Abstract
Expression of apoE in adipocytes has been shown to have an important role in modulating adipocyte triglyceride (TG) metabolism and gene expression that is independent of circulating and extracellular apoE. The impact of adipocyte expression of common human apoE isoforms was evaluated using adipocytes harvested from human apoE2, -3, and -4 knock-in mice. Expression of the apoE2 isoform was associated with an increase in adipocyte apoE gene expression and apoE synthesis. Newly synthesized apoE2 was unstable in adipocytes and demonstrated increased degradation and decreased secretion. ApoE2-expressing mice were hyperlipidemic, and had increased size of gonadal fat pads and of adipocytes, compared with apoE3 mice. In isolated cells, however, expression of the apoE2 isoform produced defective lipogenesis and increased TG hydrolysis. Incubation of adipose tissue with apoE3-containing TG-rich lipoproteins resulted in a significant increase in TG in adipose tissue from apoE3 and -E4 mice, but not apoE2 mice. Reduced capacity to internalize FFA as lipogenic substrate contributed to defective lipogenesis. Newly synthesized apoE2 is unstable in adipocytes and results in decreased adipocyte TG synthesis and defective FA uptake. These changes recapitulate those observed in apoE knockout adipocytes and have implications for understanding metabolic disturbances in humans expressing the E2 isoform.
Keywords: obesity, adipose tissue, adipogenesis, fatty acid, apolipoprotein
Obesity is becoming increasingly common and predisposes to premature cardiovascular mortality (1–3). Because of this, it is important to understand mechanisms by which adipose tissue influences organismal substrate distribution and metabolism in both physiological and pathophysiological states. Recently, the unexpected observation was made that the endogenous expression of apoE in adipose tissue and adipocytes importantly influences adipocyte differentiated function. Adipocytes that do not express endogenous apoE are smaller, contain less lipid, display a defect in lipogenesis and substrate acquisition from extracellular lipoproteins, and have alterations in the expression of genes controlling adipocyte lipid turnover (4–7). Moreover, these defects cannot be corrected by the provision of extracellular apoE in vitro or in vivo, but can be corrected by adenoviral-mediated expression of endogenous apoE in adipocytes (4, 5). Because of these effects on adipose tissue metabolism, endogenous adipocyte apoE also importantly influences the systemic distribution of substrate in intact animals (5). A physiological role for endogenous adipocyte apoE expression in the regulation of organismal metabolism is further underscored by the recent demonstration of physiologically relevant pathways for regulating adipocyte apoE expression (8–14).
In humans, apoE is a polymorphic protein, and three common isoforms have been identified: apoE2 (Cys112/Cys158), apoE3 (Cys112/Arg158), and apoE4 (Arg112/Arg158). The apoE3 isoform is by far the most common and is present in more than 75% of individuals. The presence of the apoE2 or apoE4 isoform has been associated with important human diseases and alterations in systemic lipoprotein metabolism (15–17). There are also observations in human populations suggesting a relationship between apoE isoform expression and adiposity, and a relationship between adiposity and lipid metabolism (18, 19). In addition, important differences in cellular turnover of the apoE isoforms, with related implications for differentiated cell function, have been described in macrophages (20–23) and neuronal cells (24–26). For example, in macrophages, the secretion of apoE2 is impaired, and there is an associated alteration in macrophage sterol efflux mediated by apoE2. In vivo, adipose tissue dysfunction has been associated with the systemic expression of apoE4 in mice maintained on a high-fat Western diet (27, 28).
In view of these considerations, in the current studies, we have evaluated the impact of apoE isoform expression in adipocytes on adipocyte function by taking advantage of the previously described human apoE2, apoE3, and apoE4 knock-in mouse models. In these mice, mouse apoE expression is deleted and replaced with expression of the human apoE2, -E3, and -E4 isoforms under the control of endogenous gene control elements. Using adipose tissue and adipocytes isolated from these mice, we have characterized adipocyte handling of the apoE isoforms and have evaluated the impact of apoE2 isoform expression on adipocyte lipid metabolism.
MATERIALS AND METHODS
Materials
Cell culture medium and FBS were purchased from Invitrogen (Carlsbad, CA). Organic solvents were from Thermo-Fisher (Pittsburgh, PA). Other chemicals were from Sigma (St. Louis, MO). 35[S]methionine and 14[C]glucose were obtained from PerkinElmer (Wellesley, MA). Triglyceride (TG) assay kits were obtained from Wako Chemicals USA (Richmond, VA). The Quencher based technology (QBT) assay kit was obtained from Molecular Devices (Sunnyvale, CA). Other reagents were from previously identified sources (4–14).
Animals and cell isolation and culture
C57BL/6 mice were knocked in with human apoE2, -E3, or -E4 alleles to replace native mouse apoE. These models have been described in detail in previous publications (29–33). In these mice, expression of the apoE isoform remains under the control of the endogenous apoE gene control elements. All animal protocols in this study were approved by the Institutional Animal Care and Use Committees of the University of Illinois at Chicago.
Intra-abdominal perigonadal adipose tissue was collected from age- (12–14 week-old) and gender-matched apoE2, -E3, and -E4 isoform knock-in mice. Mature adipocytes were isolated by collagenase digestion of adipose tissues, as previously described in detail (4, 6). The diameter of freshly isolated mature adipocytes was determined as previously described (5). Preadipocytes were isolated from the adipose tissue stromovascular fraction, differentiated to mature adipocytes, and maintained in culture for 10–14 days, also as previously described (4, 6). Adipose tissue explants and culture were prepared as described (4, 6). Mouse macrophages were collected after peritoneal cavity lavage with cold PBS. Macrophages were washed and cultured in DMEM and 10% FBS for 10–14 days as previously described (34).
For adipose tissue transplantation experiments, adipose tissue harvested from 10 week-old apoE2 isoform mice was transplanted into 14 week-old apoE2 or apoE3 isoform recipient mice (n = 5 for each recipient isoform type) as previously described in detail (5). After 8 weeks, transplanted adipose tissue was harvested. Adipocyte surface area in transplanted adipose tissue was measured using ImageScope (Aperio Technologies; Vista, CA) in paraffin-embedded adipose tissue sections stained with hematoxylin-eosin. For each transplanted adipose tissue genotype, a minimum of 800 cells were evaluated.
Quantitation of apoE protein and mRNA
ApoE protein and mRNA levels in adipocytes were analyzed as described previously (4, 6). Briefly, adipocyte lysates were separated by electrophoresis, transferred to nitrocellulose membranes, and incubated with goat anti-human apoE antiserum. ApoE signals were quantified by Image Quant TL v2005 software (GE Healthcare; Piscataway, NJ). Flotillin-2 was used for loading normalization.
