Abstract
This study aimed to investigate whether plasma levels of HDL cholesterol (HDL-C) were associated with the risk of intracerebral hemorrhage (ICH). Plasma HDL-C was determined via enzymatic methods, and ICH was ascertained via medical history, physical examination, and brain imaging (computed tomography or magnetic resonance imaging). The multivariable logistic regression model was used to calculate the odds ratios (OR) and 95% confidence intervals (CI) of ICH according to levels of plasma cholesterol. A total of 170 patients with ICH were identified from 6,046 participants. After adjustment for conventional cardiovascular risk factors, the OR was 2.06 (95% CI, 1.25-3.12; P < 0.01) for participants in the first tertile of HDL-C levels (<1.38 mmol/l) and 1.13 (95% CI, 0.72-1.78; P = 0.59) for participants in the second tertile (1.38-1.64 mmol/l), compared with participants in the third tertile (∩≥1.65 mmol/l). Subgroup analysis indicated that the detrimental effects of HDL-C were more significant in men and lean participants than in their corresponding controls, independent of hypertension. The results presented herein indicate that low plasma HDL-C (<1.38 mmol/l) may be associated with risk of ICH.
Keywords: blood lipid, stroke, hypertension
Intracerebral hemorrhage (ICH) is a serious cerebrovascular disease worldwide (1) and has a fatality rate of approximately 35-52% at 30 days. Fully half of these fatalities occur within the first two days (2). The proportion of this subtype of stroke is high in China, accounting for approximately 38-55% of total strokes (3, 4). Given the high mortality rate and the paucity of effective treatments, prevention of ICH is of paramount importance (5). Identification of risk factors for ICH, especially modifiable factors, is the first step in prevention. Several modifiable risk factors, such as hypertension and excessive alcohol consumption, have been demonstrated to increase the risk of ICH (6), but not all patients with ICH have these identifiable risk factors.
Abnormal lipid levels are among the list of candidate risk factors for stroke (7), but their contribution to ICH remains inconclusive. This is especially true for plasma high-density lipoprotein cholesterol (HDL-C), which, along with low-density lipoprotein cholesterol (LDL-C), is a primary component of plasma total cholesterol (TC). A low level of TC has emerged as a risk factor for ICH in several prospective studies (8–10), and an association between LDL-C and ICH has also been reported (11–13). Moreover, HDL-C improves endothelial function (14) and repairs vessel walls (15, 16), suggesting that high levels of HDL-C may be protective against ICH. To date, however, few clinical studies have explored a connection between low HDL-C levels and the risk of ICH (17).
This cross-sectional study investigated whether low HDL-C might contribute to the risk of ICH. An association between plasma HDL-C and ICH would provide a new potential target for the prevention of ICH.
METHODS
Participants
A community-based cross-sectional study was conducted in seven local communities of Xinyang County, Henan Province, China, from March to August 2005. The study protocol was reviewed and approved by the ethics committees of FuWai Hospital and local collaborative hospitals and conducted according to the Declaration of Helsinki. Informed consent was obtained from each participant before enrollment into the study.
Inclusion criteria were as follows: 1) participants resided in a household in one of these seven communities for at least 3 months; 2) participants were ages 40 to 75 years; and 3) participants were free of clinical ischemic stroke as well as coronary heart disease (CHD).
Participants were excluded if they had ischemic stroke, subarachnoid hemorrhage, or CHD. CHD was defined as the ninth International Classification of Disease (ICD-9, 1997), code 410-414; ICH, as ICD-9, code 431; ischemic stroke, as ICD-9, codes 433.0 to 434.9; subarachnoid hemorrhage, as code 430; and unclassified stroke, as code 436. All medical records and neuroimaging data (computed tomography and magnetic resonance imaging) in subjects with a reported history of ischemic stroke, subarachnoid hemorrhage, or ICH were examined by an event committee. Participants with systemic diseases, which were ascertained clinically and recorded in the medical history, were excluded as well. The systemic diseases were defined as severe inflammation (ICD-9, code 995.9); collagen disease (code 710); hepatic cirrhosis (code 571.2, 5, 6); end-stage renal disease (code 585); neoplasm disease in brain (code 191), lung (code 162), liver, colon, and rectum (code 153-5), pancreas (code 157), breast (code 174), bladder and kidney (code 188-9), and blood system (code 200-8); endocrine disease (code 253, 255); metabolic disease (code 272); and hemorrhagic disease (code 286-7).
Two physicians on the committee independently reviewed the data and decided whether the participants met all the inclusion criteria. In cases of disagreement, a third physician was asked to resolve the dispute. Of 170 patients with ICH, 7 patients required arbitration; the data of these 7 patients were included in the analysis because no significant differences were found whether or not their data were included.
Biochemical variable determination
Blood samples were collected after a 12 h overnight fast of all participants, including ICH patients who had a prior history of ICH for a median period of 2 years (range, 1-5 years). All samples were analyzed for plasma TC, HDL-C, LDL -C, triglycerides, and plasma glucose levels by enzymatic methods with an automatic analyzer (Hitachi 7060, Hitachi, Japan). All lipid levels were determined in a Centers for Disease Control and Prevention (CDC)-qualified laboratory in FuWai Hospital.
