochem. 75: 367–401. [DOI] [PubMed] [Google Scholar]
- 9.Zimmet P., Alberti K. G., Shaw J. 2001. Global and societal implications of the diabetes epidemic. Nature. 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 10.Phan C. T., Tso P. 2001. Intestinal lipid absorption and transport. Front. Biosci. 6: D299–D319. [DOI] [PubMed] [Google Scholar]
- 11.Yen C. L., Farese R. V., Jr 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 12.Yen C. L., Stone S. J., Cases S., Zhou P., Farese R. V., Jr 2002. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA. 99: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao J., Burn P., Shi Y. 2003. Properties of the mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278: 25657–25663. [DOI] [PubMed] [Google Scholar]
- 14.Cheng D., Nelson T. C., Chen J., Walker S. G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J. T., Ramaker M., Feder J. N. 2003. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278: 13611–13614. [DOI] [PubMed] [Google Scholar]
- 15.Cao J., Hawkins E., Brozinick J., Liu X. Y., Zhang H. X., Burn P., Shi Y. G. 2004. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J. Biol. Chem. 279: 18878–18886. [DOI] [PubMed] [Google Scholar]
- 16.Nelson D. W., Yen C. L. E. 2009. Triacylglycerol synthesis and energy metabolism: a gut reaction? Clinical Lipidology. 4: 683–686. [Google Scholar]
- 17.Yen C. L., Cheong M. L., Grueter C., Zhou P., Moriwaki J., Wong J. S., Hubbard B., Marmor S., Farese R. V., Jr 2009. Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat. Med. 15: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michaud E. J., Bultman S. J., Klebig M. L., van Vugt M. J., Stubbs L. J., Russell L. B., Woychik R. P. 1994. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (Ay) mutation. Proc. Natl. Acad. Sci. USA. 91: 2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir J. B. 1949. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butler A. A., Kozak L. P. 2010. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 59: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiyala K. J., Morton G. J., Leroux B. G., Ogimoto K., Wisse B., Schwartz M. W. 2010. Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 59: 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean P. S. 2010. Comment on Kaiyala et al. (2010). Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes. 59: 1657–1666. Diabetes.60: e3; author reply e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zar J. 1996. Biostatistical Analysis. 3rd edition. Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- 24.Reed G. W., Hill J. O. 1996. Measuring the thermic effect of food. Am. J. Clin. Nutr. 63: 164–169. [DOI] [PubMed] [Google Scholar]
- 25.Schutz Y., Bessard T., Jequier E. 1984. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am. J. Clin. Nutr. 40: 542–552. [DOI] [PubMed] [Google Scholar]
- 26.Glick Z., Teague R. J., Bray G. A. 1981. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science. 213: 1125–1127. [DOI] [PubMed] [Google Scholar]
- 27.Landsberg L., Saville M. E., Young J. B. 1984. Sympathoadrenal system and regulation of thermogenesis. Am. J. Physiol. 247: E181–E189. [DOI] [PubMed] [Google Scholar]
- 28.Himms-Hagen J. 1985. Brown adipose tissue metabolism and thermogenesis. Annu. Rev. Nutr. 5: 69–94. [DOI] [PubMed] [Google Scholar]
- 29.Lidell M. E., Enerback S. 2010. Brown adipose tissue–a new role in humans? Nat. Rev. Endocrinol. 6: 319–325. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz R. S., Halter J. B., Bierman E. L. 1983. Reduced thermic effect of feeding in obesity: role of norepinephrine. Metabolism. 32: 114–117. [DOI] [PubMed] [Google Scholar]
- 31.Tappy L. 1996. Thermic effect of food and sympathetic nervous system activity in humans. Reprod. Nutr. Dev. 36: 391–397. [DOI] [PubMed] [Google Scholar]
- 32.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V. 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25: 87–90. [DOI] [PubMed] [Google Scholar]
- 34.Lee B., Fast A. M., Zhu J., Cheng J. X., Buhman K. K. 2010. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1−/− mice. J. Lipid Res. 51: 1770–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okawa M., Fujii K., Ohbuchi K., Okumoto M., Aragane K., Sato H., Tamai Y., Seo T., Itoh Y., Yoshimoto R. 2009. Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem. Biophys. Res. Commun. 390: 377–381. [DOI] [PubMed] [Google Scholar]
- 36.Field B. C., Chaudhri O. B., Bloom S. R. 2010. Bowels control brain: gut hormones and obesity. Nat. Rev. Endocrinol. 6: 444–453. [DOI] [PubMed] [Google Scholar]
- 37.Stanley S., Wynne K., McGowan B., Bloom S. 2005. Hormonal regulation of food intake. Physiol. Rev. 85: 1131–1158. [DOI] [PubMed] [Google Scholar]
- 38.Frigeri L. G., Wolff G. L., Teguh C. 1988. Differential responses of yellow Avy/A and agouti A/a (BALB/c X VY) F1 hybrid mice to the same diets: glucose tolerance, weight gain, and adipocyte cellularity. Int. J. Obes. 12: 305–320. [PubMed] [Google Scholar]
- 39.Lockwood J. F., Cao J., Burn P., Shi Y. 2003. Human intestinal monoacylglycerol acyltransferase: differential features in tissue expression and activity. Am. J. Physiol. Endocrinol. Metab. 285: E927–E937. [DOI] [PubMed] [Google Scholar]