Total RNA was extracted from adipocytes, macrophages, or livers using the Qiagen RNeasy kit (Valencia, CA). First-strand cDNA was synthesized according to the manufacturer's instructions (Fermantas; Hanover, MD). Real-time PCR was performed in triplicate using Brilliant SYBR Green QRT-PCR Master Mix II on Stratagene MX 3000P (Agilent Technologies; La Jolla, CA). Primers have been previously described (4, 6). PCR conditions were 95°C for 1 min (1 cycle), followed by 94°C for 30 s, 61°C for 30 s, and 72°C for 30 s (40 cycles). Data were analyzed using comparative critical threshold (Ct), normalized to β-actin, and were calculated by 2–ΔΔCt.
Analysis of adipocyte apoE synthesis and turnover
Cultured adipocytes were pulse-labeled with 200 μCi/ml 35[S]methionine in DMEM (methionine-free) for 45 min, followed by 60 min chase in DMEM containing 500 μM unlabeled methionine. Biosynthetically labeled apoE in the cell and medium was measured as previously described (34, 35). Briefly, cell lysates and culture medium were immunoprecipitated with a goat anti-human apoE antibody. Immune complexes were then pulled down with recombinant protein G agarose beads. ApoE was eluted from the beads by boiling in sample buffer, followed by electrophoresis on 10% SDS-PAGE gel. Labeled apoE was quantitated using Image Quant TL v2005 software (Amersham Biosciences; Piscataway, NJ). ApoE synthesis was measured as the level of biosynthetically labeled apoE present in cell lysates after a 45 min pulse and no chase incubation. Secreted apoE was measured as labeled apoE in the medium after a 60 min chase incubation. Retained apoE was measured as labeled apoE in cell lysates after a 60 min chase incubation. ApoE degradation was calculated by subtracting secreted and retained apoE (at 60 min chase) from total synthesized cellular apoE present at the start of the chase incubation (34, 35).
VLDL isolation
Human VLDL was isolated from plasma as previously described (4, 6). The apoE genotype for plasma donors was determined by PCR as described (36, 37). The VLDL preparations used in this series of experiments were obtained from E3/E3 donors.
Adipocyte TG synthesis and hydrolysis
To measure adipocyte TG synthesis, adipocytes were incubated with 0.5 μCi/ml 14[C]glucose with or without 100 μg/ml VLDL in DMEM (5 mM glucose) and 0.1% BSA for 6 h. Cells were washed, and lipids were extracted. TG was separated by TLC in a solvent system of hexane-ethyl ether-acetic acid (90:30:1) as previously described (6). TG spots were visualized and harvested, and the radioactivity of the spots was measured in a scintillation counter. TG hydrolysis was estimated by measuring the release of glycerol into incubation medium over 2 h (6).
Adipocyte FA uptake
FA uptake by adipocytes was measured using the QBT FA uptake assay kit according to the manufacturer's instructions (6). The kit uses a biodipy-dodecanoic FA fluorescent analog that remains quenched until taken up by cells. Mature adipocytes were incubated with 400 μM oleic acid (FA:BSA molar ratio = 2:1) along with QBT FA uptake solution. Readings of FA uptake were recorded over a period of 15 min using a Synergy HT multimode plate reader (BioTek; Winooski, VT).
Lipid, protein, and DNA estimations
TG levels were quantitated using a kit from Wako Chemicals. DNA was isolated with Qiagen DNeasy kit according to the manufacturer's instructions. The amount of DNA was measured by a PicoGreen DNA assay kit (Invitrogen; Carlsbad, CA). Cell protein was measured using a detergent compatible (DC) protein assay kit (Bio-Rad; Hercules, CA).
Statistical analysis
The results are expressed as mean ± SD of triplicate determinations for each group unless indicated in the figure legends. Results presented are representative of two or three experiments. Statistical differences were analyzed by Student t-test or ANOVA using SPSS 17.0 (Chicago, IL) or Microsoft Excel. P < 0.05 was considered significant.
RESULTS
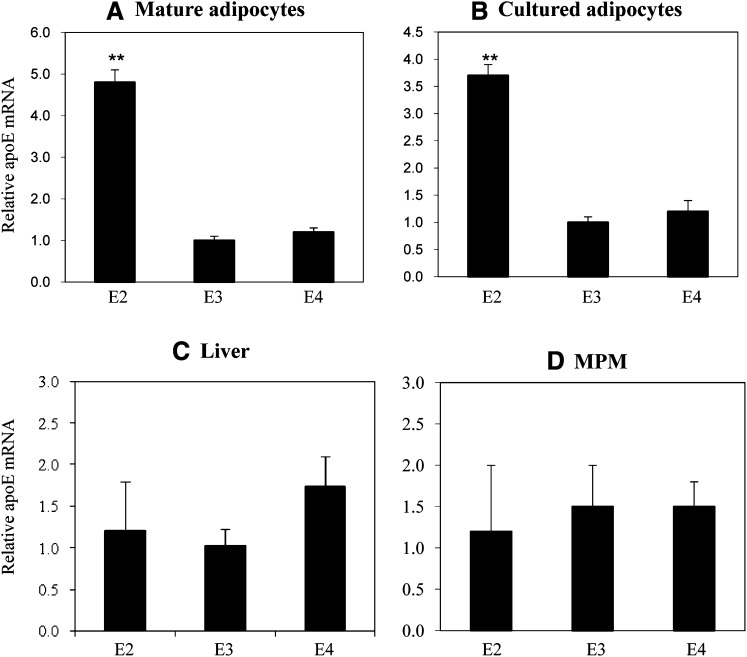