Clinical data collection
Demographic data and vascular risk factors, including age, gender, weight, height, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded. Blood pressure (BP) data analyzed in this study were obtained from all participants, including ICH patients who had a prior history of ICH for a median period of 2 years.
BP was measured three times in seated patients after a rest period of 5 min using a mercury column sphygmomanometer (18); an average of the last two readings was taken as the analyzed BP level. Medical history, alcohol use, and cigarette use were obtained from all participants using a standardized questionnaire.
Hypertension was defined as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg on two visits, with the interval between the two visits of more than 2 weeks (19). Alternatively, hypertension was defined as a history of hypertension with or without antihypertensive treatment (18). Hypercholesterolemia was defined according to the Adult Treatment Panel III guidelines (20). Family history of stroke was defined as a history of any of the patient's first-degree relatives (father, mother, or brother/sister) having suffered a stroke (fatal or nonfatal strokes).
Statistical analyses
Statistical analyses were based on individuals with ICH divided according to the tertile category of HDL-C levels and other lipid profiles: TC, LDL-C, and triglycerides. The participants were categorized into three groups of approximately equal number based on the distribution of TC, LDL-C, and HDL-C levels. The distribution of triglyceride levels was skewed, and log transformation was used. Continuous variables were compared using the ANOVA (ANOVA) test and category characteristics by the chi-square/Mantel-Haenszel analysis. The Dunnett posthoc test was performed among multiple comparisons after the ANOVA analysis showed significance. Variables with a univariate association with ICH were entered stepwise into a logistic model if their contribution to the model was significant at the α = 0.05 level after mutual adjustment. The binary logistic regression model was applied to adjust for conventional risk factors for ICH and to calculate the odds ratios (OR).
Two logistic models were used to calculate the OR of ICH in the subgroups divided according to plasma lipid profiles. First, age and gender were used as covariate factors for the plasma lipid group for calculation of the adjusted OR. Second, age, gender, and other confounding vascular risk factors, including SBP, BMI, hypercholesterolemia, current smoking, current drinking, and family history of stroke, were adjusted in the logistic regression model. In subgroup analysis stratified by gender, hypertensive status, and BMI, the linear trend across HDL-C tertiles was tested by introducing the median plasma HDL-C concentration of each category as continuous variables within the multivariable models (21). The departure from the linear trend was also evaluated. A two-tailed P value of 0.05 or less was considered significant. Statistical analyses were carried out using SPSS version 13.0 (SPSS Inc., Chicago, IL).
RESULTS
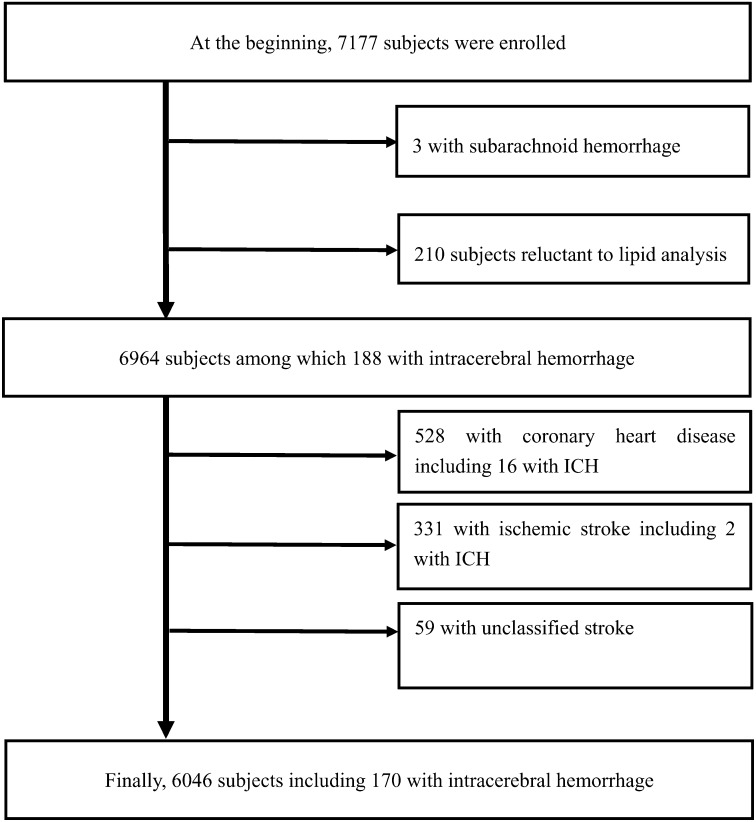
At the beginning of the study, 7,177 participants were enrolled. Of these, 3 participants with subarachnoid hemorrhage and 210 participants without plasma lipid analyses were excluded. After further excluding 528 participants with CHD, 331 with ischemic stroke, and 59 with unclassified stroke, 6,046 participants were available for the study (Fig. 1). Of these, 170 participants were identified as having ICH.
Fig. 1.
Flowchart of subject enrollment and exclusion.