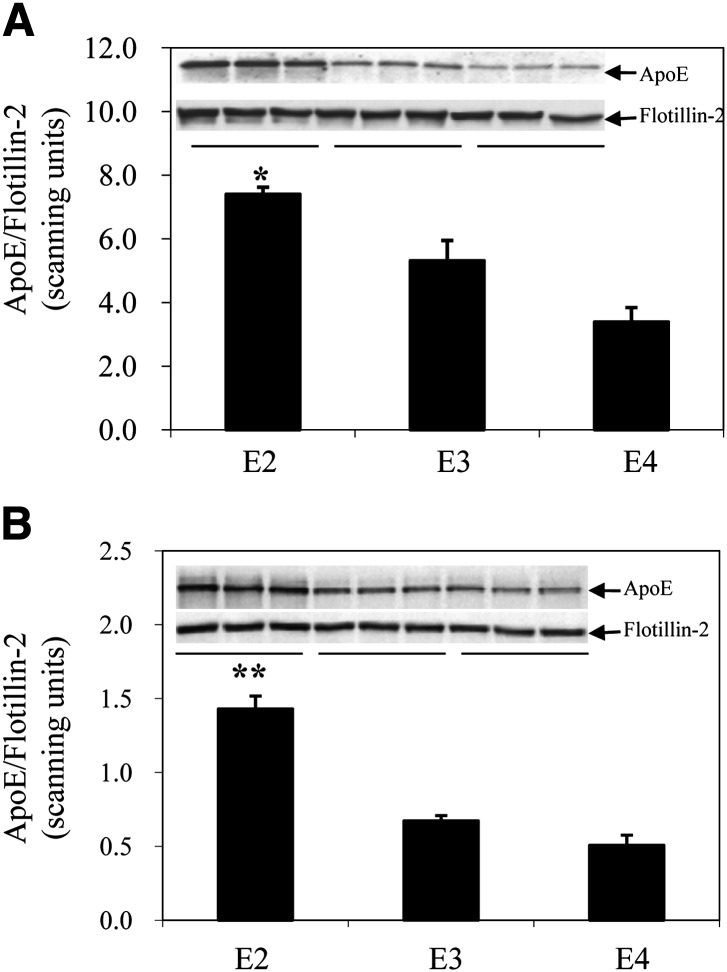
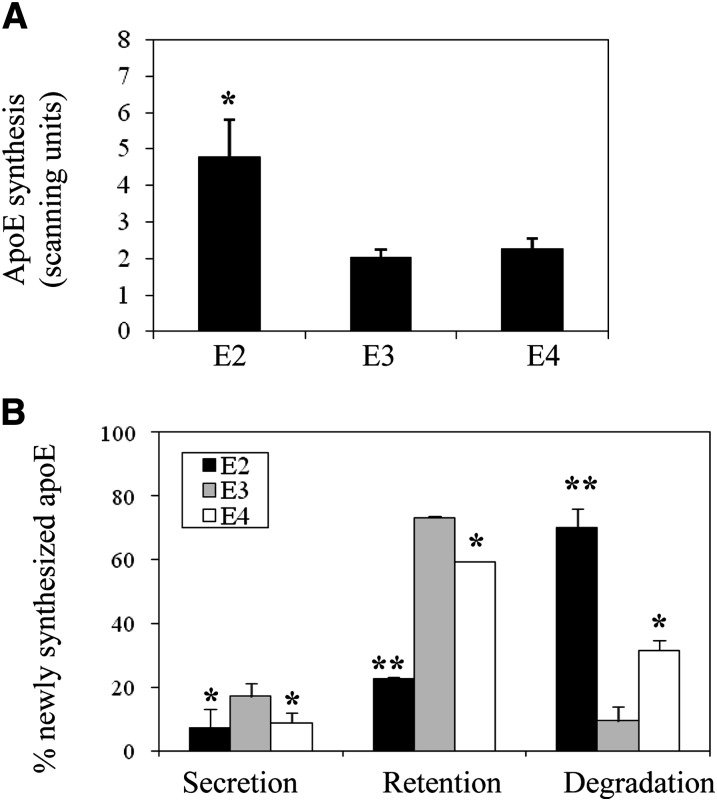
Regulation of apoE genes in these knock-in mice remains under the control of endogenous apoE gene regulatory elements (29–33). We, therefore, first evaluated level of gene expression, apoE synthesis, and cellular apoE turnover in adipocytes isolated from apoE2, -E3, and -E4 knock-in mice. Figure 1 shows apoE mRNA levels in freshly isolated mature adipocytes, cultured adipocytes, liver, and mouse peritoneal macrophages harvested from the apoE isoform mice. ApoE2 mRNA levels are substantially elevated in fresh and cultured adipocytes compared with apoE3 and apoE4 isoforms. There is no such elevation observed in liver or macrophages. Figure 2 shows results of a Western blot analysis of apoE protein levels in freshly isolated mature and cultured adipocytes. ApoE protein levels are significantly elevated in adipocytes expressing the apoE2 isoform, compared with adipocytes expressing the E3 and E4 isoforms. The large increment in apoE mRNA levels, compared with a smaller, but still significant increase in protein levels for apoE2, suggested differences in posttranslational processing of the apoE2 isoform. This was examined more carefully using pulse-chase analysis in cultured adipocytes. First, the synthesis of the apoE isoforms was evaluated by pulse-labeling cells for 45 min and evaluating the amount of labeled apoE present in cell lysates. As shown in Fig. 3A, the synthetic rate of apoE2 was elevated approximately 2.5-fold compared with apoE3 and apoE4. Using a 45 min pulse-labeling followed by a 60 min chase, we directly measured apoE secretion and cellular retention, and calculated degradation rate for newly synthesized apoE as described in Materials and Methods. Figure 3B shows that secretion of both apoE2 and apoE4 were significantly lower than that measured for the apoE3 isoform. ApoE2 retention in the cell was also significantly lower than that of both apoE3 and apoE4. The largest difference between the isoforms was for degradation rate of newly synthesized apoE2, which was substantially elevated, compared with degradation rates observed for both apoE3 and apoE4. These results indicate that newly synthesized apoE2 in adipocytes is highly unstable and undergoes rapid degradation prior to its secretion. The reduction in apoE4 secretion is related to smaller changes in both retention and degradation, compared with apoE3.
Fig. 1.
ApoE mRNA levels in adipocytes, macrophages, and liver of apoE isoform knock-in mice. RNA was isolated from (A) freshly isolated mature adipocytes, (B) adipocytes differentiated from preadipocytes and maintained in culture, (C) liver, or (D) peritoneal macrophages of apoE knock-in mice. ApoE mRNA levels were analyzed by quantitative RT-PCR as described in Materials and Methods. The results are from five mice per group, and each sample was analyzed in triplicate. **P < 0.01 compared with apoE3.
Fig. 2.
ApoE protein levels in freshly isolated mature and cultured adipocytes from apoE isoform knock-in mice. A: Freshly isolated mature adipocytes isolated from the perigonadal fat pad or (B) adipocytes cultured from preadipocytes isolated from the same depot and maintained in culture were utilized for Western blot for apoE as described in Materials and Methods. *P < 0.05, **P < 0.01 compared with apoE3.
Fig. 3.
ApoE synthesis and turnover in adipocytes from apoE isoform knock-in mice. A: Cultured adipocytes were pulse-labeled with 200 μCi/ml 35[S]methionine for 45 min and immediately harvested to measure incorporation of label into newly synthesized cellular apoE. B: Cultured adipocytes were pulse-labeled as described above followed by a 60 min chase incubation in DMEM supplemented with 500 μM unlabeled methionine. The amount of apoE secreted and retained in cells was directly measured, and the amount degraded was calculated as described in Materials and Methods. *P < 0.05, **P < 0.01 for comparison to apoE3.
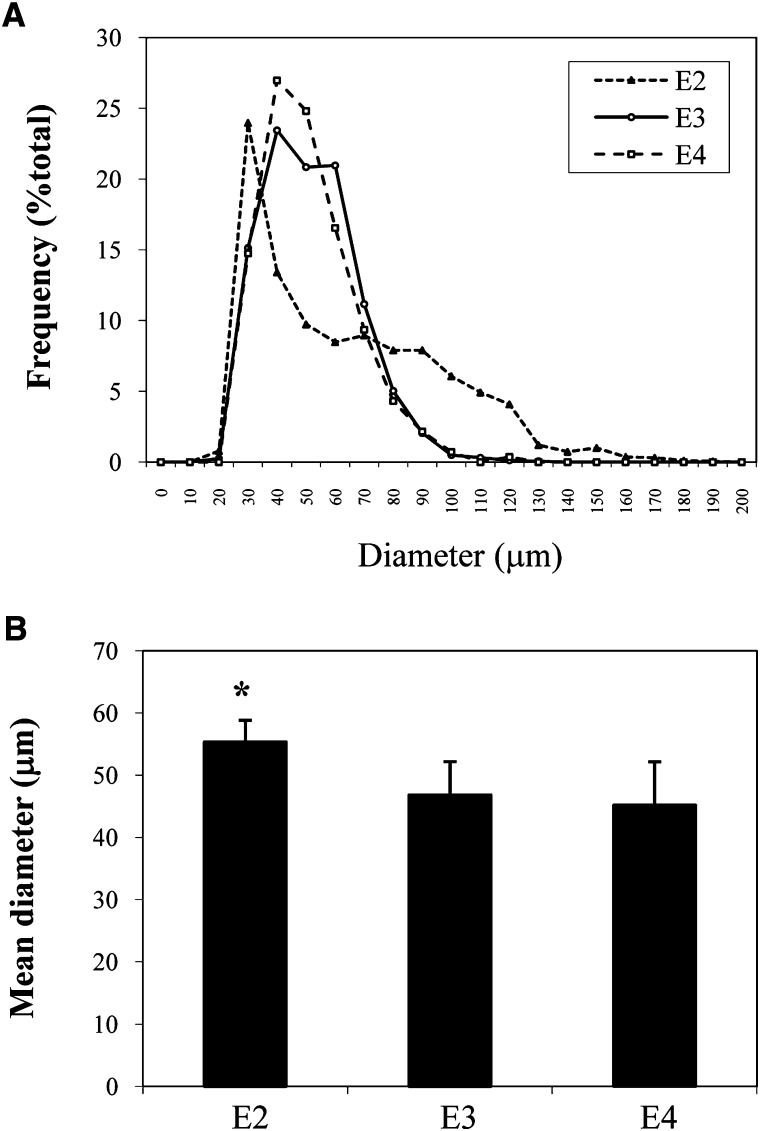
We next evaluated the impact of apoE isoform expression on adipocyte TG metabolism. Table 1 presents results for plasma lipid levels, body weight, and gonadal fat pad weight in the apoE isoform knock-in mice. The plasma total cholesterol and TG were significantly higher in apoE2 mice compared with apoE3 and apoE4 mice. There was no difference in circulating FFA level. It has already been reported that circulating apoE levels in apoE2 mice are significantly elevated (up to 16-fold) compared with apoE3 mice (31). Total body weight was not different among the three types of mice. However, gonadal fat pad weight was significantly higher in the apoE2 isoform mice. Figure 4 further explores the significance of this change in gonadal fat pad weight by presenting size distribution for mature adipocytes isolated from the gonadal fat pad of apoE2, apoE3, and apoE4 isoform mice. As shown in Fig. 4A, compared with apoE3 and apoE4 mice, apoE2 mice demonstrated altered distribution in overall adipocyte diameter with a shift to larger size adipocytes; this shift was reflected in a higher mean adipocyte diameter (Fig. 4B) compared with apoE3 and apoE4 mice.
TABLE 1.
Plasma lipids, body weight, and perigonadal fat mass in apoE knock-in mice
| TC | TG | FFA | Body Weight | Fat Pad | |
| mg/dl | mg/dl | mmol/dl | g | g | |
| E2 | 162 ± 24a | 117 ± 36a | 67 ± 16 | 19.9 ± 2.6 | 0.23 ± 0.08a |
| E3 | 30 ± 12 | 70 ± 20 | 65 ± 12 | 20.2 ± 1.1 | 0.13 ± 0.06 |
| E4 | 24 ± 10 | 51 ± 16 | 78 ± 26 | 19.3 ± 2.6 | 0.14 ± 0.05 |
P < 0.05 versus apoE3. TC, total cholesterol.
Fig. 4.