Baseline characteristics
To determine the relationship between plasma HDL-C levels and ICH, participants were categorized into tertile groups based on the distribution of HDL-C levels as follows: Tertile-1 group, <1.38 mmol/l; Tertile-2 group, 1.38-1.64 mmol/l; and Tertile-3 group, ≥1.65 mmol/l (Table 1). Only 8.6% (519/6046) of all participants received lipid-lowering medications; however, 17.1% (29/170) of the ICH patients were on lipid-lowering medications.
TABLE 1.
Baseline characteristics according to HDL-C tertiles
| Variables | HDL-C | ||||
| Tertile-3 | Tertile-2 | Tertile-1 | Pa | Pb | |
| Number of subjects | 2008 | 1996 | 2042 | – | – |
| Men, % (n) | 32.9 (660) | 34.9 (696) | 40.3 (823)d | 0.18 | <0.01 |
| Mean (SD) | – | – | – | – | – |
| Age, years | 58.0 (9.1) | 56.9 (9.8) | 56.2 (10.0) | <0.01 | <0.01 |
| BMI, kg/m | 24.8 (3.6) | 25.9 (3.6) | 26.7 (3.5)d | <0.01 | <0.01 |
| SBP, mm Hg | 157.4 (28.1) | 154.6 (28.6) | 153.6 (29.5) | <0.01 | <0.01 |
| DBP, mm Hg | 94.5 (13.8) | 93.9 (14.2) | 93.5 (14.4) | 0.51 | 0.06 |
| TC, mmol/l | 5.82 (1.11) | 5.42 (1.04) | 4.98 (1.06)d | <0.01 | <0.01 |
| LDL-C, mmol/l | 3.11 (0.94) | 3.11 (0.85) | 2.85 (0.82)d | 1.00 | <0.01 |
| HDL-C, mmol/l | 1.93 (0.25) | 1.52 (0.08) | 1.21 (0.13)d | <0.01 | <0.01 |
| TG, mmol/l | 1.63 (1.33) | 1.52 (0.92) | 2.10 (1.90)d | <0.01 | <0.01 |
| FBG, mmol/l | 5.39 (1.71) | 5.39 (1.73) | 5.46 (1.82) | 0.06 | 0.06 |
| % (n) | – | – | – | – | – |
| Hypertension | 59.3(1191) | 59.2 (1181) | 58.5 (1194) | 0.92 | 0.59 |
| Antihypertensive treatment | 30.3 (608) | 33.1 (660) | 33.7 (688) | 0.06 | 0.02 |
| Diabetes | 2.0 (20) | 3.4 (68) | 4.7 (96)c | <0.01 | <0.01 |
| Hypercholesterolemia | 9.5 (191) | 12.6 (252) | 16.4 (334)d | <0.01 | <0.01 |
| Lipid-lowering drugs | 7.1 (143) | 8.0 (160) | 10.6 (216)d | <0.01 | <0.01 |
| Family history of stroke | 15.2 (305) | 14.6 (292) | 15.8 (322) | 0.62 | 0.63 |
| Current smoker | 10.2 (204) | 10.2 (203) | 14.7 (300)d | 0.69 | <0.01 |
| Current drinker | 14.9 (300) | 13.8 (276) | 15.7 (321)d | 0.38 | 0.02 |
Comparisons were performed between different groups of continuous variables using AVONA; category characteristics were compared using the Mantel-Haenszel chi-square test. TG levels were determined using the log-transformed value. To convert TG millimoles per liter to milligrams per deciliter, values were multiplied by 88.54. To convert cholesterol millimoles per liter to milligrams per deciliter, values were multiplied by 38.67.
Tertile-2 compared with Tertile-3.
Tertile-1 compared with Tertile-3.
Indicates P < 0.05 for Tertile-1 compared with Tertile-2.
Indicates P < 0.01 for Tertile-1 compared with Tertile-2.
The Tertile-3 group had much fewer men than the Tertile-1 group (32.9% versus 40.3%, P < 0.01). Mean SBP was higher in the Tertile-3 group than in the Tertile-1 group (157.4 versus 153.6 mm Hg, P < 0.01). Frequency of antihypertensive treatment was lower in the Tertile-3 group than in the Tertile-1 group (30.3% versus 33.7%, P = 0.02). Use of lipid-lowering medications was lower in the Tertile-3 group than in the Tertile-1 group (7.1% versus 10.6%, P < 0.01). Current cigarette smoking was lower in the Tertile-3 group than in the Tertile-1 group (10.2% versus 14.7%, P < 0.01). Significantly fewer participants in the Tertile-2 group received lipid-lowering medications than did participants in the Tertile-3 group (8.0% versus 10.6%, P < 0.01). No significant differences were found in family history of stroke or hypertension among the HDL-C tertiles.
Inverse association of HDL-C with ICH
The odds of a prior history of ICH decreased with increasing plasma HDL-C levels. ICH was more prevalent in the Tertile-1 group than in the Tertile-2 (3.97% versus 2.45%; P < 0.01) and Tertile-3 groups (3.97% versus 1.99%; P < 0.01). The unadjusted OR of ICH was 1.24 (95% CI, 0.81-1.89; P = 0.32) in the Tertile-2 group and 2.03 (95% CI, 1.38-2.98; P < 0.01) in the Tertile-1 group, compared with the Tertile-3 group.