Adipocyte size in apoE isoform knock-in mice. Mature adipocytes were freshly isolated from the perigonadal fat pad of five apoE isoform mice of each genotype as described in Materials and Methods. A: Adipocyte diameter was measured as described in Materials and Methods. B: Mean adipocyte diameter. *P < 0.05 compared with apoE3
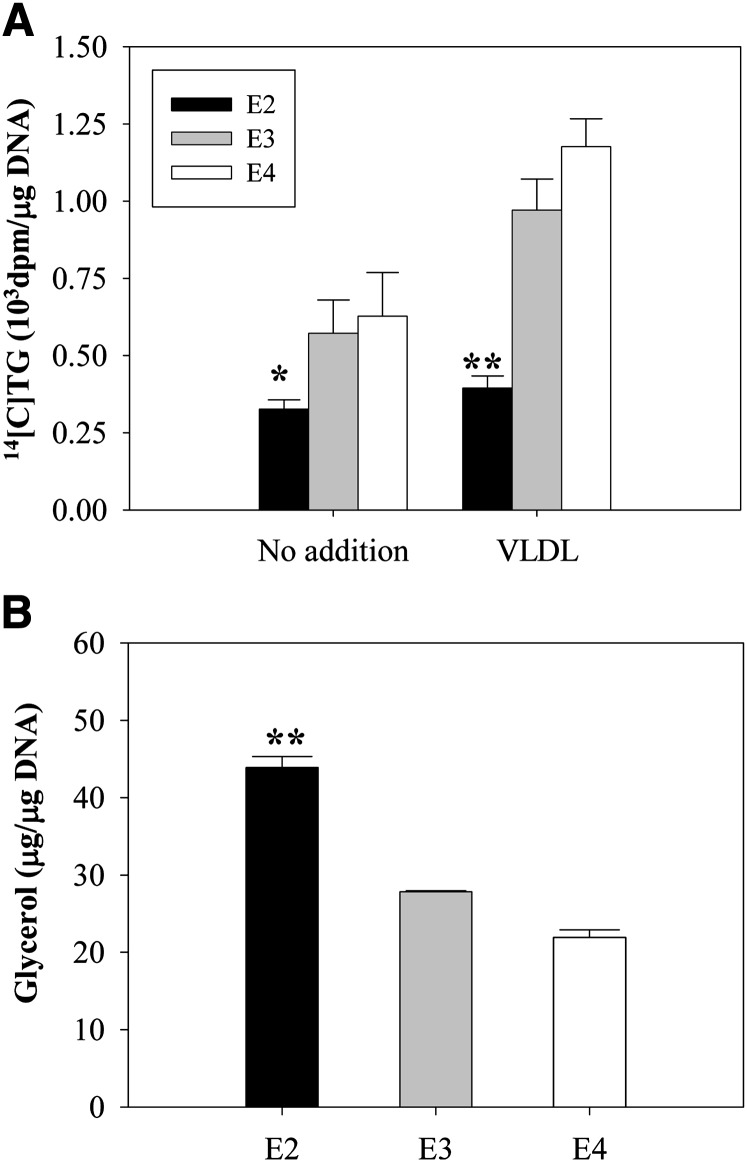
Changes in adipocyte size and overall body fat mass in apoE2 mice could result from increased circulating lipid and apoE levels, noted in Table 1. However, a role for endogenous adipocyte apoE in regulating adipocyte size and overall body fat mass, independent of circulating lipid levels, has been established (4–6). We, therefore, directly examined the impact of apoE isoform expression on adipocyte lipid metabolism in adipocytes isolated from apoE isoform mice. We have previously shown that in apoE knockout (EKO) adipocytes, TG synthesis is lower in both the presence and the absence of extracellular VLDL, and that TG hydrolysis is higher (6). Figure 5A shows that adipocytes expressing the apoE2 isoform synthesized significantly less TG in both the absence and the presence of extracellular apoE3/E3 VLDL compared with apoE3 adipocytes. Figure 5B shows that TG hydrolysis, as measured by release of glycerol to the extracellular medium over 2 h, was elevated in adipocytes expressing apoE2, compared with those expressing apoE3. Therefore, despite significantly expanded apoE synthesis and increased cellular levels of the apoE2 isoform, adipocytes expressing this isoform demonstrate TG turnover characteristics similar to those observed in EKO adipocytes, and as previously observed in EKO adipocytes, the defect in TG synthesis is maintained despite the provision of extracellular apoE3 in VLDL particles.
Fig. 5.
TG turnover in apoE isoform knock-in adipocytes. A: TG synthesis was measured in apoE2, -E3, and -E4 freshly isolated mature adipocytes in the absence or presence of 100 μg/ml human VLDL isolated from an apoE3/E3 donor, as described in Materials and Methods. B: TG hydrolysis was measured in freshly isolated mature adipocytes isolated from apoE isoform mice as described in Materials and Methods. *P < 0.05, **P < 0.01 for comparison to E3 under the same incubation conditions.
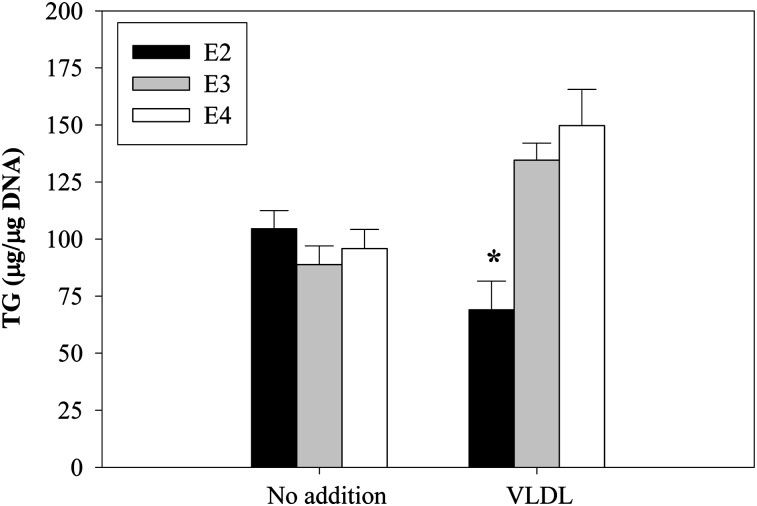
To further confirm differences in VLDL-mediated lipogenesis in the context of intact adipose tissue, we next examined the impact of incubating perigonadal fat isolated from apoE2, apoE3, and apoE4 mice with apoE3/E3 VLDL on TG mass. After 24 h incubation in the absence of VLDL, there was no significant difference in adipose tissue TG mass comparing the apoE isoform adipose tissue types, although this level tended to be somewhat higher in E2 compared with E3 and E4 adipose tissue (Fig. 6). A 24 h incubation in VLDL, however, significantly increases TG mass in E3 and E4 adipose tissue. TG mass does not increase, and appears to decrease, in E2 adipose tissue incubated with VLDL. This result in intact adipose tissue is consistent with results in isolated cells showing that E2 adipocytes manifest impaired lipogenesis in response to VLDL compared with E3 and E4 adipocytes.
Fig. 6.
Effect of incubation with VLDL on TG mass in adipose tissue explants from apoE isoform knock-in mice. ApoE2, -E3, or -E4 perigonadal adipose tissue explants were prepared and incubated with human VLDL (100 μg/ml) harvested from an apoE3/E3 donor in 0.2% BSA for 24 h. After incubation, TG mass was measured. *P < 0.05 for the change in TG mass due to inclusion of VLDL.
The defect in lipogenesis in apoE2-expressing adipocytes would predict the presence of smaller adipocytes. However, freshly isolated mature adipocytes from E2 mice are significantly larger (by about 15%) compared with adipocytes harvested from E3 or E4 isoform mice (Fig. 4). The results shown in Figs. 5 and 6 implicate the chronic hyperlipidemia resulting from systemic expression of apoE2 as the driver of larger adipocyte size in apoE2 isoform mice. To more directly evaluate this question, we transplanted adipose tissue from hyperlipidemic apoE2 isoform mice into normolipidemic apoE3 isoform mice. For a control, we also transplanted apoE2 adipose tissue into apoE2 recipient mice. After 8 weeks in the normolipidemic E3 in vivo milieu, the surface area of the transplanted E2 adipocytes decreased by 16% (P < 0.02) compared with E2 adipocytes transplanted into hyperlipidemic E2 recipients (not shown). This result supports the importance of chronic hyperlipidemia for driving larger adipocyte size in E2 isoform mice.
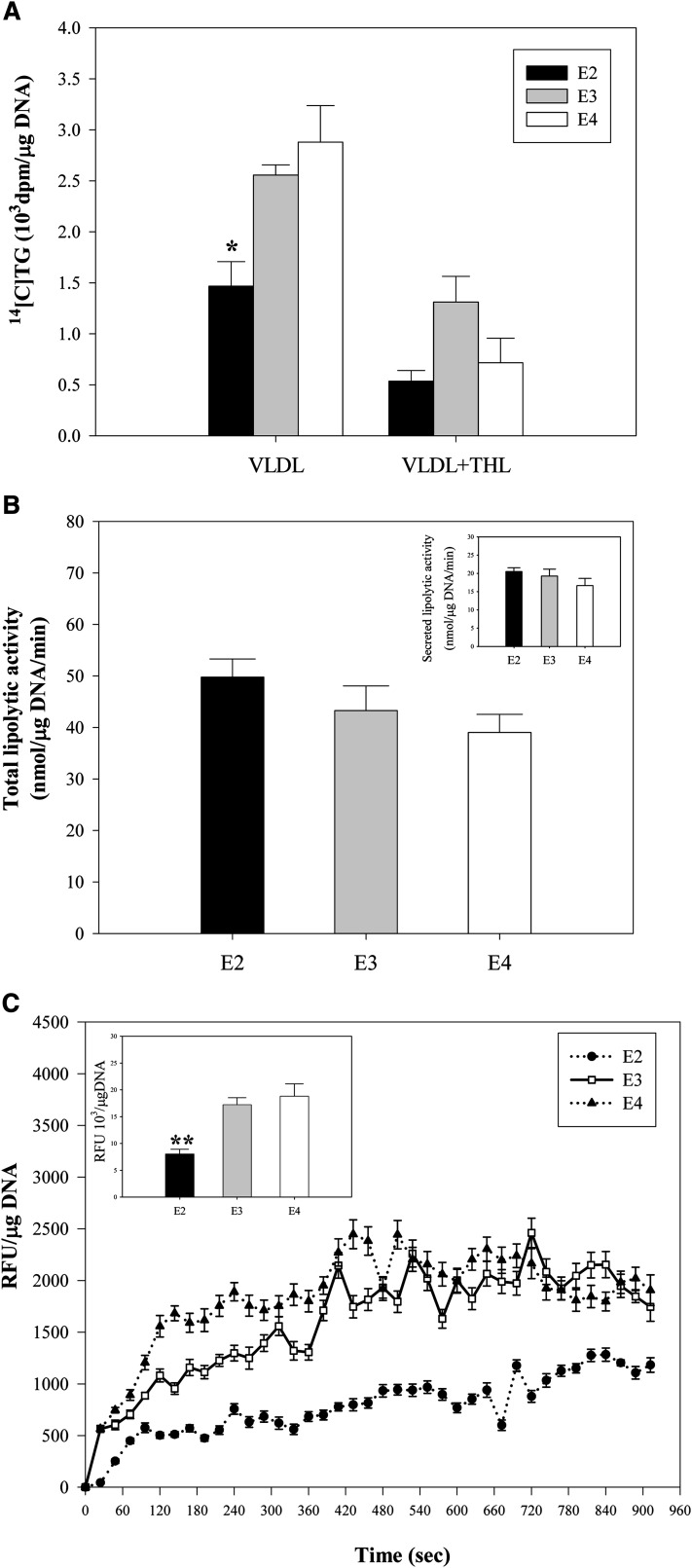
In EKO adipocytes, it has been demonstrated that the defect in TG synthesis is related to impaired uptake of the FFA released by LPL-mediated hydrolysis of TG present in extracellular VLDL particles (6). We analyzed this mechanism for the defect in TG synthesis observed in E2 cells. Figure 7A examines the importance of LPL-mediated hydrolysis of VLDL for producing the difference in TG synthesis between apoE isoform adipocytes by utilizing the LPL inhibitor, tetrahydrolipstatin (THL). In the presence of VLDL, but the absence of THL, TG synthesis is significantly lower in the apoE2 isoform adipocytes (consistent with results shown in Fig. 5A). In the presence of THL, TG synthesis is substantially suppressed in each of the apoE isoform adipocytes, and there are no longer significant differences between them, suggesting that LPL-mediated hydrolysis of VLDL contributed to the difference in TG synthesis observed between apoE2 and apoE3 adipocytes. To determine whether this result could be explained by differences in LPL expression, we directly measured total and secreted LPL activity in the apoE isoform adipocytes. Figure 7B shows that total lipolytic activity, as well as secreted lipolytic activity, was identical in the apoE2, apoE3, and apoE4 adipocytes. Therefore, differences in LPL expression cannot account for the result shown in Fig. 7A. The adipocyte lipogenesis that results from LPL hydrolysis of extracellular VLDL depends on adipocyte internalization of the released FFA. We, therefore, next measured the internalization of FFA directly. FA internalization was not different between apoE3 and apoE4 adipocytes; however, it was markedly reduced in apoE2 adipocytes (Fig. 7C). This result indicates that a defect in the internalization of FAs released by LPL hydrolysis of VLDL contributes to the impaired lipogenesis in apoE2 adipocytes incubated with VLDL. This defect is identical to that observed in EKO adipocytes (6).
Fig. 7.
LPL activity and FA internalization in apoE isoform knock-in adipocytes. A: Mature adipocytes were freshly isolated from perigonadal fat pads of apoE isoform mice, and TG synthesis was measured over 6 h during incubation with 100 μg/ml VLDL isolated from an apoE3/E3 donor with or without the LPL inhibitor THL. B: Total LPL activity was measured from mature apoE isoform knock-in adipocytes. The insert shows secreted LPL activity. C: FA uptake was measured in freshly isolated mature apoE isoform adipocytes as described in Materials and Methods. A representative measurement of uptake from triplicate samples is shown as a function of time. The inset shows total FA uptake mean ± SD over the monitoring period from two separate experiments, each performed in triplicate using freshly isolated mature adipocytes pooled from five mice. *P < 0.05, **P < 0.01 for comparison to E3 adipocytes under the same incubation conditions.
DISCUSSION
Recently, an important role has been established for endogenous adipocyte apoE expression, separate from circulating (using in vivo experimental systems) and extracellular apoE (using in vitro experimental systems), for regulating adipocyte substrate metabolism (4–7). There are three common apoE isoforms in human populations, with apoE3 being the most common. Human expression of the apoE2 and E4 isoforms has been associated with important human diseases (15–17). Isoform-specific effects on cellular-differentiated function have been established for neurons and macrophages (20–26), and there are isoform-specific differences in the posttranslational processing of apoE.