Linear regression analysis performed before multivariable logistic regression analysis revealed no significant colinearity between the continuous variables (age, SBP, and BMI) and HDL-C (data not shown). The OR of ICH in the Tertile-1 group was significantly higher than in the Tertile-3 group (OR 1.88; 95% CI, 1.27-2.78; P < 0.01) after adjustment for age and gender. After further adjustment for SBP, BMI, hypercholesterolemia, current smoking, current drinking, and family history of stroke, the OR of ICH in Tertile-1 remained significantly higher than in the Tertile-3 group (OR 2.06; 95% CI, 1.35-3.12; P < 0.01). The multivariable-adjusted OR of ICH was 1.13 (95% CI, 0.72-1.78; P = 0.59) in Tertile-2 compared with Tertile-3 (Table 2). To exclude the effect of lipid-lowering medications on levels of plasma lipids, a multivariable logistic regression analysis was performed after excluding of 519 participants (including 29 with ICH) who received lipid-lowering medications. The results demonstrated that the association between plasma lipids and ICH was not altered by the lipid-lowering medications (supplementary Table I).
TABLE 2.
OR (95% CI) of ICH according to plasma lipid tertiles
| Variables | Tertiles of Plasma Lipid | ||||
| Tertile-3 | Tertile-2 | Tertile-1 | Pa | Pb | |
| LDL-C (mmol/l) | ≥3.20 | 2.61–3.19 | <2.61 | – | – |
| ICH (n) | 69 | 54 | 47 | – | – |
| Unadjusted | 1.0 | 0.78 (0.5–1.11) | 0.66 (0.45–0.96) | 0.17 | 0.03 |
| Model 1 | 1.0 | 0.77 (0.53–1.11) | 0.62 (0.42–0.92) | 0.15 | 0.02 |
| Model 2 | 1.0 | 0.83 (0.57–1.22) | 0.72 (0.48–1.10) | 0.34 | 0.13 |
| HDL-C (mmol/l) | ≥1.65 | 1.38–1.64 | <1.38 | – | – |
| ICH (n) | 40 | 49 | 81 | – | – |
| Unadjusted | 1.0 | 1.24 (0.81–1.89) | 2.03 (1.38–2.98) | 0.32 | <0.01 |
| Model 1 | 1.0 | 1.21 (0.79–1.85) | 1.88 (1.27–2.78) | 0.39 | <0.01 |
| Model 2 | 1.0 | 1.13 (0.72–1.78) | 2.06 (1.35–3.12) | 0.59 | <0.01 |
| TC (mmol/l) | ≥5.77 | 4.87–5.76 | <4.87 | – | – |
| ICH (n) | 57 | 59 | 54 | – | – |
| Unadjusted | 1.0 | 1.06 (0.73–1.53) | 0.95 (0.65–1.38) | 0.76 | 0.77 |
| Model 1 | 1.0 | 1.07 (0.73–1.55) | 0.88 (0.60–1.31) | 0.74 | 0.54 |
| Model 2 | 1.0 | 1.14 (0.77–1.70) | 1.11 (0.73–1.67) | 0.51 | 0.63 |
| TG (mmol/l) | ≥1.24 | 1.03–1.23 | <1.03 | – | – |
| ICH (n) | 63 | 57 | 50 | – | – |
| Unadjusted | 1.0 | 0.92 (0.64–1.33) | 0.79 (0.54–1.16) | 0.66 | 0.23 |
| Model 1 | 1.0 | 0.85 (0.58–1.23) | 0.75 (0.51–1.10) | 0.39 | 0.14 |
| Model 2 | 1.0 | 0.87 (0.58–1.30) | 0.86 (0.55–1.33) | 0.49 | 0.49 |
Model 1 was used to adjust for age and gender. Model 2 was used to adjust for age, gender, and other vascular risk factors, including SBP, BMI, hypercholesterolemia, current smoking, current drinking, and family history of stroke.
Values were calculated using the multivariable logistic regression model and compared Tertile-2 with Tertile-3, the reference group.
Tertile-1 compared with Tertile-3, the reference group.
In the final multivariable-adjusted model, SBP was found to have the most significant association with ICH (increment per 10 mm Hg: OR 1.21; 95% CI, 1.15-1.29; P < 0.01) (Table 3); female gender and high BMI were identified as protective factors for ICH (women: OR 0.50; 95% CI, 0.33-0.77; P < 0.01; high BMI: OR 0.93; 95% CI, 0.88-0.97; P < 0.01).
TABLE 3.
Contribution of confounding variables to ICH in the final multivariable logistic regression model
| Variables | OR | 95% CI | P |
| Age (increment per year) | 1.00 | 0.99–1.02 | 0.66 |
| Female (0, 1) | 0.50 | 0.33–0.77 | <0.01 |
| BMI (kg/m2) | 0.93 | 0.88–0.97 | <0.01 |
| SBP (increment per 10 mm Hg) | 1.21 | 1.15–1.29 | <0.01 |
| HDL-C (increment per 1 mmol/l) | 0.47 | 0.28–0.78 | <0.01 |
| Hypercholesterolemia (0, 1) | 1.85 | 1.24–2.76 | <0.01 |
| Current smoking, (0, 1) | 1.02 | 0.63–1.64 | 0.95 |
| Current drinking (0, 1) | 1.29 | 0.81–2.04 | 0.28 |
| Family history of stroke (0, 1) | 1.06 | 0.69–1.61 | 0.80 |
0, no; 1, yes.