In the current set of studies, we establish that the expression of the apoE2 isoform under the control of its endogenous gene control elements has a significant impact on adipocyte apoE synthesis and degradation. In vivo, we demonstrate that systemic expression of the apoE2 isoform leads to a perturbation in adipocyte size distribution and a small but significant increase in mean diameter of freshly isolated mature adipocytes. This result could have been related to the disordered systemic lipoprotein metabolism and hyperlipidemia associated with systemic expression of the apoE2 isoform. Alternatively, this result could have been related to an adipocyte-specific effect of endogenous apoE2 expression, based on previously reported observations establishing a role for endogenous adipocyte apoE in supporting adipocyte substrate acquisition and lipogenesis. The results in this manuscript establish that the adipocyte-specific effects of the endogenous expression of the apoE2 isoform cannot account for the increased adipocyte size or lipid content in the intact E2-expressing mouse; and adipocyte-specific effects of expression of this isoform would actually predict the presence of smaller adipocytes and less adipose tissue. Expression of the apoE2 isoform in adipocytes is associated with defective lipogenesis, compared with expression of the E3 isoform, and it is important to emphasize that this decreased lipogenesis cannot be ascribed to reduced apoE2 secretion with subsequent lack of extracellular apoE, inasmuch as it remains decreased even in the presence of apoE-containing VLDL. Further, the transplantation of adipose tissue from hyperlipidemic apoE2 mice into normolipidemic apoE3 mice leads to a significant decrease in adipocyte size. Therefore, in apoE2 mice, the presence of larger adipocytes and increased adipose tissue probably results from the disordered lipoprotein metabolism accompanying systemic apoE2 expression, with attendant hyperlipidemia and increased availability of circulating lipogenic substrate.
The defect in adipocyte apoE2 secretion and the instability of the newly synthesized apoE2 isoform have also been reported in macrophages (21). We were surprised, however, by the increase in apoE gene expression and synthesis that accompanied expression of the apoE2 gene under control of its endogenous control elements. A number of physiologically relevant stimuli and regulatory pathways have been established for regulation of adipocyte apoE gene expression (8–14). These include pathways that impact on functional LXR, PPARγ, and NFκB response elements identified within apoE gene-regulatory sequences. We speculate that the large increase in apoE2 gene expression may relate to altered adipocyte lipid metabolism in E2 adipocytes, with defective generation of a bioactive lipid that functions in a regulatory feedback loop at one of these regulatory sequences. This conceptual construct will require additional investigation.
It is also of interest that a major contributor to defective VLDL-mediated lipogenesis in adipocytes expressing the apoE2 isoform is ineffective internalization of FA substrate. This is the precise defect identified in the ineffective lipogenesis measured in EKO adipocytes, and in these cells appears to be related to alterations in caveolin function (4, 7). By comparing EKO and wild-type adipocytes, we have previously established that endogenous expression of adipocyte apoE has an important impact on adipocyte caveolin expression and caveolar number (7). Further, endogenously synthesized adipocyte apoE can be colocalized with caveolin at the adipocyte cell surface (7). Alterations in secondary structure and stability of the apoE2 isoform may lead to a defect in establishing these associations with caveolin. The experimental examination of this question by comparing E2, E3, and E4 isoform adipocytes, however, is importantly confounded by the significant expansion of adipocyte apoE2 level.
In summary, in this manuscript, we establish that abnormalities in adipose tissue mass and adipocyte size that accompany the systemic expression of the apoE2 isoform cannot be ascribed to adipocyte-specific effects of this isoform on adipocyte lipid metabolism, and most likely result from long-term exposure to elevated circulating TG and apoE levels. Importantly, we also establish that gene expression and posttranslational handling of the apoE2 isoform are significantly different from those of the apoE3 and apoE4 isoforms. Expression of the apoE2 isoform is also associated with significant changes in adipocyte TG synthesis, TG hydrolysis, and FA internalization. Interpretation of human studies describing metabolic changes in apoE2, apoE3, and apoE4 subjects will need to account for adipocyte- and adipose tissue-specific effects of the apoE isoforms when considering pathophysiological pathways accounting for these changes.
Acknowledgments
The authors thank Stephanie Thompson for assistance with manuscript preparation.
Footnotes
Abbreviations:
- EKO
- apoE
- knockout
- TG
- triglyceride
- THL
- tetrahydrolipstatin
This work was supported by Grant DK-71711 (T.M.) and Grant HL-42630 (N.M.) from the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Ford E. S., Mokdad A. H. 2008. Epidemiology of obesity in the Western Hemisphere. J. Clin. Endocrinol. Metab. 93: S1–S8. [DOI] [PubMed] [Google Scholar]
- 2.Despres J. P., Lemieux I., Bergeron J., Pibarot P., Mathieu P., Larose E., Rodes-Cabau J., Bertrand O. F., Poirier P. 2008. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 28: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 3.Canoy D., Boekholdt S. M., Wareham N., Luben R., Welch A., Bingham S., Buchan I., Day N., Khaw K. T. 2007. Body fat distribution and risk of coronary heart disease in men and women in the European Prospective Investigation Into Cancer and Nutrition in Norfolk cohort: a population-based prospective study. Circulation. 116: 2933–2943. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z. H., Reardon C. A., Mazzone T. 2006. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 55: 3394–3402. [DOI] [PubMed] [Google Scholar]
- 5.Huang Z. H., Gu D., Mazzone T. 2009. Role of adipocyte-derived apoE for modulating adipocyte size, lipid metabolism, and gene expression in vivo. Am. J. Physiol. Endocrinol. Metab. 296: E1110–E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z. H., Minshall R. D., Mazzone T. 2009. Mechanism for endogenously expressed APOE modulation of adipocyte VLDL metabolism: role in endocytic and lipase-mediated metabolic pathways. J. Biol. Chem. 284: 31512–31522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue L., Mazzone T. 2011. Endogenous adipocyte apolipoprotein E is colocalized with caveolin at the adipocyte plasma membrane. J. Lipid Res. 52: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yue L., Rassouli N., Ranganathan G., Kern P. A., Mazzone T. 2004. Divergent effects of PPARγ agonists and TNFα on adipocyte apoE expression. J. Biol. Chem. 279: 47626–47632. [DOI] [PubMed] [Google Scholar]