Subgroup analysis by gender, hypertension, and BMI
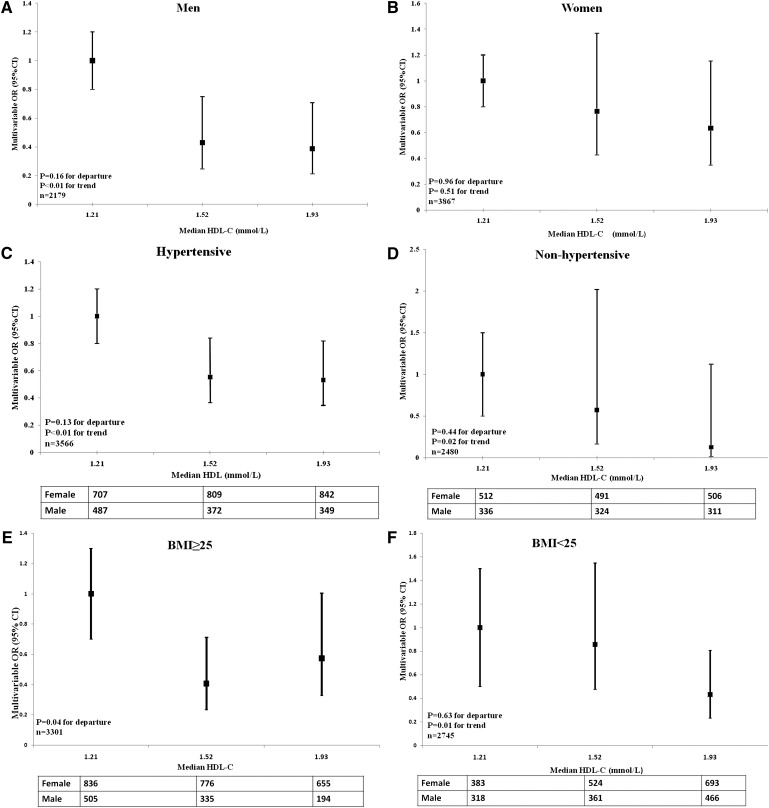
The risk factors for ICH, including male gender, hypertension, and BMI, were not distributed normally across the three HDL-C groups. Therefore, a stratification test taking these three factors into account was performed (Fig. 2). In the stratification test, the significant inverse association of HDL-C with ICH was found only in men (n = 2179; P < 0.01 for trend), and HDL-C levels conformed to an increasing dose-dependent relationship across the three tertile groups. The inverse association between HDL-C and ICH was also significant in subjects with low BMI (<25 kg/m2; P = 0.01 for trend), but no linear trend was documented in participants with high BMI (P = 0.04 for departure). The inverse association of HDL-C with ICH was demonstrated in both hypertensive and nonhypertensive participants (P < 0.01 and P = 0.02, respectively).
Fig. 2.
Multivariable-adjusted ORs of ICH with different HDL-C categories, stratified by gender, hypertension status, and BMI. Solid-square points indicate multivariable ORs of ICH in Tertile-2 and Tertile-3 compared with Tertile-1 (reference). Bars indicate 95% CI. Median HDL-C values of the different groups are indicated on the horizontal axis. A: The OR of ICH significantly decreased as median plasma levels of HDL-C increased in men. B: The linear trend of association between HDL-C and ICH remained nonsignificant in women. C: The inverse trend of association between ICH and HDL-C in hypertensive participants was significant. D: The inverse trend of association between ICH and HDL-C in nonhypertensive participants was also significant. E: No linear association was found between ICH and HDL-C in participants with BMI ≥ 25 kg/m2 (P = 0.04 for departure from linear trend). F: The inverse trend of association in participants with BMI < 25 kg/m2 was significant. Multivariable OR was adjusted by gender, age, SBP, BMI, hypercholesterolemia, current smoking, current drinking, and family history of stroke, excluding the stratifying factor. P < 0.05 for departure indicates nonlinearity across three groups. The departure from the linear trend was evaluated using x2 statistic, which was obtained by subtracting the trend chi-square from the overall chi-square with one (k-2) degree of freedom.
DISCUSSION
The major finding of the present study is that plasma HDL-C levels were inversely associated with a history of ICH within the prior 1-5 years, indicating a potential risk factor of low plasma HDL-C on the development of ICH. Further subgroup analysis showed that the effect was primarily evident in men and in participants with low BMI. Furthermore, only 8.6% of the participants (519/6046) received lipid-lowering medications, suggesting that the plasma HDL-C levels were not influenced by lipid-lowering medications in the present study. The potential detrimental effects of low plasma HDL-C on ICH suggest that raising HDL-C levels would provide a new prevention treatment for ICH, although more prospective studies would be required to confirm this.
Previously, low HDL-C concentrations were found to be associated with the risk of ischemic stroke in a Japanese population (22) as well as in elderly patients with diabetes (23). The results from a study that employed English males as participants support the above conclusion, in which subjects with high levels of HDL-C (top-fifth percentile) had a 50% reduction in nonfatal stroke risk compared with subjects with low levels of HDL-C (bottom-fifth percentile) (24). Furthermore, in subjects with low levels of LDL-C attained through the use of statins, low plasma HDL-C correlated with the risk of cardiovascular disease (25).
Although the studies mentioned above favor a detrimental effect of low HDL-C levels on stroke risk, several studies contradict this view (26–31). For example, plasma lipids have been not associated with ischemic or hemorrhagic strokes in a case-controlled study (26). However, the case-controlled study was different from ours in its antihypertensive trial design and inclusion of subjects with CHD and ischemic stroke. Other studies reported either no association between HDL-C and ICH incidence (27, 28) or an increase in the risk of hemorrhagic stroke with high HDL-C (29). However, heterogeneity in patient ethnicity may set these studies apart from the our study.
The level of plasma HDL-C was also not a predictor of residual vascular risk in the rosuvastatin (30), and no significant association was found between changes in HDL-C levels and stroke reduction in a recent meta-analysis of statin trials (31). However, the nature of lipid-lowering trials may be different from the population-based study described herein, which had relatively few participants on lipid-lowering therapy. The pharmacologic increase in HDL-C levels may, in addition, result in a functionally distinct form of HDL-C compared with the “native” HDL-C that is the focus in the present study. To this point, the conversion of HDL to a dysfunctional form that is no longer cardioprotective may be involved in the increasing risk of CHD (32), although few confirmed methods have been identified to determine the function of converted HDL-C (33).
In accordance with other studies (6, 11), SBP was confirmed to be the most important risk factor for ICH in our study (Table 3). The results suggest that the level of HDL-C may be a “natural” antihypertensive agent. This suggestion is also supported by a recent prospective study in which subjects with high-normal BP (130 ≤ SBP < 140 mm Hg or 85 ≤ DBP < 90 mm Hg) had a nonsignificantly higher risk of mortality compared with those with optimal BP (SBP <1 20 and DBP < 80 mm Hg). The combination of low HDL-C and a high-normal BP has been associated with a 2-fold-higher risk of mortality compared with a optimal BP in the follow-up study of 7.6 years (34).
The vast majority of ICH is due to the formation of microaneurysms that are caused by the degeneration and necrosis of cerebral small arteries, both of which are accompanied by hypertension and arteriosclerosis. The lipid toxicity and inflammatory function of cholesterol may account for the angio-degeneration and arteriosclerosis. In recent cell culture studies with animal and human cells, HDL promoted cholesterol efflux from macrophage foam cells in atheromatous vessels, reducing the cholesterol burden and macrophage-driven inflammation (33, 35). HDL also inhibits the type I interferon response pathway independently of macrophage cholesterol stores (36) and activates the complement cascade (37, 38). Furthermore, HDL-C not only inhibits LDL-induced lipid hydroperoxide formation, monocyte adherence, and monocyte chemotactic activity but also quenches the fluorescent signal of oxidized phospholipids in cell-based and cell-free studies (39).
In addition to hypertension, other major causes of ICH include anticoagulants, bleeding disorders, cerebral amyloid angiopathy (CAA), ruptured arterial aneurysms, arteriovenous malformations, and other vascular anomalies (40). Hypertensive degenerative changes in small cerebral arteries coexist with CAA in patients with ICH (41). A sudden elevation of BP can result in ruptured microaneurysms in such patients, resulting in a greatly enhanced risk of ICH. High HDL-C levels might reduce the risk of ICH by decreasing CAA, given that 2-fold increases in plasma HDL-C levels attenuated CAA by approximately 50% in mice (42).
Strengths and limitations of the current study
The collection of BP and HDL-C data at a median time of 2 years after the onset of ICH can be considered a drawback of the present study. Because this is a cross-sectional study, the low levels of plasma HDL-C identified in patients with ICH may have been either the cause or the result of ICH. The causal relationship between plasma HDL-C and ICH cannot be established in this kind of study design; it can only be drawn in prospective studies with large number of samples free of ICH at the baseline.
Second, survivor bias is a limitation of the present study, and the results must be interpreted with caution. The hypothesis may be advanced that patients with ICH who survived to participate in the study had lower plasma HDL-C levels than did patients who were excluded from the study because they died from the disorder. However, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study showed that low baseline HDL-C was the strongest predictor of recurrent stroke, including fatal stroke, in patients without a prior history of CHD disease (43), attenuating the hypothesis. Survivor bias can be reduced through the use of time-dependent covariate analyses in follow-up studies.
The strength of present study is that it was performed in a large community-based sample of participants who did not employ many interventions to alter lipids profiles or blood pressure. These interventions would likely have an impact on the relationship between HDL-C levels and ICH risk.
In conclusion, this study showed that HDL-C levels were inversely associated with a history of prior ICH. Hence, low plasma levels of HDL-C (<1.38 mmol/l) may be associated with the development of ICH, especially in men and lean participants.