- 9.Huang Z. H., Luque R. M., Kineman R. D., Mazzone T. 2007. Nutritional regulation of adipose tissue apolipoprotein E expression. Am. J. Physiol. Endocrinol. Metab. 293: E203–E209. [DOI] [PubMed] [Google Scholar]
- 10.Rao P., Huang Z. H., Mazzone T. 2007. Angiotensin II regulates adipocyte apolipoprotein E expression. J. Clin. Endocrinol. Metab. 92: 4366–4372. [DOI] [PubMed] [Google Scholar]
- 11.Yue L., Christman J. W., Mazzone T. 2008. Tumor necrosis factor-alpha-mediated suppression of adipocyte apolipoprotein E gene transcription: primary role for the nuclear factor (NF)-kappaB pathway and NFkappaB p50. Endocrinology. 149: 4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espiritu D. J., Mazzone T. 2008. Oxidative stress regulates adipocyte apolipoprotein E and suppresses its expression in obesity. Diabetes. 57: 2992–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yue L., Mazzone T. 2009. Peroxisome proliferator-activated receptor {gamma} stimulation of adipocyte ApoE gene transcription mediated by the liver receptor X pathway. J. Biol. Chem. 284: 10453–10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espiritu D. J., Huang Z. H., Zhao Y., Mazzone T. 2010. Hyperglycemia and advanced glycosylation end products suppress adipocyte apoE expression: implications for adipocyte triglyceride metabolism. Am. J. Physiol. Endocrinol. Metab. 299: E615–E623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y. 2010. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr. Opin. Lipidol. 21: 337–345. [DOI] [PubMed] [Google Scholar]
- 16.Sima A., Iordan A., Stancu C. 2007. Apolipoprotein E polymorphism–a risk factor for metabolic syndrome. Clin. Chem. Lab. Med. 45: 1149–1153. [DOI] [PubMed] [Google Scholar]
- 17.Araki S., Moczulski D. K., Hanna L., Scott L. J., Warram J. H., Krolewski A. S. 2000. APOE polymorphisms and the development of diabetic nephropathy in type 1 diabetes: results of case-control and family-based studies. Diabetes. 49: 2190–2195. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan S. R., Ehnholm C., Elkasabany A., Berenson G. S. 2001. Apolipoprotein E polymorphism modulates the association between obesity and dyslipidemias during young adulthood: the Bogalusa Heart Study. Metabolism. 50: 696–702. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho-Wells A. L., Jackson K. G., Gill R., Olano-Martin E., Lovegrove J. A., Williams C. M., Minihane A. M. 2010. Interactions between age and apoE genotype on fasting and postprandial triglycerides levels. Atherosclerosis. 212: 481–487. [DOI] [PubMed] [Google Scholar]
- 20.Hara M., Matsushima T., Satoh H., Iso-o N., Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. 2003. Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23: 269–274. [DOI] [PubMed] [Google Scholar]
- 21.Fan D., Qiu S., Overton C. D., Yancey P. G., Swift L. L., Jerome W. G., Linton M. F., Fazio S. 2007. Impaired secretion of apolipoprotein E2 from macrophages. J. Biol. Chem. 282: 13746–13753. [DOI] [PubMed] [Google Scholar]
- 22.Cullen P., Cignarella A., Brennhausen B., Mohr S., Assmann G., von Eckardstein A. 1998. Phenotype-dependent differences in apolipoprotein E metabolism and in cholesterol homeostasis in human monocyte-derived macrophages. J. Clin. Invest. 101: 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida H., Hasty A. H., Major A. S., Ishiguro H., Su Y. R., Gleaves L. A., Babaev V. R., Linton M. F., Fazio S. 2001. Isoform-specific effects of apolipoprotein E on atherogenesis: gene transduction studies in mice. Circulation. 104: 2820–2825. [DOI] [PubMed] [Google Scholar]
- 24.Gong J. S., Kobayashi M., Hayashi H., Zou K., Sawamura N., Fujita S. C., Yanagisawa K., Michikawa M. 2002. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J. Biol. Chem. 277: 29919–29926. [DOI] [PubMed] [Google Scholar]
- 25.Ji Z. S., Miranda R. D., Newhouse Y. M., Weisgraber K. H., Huang Y., Mahley R. W. 2002. Apolipoprotein E4 potentiates amyloid beta peptide-induced lysosomal leakage and apoptosis in neuronal cells. J. Biol. Chem. 277: 21821–21828. [DOI] [PubMed] [Google Scholar]
- 26.Zhong N., Ramaswamy G., Weisgraber K. H. 2009. Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function. J. Biol. Chem. 284: 27273–27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Maeda N. 2008. Differential modulation of diet-induced obesity and adipocyte functionality by human apolipoprotein E3 and E4 in mice. Int. J. Obes. (Lond). 32: 1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arbones-Mainar J. M., Johnson L. A., Altenburg M. K., Kim H. S., Maeda N. 2010. Impaired adipogenic response to thiazolidinediones in mice expressing human apolipoproteinE4. FASEB J. 24: 3809–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malloy S. I., Altenburg M. K., Knouff C., Lanningham-Foster L., Parks J. S., Maeda N. 2004. Harmful effects of increased LDLR expression in mice with human APOE*4 but not APOE*3. Arterioscler. Thromb. Vasc. Biol. 24: 91–97. [DOI] [PubMed] [Google Scholar]
- 30.Knouff C., Hinsdale M. E., Mezdour H., Altenburg M. K., Watanabe M., Quarfordt S. H., Sullivan P. M., Maeda N. 1999. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Invest. 103: 1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan P. M., Mezdour H., Quarfordt S. H., Maeda N. 1998. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J. Clin. Invest. 102: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan P. M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R. L., Quarfordt S. H., Maeda N. 1997. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272: 17972–17980. [DOI] [PubMed] [Google Scholar]
- 33.Knouff C., Briand O., Lestavel S., Clavey V., Altenburg M., Maeda N. 2004. Defective VLDL metabolism and severe atherosclerosis in mice expressing human apolipoprotein E isoforms but lacking the LDL receptor. Biochim. Biophys. Acta. 1684: 8–17. [DOI] [PubMed] [Google Scholar]
- 34.Huang Z. H., Gu D., Mazzone T. 2004. Oleic acid modulates the post-translational glycosylation of macrophage apoE to increase its secretion. J. Biol. Chem. 279: 29195–29201. [DOI] [PubMed] [Google Scholar]
- 35.Mazzone T., Pustelnikas L., Reardon C. A. 1992. Post-translational regulation of macrophage apoprotein E production. J. Biol. Chem. 267: 1081–1087. [PubMed] [Google Scholar]
- 36.Clavel C., Durlach A., Durlach V., Birembaut P. 1995. Rapid and safe determination of human apolipoprotein E genotypes by miniaturised SDS-PAGE in non-insulin dependent diabetes mellitus. J. Clin. Pathol. 48: 295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hixson J. E., Vernier D. T. 1990. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 31: 545–548. [PubMed] [Google Scholar]