Supplementary Material
Acknowledgments
The three anonymous reviewers are greatly appreciated for their constructive comments. All participants in this study are appreciated for their valuable contribution.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- CAA
- cerebral amyloid angiopathy
- CHD
- coronary heart disease
- CI
- confidence interval
- DBP
- diastolic blood pressure
- FBG
- fasting blood glucose
- HDL-C, HDL
- cholesterol
- ICH
- intracerebral hemorrhage
- LDL-C, LDC
- cholesterol
- OR
- odds ratio
- SBP
- systolic blood pressure
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by Grant 2006AA02Z477 (R.H.) from the National High Technology Research and Development Program (863 Program) of China and by Grant 2006CB503805 (R.H.) from the Ministry of Science and Technology of China.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one table.
REFERENCES
- 1.Qureshi A. I., Mendelow A. D., Hanley D. F. 2009. Intracerebral haemorrhage. Lancet. 373: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broderick J., Connolly S., Feldmann E., Hanley D., Kase C., Krieger D., Mayberg M., Morgenstern L., Ogilvy C. S., Vespa P., et al. 2007. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 116: e391–e413. [DOI] [PubMed] [Google Scholar]
- 3.He J., Gu D., Wu X., Reynolds K., Duan X., Yao C., Wang J., Chen C. S., Chen J., Wildman R. P., et al. 2005. Major causes of death among men and women in China. N. Engl. J. Med. 353: 1124–1134. [DOI] [PubMed] [Google Scholar]
- 4.Jiang B., Wang W. Z., Chen H., Hong Z., Yang Q. D., Wu S. P., Du X. L., Bao Q. J. 2006. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 37: 63–68. [DOI] [PubMed] [Google Scholar]
- 5.Thrift A. G. 2003. Editorial comment--Minor risk factors for intracerebral hemorrhage: the jury is still out. Stroke. 34: 2065–2066. [DOI] [PubMed] [Google Scholar]
- 6.Ariesen M. J., Claus S. P., Rinkel G. J., Algra A. 2003. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 34: 2060–2065. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P., Labreuche J. 2009. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 8: 453–463. [DOI] [PubMed] [Google Scholar]
- 8.Iso H., Jacobs D. R. J., Wentworth D., Neaton J. D., Cohen J. D. 1989. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N. Engl. J. Med. 320: 904–910. [DOI] [PubMed] [Google Scholar]
- 9.Lewington S., Whitlock G., Clarke R., Sherliker P., Emberson J., Halsey J., Qizilbash N., Peto R., Collins R. 2007. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 370: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Patel A., Horibe H., Wu Z., Barzi F., Rodgers A., MacMahon S., Woodward M. 2003. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int. J. Epidemiol. 32: 563–572. [DOI] [PubMed] [Google Scholar]
- 11.Sturgeon J. D., Folsom A. R., Longstreth W. T. J., Shahar E., Rosamond W. D., Cushman M. 2007. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 38: 2718–2725. [DOI] [PubMed] [Google Scholar]
- 12.Amarenco P., Bogousslavsky J., Callahan 3rd A., Goldstein L. B., Hennerici M., Rudolph A. E., Sillesen H., Simunovic L., Szarek M., Welch K. M., et al. 2006. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 355: 549–559. [DOI] [PubMed] [Google Scholar]
- 13.Noda H., Iso H., Irie F., Sairenchi T., Ohtaka E., Doi M., Izumi Y., Ohta H. 2009. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: the Ibaraki Prefectural Health Study. Circulation. 119: 2136–2145. [DOI] [PubMed] [Google Scholar]
- 14.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., et al. 2001. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7: 853–857. [DOI] [PubMed] [Google Scholar]
- 15.Seetharam D., Mineo C., Gormley A. K., Gibson L. L., Vongpatanasin W., Chambliss K. L., Hahner L. D., Cummings M. L., Kitchens R. L., Marcel Y. L., et al. 2006. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ. Res. 98: 63–72. [DOI] [PubMed] [Google Scholar]
- 16.Petoumenos V., Nickenig G., Werner N. 2008. High density lipoprotein exerts vasculoprotection via endothelial progenitor cells. J. Cell Mol. Med. 13: 4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarenco P., Labreuche J., Touboul P. J. 2008. High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis. 196: 489–496. [DOI] [PubMed] [Google Scholar]
- 18.Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L. J., Jones D. W., Materson B. J., Oparil S., Wright J. T. J., et al. 2003. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 42: 1206–1252. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Sandoval J. L., Romero-Vargas S., Chiquete E., Padilla-Martinez J. J., Villarreal-Careaga J., Cantu C., Arauz A., Barinagarrementeria F. 2006. Hypertensive intracerebral hemorrhage in young people: previously unnoticed age-related clinical differences. Stroke. 37: 2946–2950. [DOI] [PubMed] [Google Scholar]
- 20.Grundy S. M., Cleeman J. I., Merz C. N., Brewer H. B. J., Clark L. T., Hunninghake D. B., Pasternak R. C., Smith S. C., Jr, Stone N. J. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 110: 227–239. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N. 1963. Chi-square tests with one degree of freedom: extensions of the Mantel-Haenszel procedure. J. Am. Stat. Assoc. 58: 690–700. [Google Scholar]
- 22.Soyama Y., Miura K., Morikawa Y., Nishijo M., Nakanishi Y., Naruse Y., Kagamimori S., Nakagawa H. 2003. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: the Oyabe Study. Stroke. 34: 863–868. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T., Kawashima S., Itoh H., Yamada N., Sone H., Watanabe H., Hattori Y., Ohrui T., Yokote K., Nomura H., et al. 2009. Low HDL cholesterol is associated with the risk of stroke in elderly diabetic individuals: changes in the risk for atherosclerotic diseases at various ages. Diabetes Care. 32: 1221–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wannamethee S. G., Shaper A. G., Ebrahim S. 2000. HDL-Cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke. 31: 1882–1888. [DOI] [PubMed] [Google Scholar]
- 25.Jafri H., Alsheikh-Ali A. A., Karas R. H. 2010. Meta-analysis: statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann. Intern. Med. 153: 800–808. [DOI] [PubMed] [Google Scholar]
- 26.Patel A., Woodward M., Campbell D. J., Sullivan D. R., Colman S., Chalmers J., Neal B., MacMahon S. 2005. Plasma lipids predict myocardial infarction, but not stroke, in patients with established cerebrovascular disease. Eur. Heart J. 26: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 27.Tirschwell D. L., Smith N. L., Heckbert S. R., Lemaitre R. N., Longstreth W. T. J., Psaty B. M. 2004. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 63: 1868–1875. [DOI] [PubMed] [Google Scholar]
- 28.Bots M. L., Elwood P. C., Nikitin Y., Salonen J. T., Freire de Concalves A., Inzitari D., Sivenius J., Benetou V., Tuomilehto J., Koudstaal P. J., et al. 2002. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J. Epidemiol. Community Health. 56(Suppl. 1): i19–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodward M., Barzi F., Feigin V., Gu D., Huxley R., Nakamura K., Patel A., Ho S., Jamrozik K. 2007. Associations between high-density lipoprotein cholesterol and both stroke and coronary heart disease in the Asia Pacific region. Eur. Heart J. 28: 2653–2660. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P. M., Genest J., Boekholdt S. M., Libby P., Gotto A. M., Nordestgaard B. G., Mora S., MacFadyen J. G., Glynn R. J., Kastelein J. J. 2010. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet. 376: 333–339. [DOI] [PubMed] [Google Scholar]
- 31.De Caterina R., Scarano M., Marfisi R., Lucisano G., Palma F., Tatasciore A., Marchioli R. 2010. Cholesterol-lowering interventions and stroke: insights from a meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 55: 198–211. [DOI] [PubMed] [Google Scholar]
- 32.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. 2004. Antiinflammatory properties of HDL. Circ. Res. 95: 764–772. [DOI] [PubMed] [Google Scholar]
- 33.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim N. H., Cho H. J., Kim Y. J., Cho M. J., Choi H. Y., Eun C. R., Kim J. H., Yang S. J., Yoo H. J., Kim H. Y., et al. Combined effect of high-normal blood pressure and low HDL cholesterol on mortality in an elderly Korean population: the South-West Seoul (SWS) Study. Am. J. Hypertens.. Epub ahead of print. April 28, 2011; doi:10.1038/ajh.2011.78. [DOI] [PubMed] [Google Scholar]
- 35.Pagler T. A., Wang M., Mondal M., Murphy A. J., Westerterp M., Moore K. J., Maxfield F. R., Tall A. R. 2010. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ. Res. 108: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M., Pritchard D. K., Becker L., Hoofnagle A. N., Tanimura N., Bammler T. K., Beyer R. P., Bumgarner R., Vaisar T., de Beer M. C., et al. 2010. High-density lipoprotein suppresses the type I interferon response, a family of potent antiviral immunoregulators, in macrophages challenged with lipopolysaccharide. Circulation. 122: 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alipour A., van Oostrom A. J., Izraeljan A., Verseyden C., Collins J. M., Frayn K. N., Plokker T. W., Elte J. W., Castro Cabezas M. 2008. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler. Thromb. Vasc. Biol. 28: 792–797. [DOI] [PubMed] [Google Scholar]
- 38.Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., et al. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navab M., Hama S. Y., Hough G. P., Subbanagounder G., Reddy S. T., Fogelman A. M. 2001. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 42: 1308–1317. [PubMed] [Google Scholar]
- 40.Donnan G. A., Fisher M., Macleod M., Davis S. M. 2008. Stroke. Lancet. 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 41.Takebayashi S., Kaneko M. 1983. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke. 14: 28–36. [DOI] [PubMed] [Google Scholar]
- 42.Lewis T. L., Cao D., Lu H., Mans R. A., Su Y. R., Jungbauer L., Linton M. F., Fazio S., LaDu M. J., Li L. 2010. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J. Biol. Chem. 285: 36958–36968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amarenco P., Goldstein L. B., Callahan 3rd A., Sillesen H., Hennerici M. G., O'Neill B. J., Rudolph A. E., Simunovic L., Zivin J. A., Welch K. M. 2009. Baseline blood pressure, low- and high-density lipoproteins, and triglycerides and the risk of vascular events in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Atherosclerosis. 204: 515–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.