Abstract
Dietary and xenobiotic compounds may alter endocrine signaling and lipid homeostasis, thus inducing obesity. We describe a short-term assay method, the zebrafish obesogenic (ZO) test, for examining the effects of diet, drugs, and environmental contaminants, singly or in combination, on white adipose tissue (WAT) dynamics in live larvae. The ZO test is an intermediate step in obesity research, between in vitro and rodent assays, and may be also used to study the effect of environmental toxicants on the adiposity of aquatic species. The procedure, using Nile Red (NR) fluorescent probe to reveal adipocyte lipid droplets, is suitable for pharmaceutical or toxicological screening. Larvae treated at an environmentally-relevant concentration of tributyltin chloride (TBT), an environmental obesogen, exhibited a remarkable increase in adiposity, irrespective of the lipid composition of the background diet. Exogenous compounds, e.g., rosiglitazone or TBT, known to increase adiposity in the fasting state, were classified as obesogenic. Anti-obesogenic compounds favored a decrease in adiposity in the fasting state. The ZO test, using adipocyte lipid droplet size and adiposity as its endpoints, is a whole-organism alternative testing assay for obesogenic and anti-obesogenic compounds and mixtures and provides relevant information for environmental and human risk assessments.
Keywords: obesity, adipose tissue, tributyltin, endocrine disrupting chemicals, Nile Red, adipocyte, lipid droplet, organotins, human risk assessment
Obesity is the result of interplay between genetic and environmental factors and is characterized by excess fat storage in white adipocytes. Due to the increasing prevalence of obesity all over the world, research into this issue has become one of the main public health priorities. Food quality and quantity is probably the main cause of obesity; however, increasing evidence for environmental factors has recently been revealed, such as exposure to endocrine disrupting chemicals (EDC) during early life (1, 2). Obesogens are a special class of EDCs that target adipose tissue formation and the lipid metabolism (2).
Overweight and obesity are characterized by overrepresentation of white adipose tissue (WAT). Several studies have investigated xenobiotic molecules able to modulate adipogenesis and the adipocyte lipid metabolism. Some obesity-causing chemicals are hypothesized to act as peroxisome proliferator-activated receptor-γ (PPARγ) agonists or estrogen receptor-α antagonists (2). In addition to rodent assays, most of these studies used cell models and focused mainly on adipocyte differentiation (3). The Caenorhabditis elegans nematode was recently used as a whole organism to screen for molecules able to regulate fat storage (4). However, one limitation of this nonvertebrate model is that the major fat storage compartment is the intestine. Evidence supporting the action mechanism of obesity-causing chemicals is under active investigation, but there is not, as yet, any convenient in vivo method for screening the obesogenic and anti-obesogenic molecules that target vertebrate WAT.
Zebrafish, a model organism of vertebrate development and organogenesis, is receiving increasing attention as a model for human diseases, drug discovery, and toxicology studies. Thus, zebrafish are increasingly used in drug-screening assays as an intermediate step after cell-based evaluation to prioritize drug candidates for conventional animal testing, thus reducing the number and cost of mammalian studies (5). Semitransparent zebrafish larvae offer a unique opportunity to study the effects of molecules on adipocyte biology and whole-organism adiposity in live vertebrates, as data on WAT development are now available for this species (6, 7). WAT is not found in embryos and early larvae (6, 7), although these stages are sensitive to fat metabolism effectors (8). An obesity syndrome may be induced at adult stages by genetic and dietary factors (9, 10).
We describe a simple, rapid zebrafish larva bioassay, the zebrafish obesogenic (ZO) test, for in vivo assessment of the potential impact of diet composition, chemical pollutants, and drugs on white adipocyte lipid droplet size and adiposity.
METHODS
Animals
All zebrafish experiments were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committee of the Université Bordeaux 1 (UFR Biologie). Wild-type adult zebrafish (Danio rerio) were initially purchased from a commercial source (Exomarc, Lormont, France). In some cases, the transparent casper line was used (11). Embryos and larvae were obtained by natural mating and raised in embryo water (90 µg/ml Instant Ocean [Aquarium Systems, Sarrebourg, France], 0.58 mM CaSO4, 2H2O, dissolved in reverse-osmosis purified water) at 28.5°C with an 11L:13D photoperiod. Animal stages were recorded according to standard length (SL), i.e., the distance from the rostral tip of the larva to the base of the caudal fin. From five days postfertilization until they were used in the ZO test, larvae were maintained in static 5 l tanks at a density of around 30 larvae per liter and fed ad libitum with a commercial formulated food adapted for zebrafish larvae (ZF Biolabs, Tres Cantos, Spain).
Chemicals and larva treatment
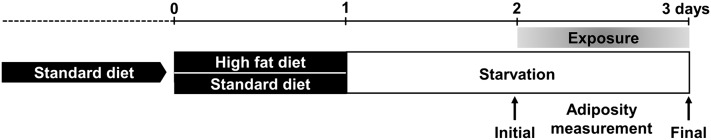
Rosiglitazone (71740) and T0070907 (10026) were purchased from SPI-BIO (Montigny le Bretonneux, France). Phenylephrine (P6126) and tributyltin chloride (TBT) (T50202) were from Sigma-Aldrich (St. Louis, MO). 1000× stock solutions of 1 mM rosiglitazone, 10 mM T0070907, and 0.05 mM TBT were prepared in DMSO on the day of the experiment. A 20 mM stock solution of phenylephrine was prepared in purified water. The three-day animal treatment protocol included adiposity recordings before and after chemical treatment (Fig. 1). Larvae with an SL between 7.5 and 9 mm were selected for enrollment in the ZO test and incubated individually in glass containers containing 25 ml water. There was no significant variation in SL during the three-day experimental period. The first day of the protocol was devoted to ad libitum feeding with a standard diet (SD) for late larvae (TetraMin Baby, Tetra GmbH, Melle, Germany) or hard-boiled chicken egg yolk as a high-fat diet (HFD), with food remaining inside the container at the end of the period. It was not possible to detect differences in feeding rates between SD and HFD fish, and large amounts of food were detectable in the lumen of the intestine under the microscope, irrespective of the nutrient used. Total fat content by weight of SD and HFD was 10% and 55%, respectively. The starvation period started on the second day and initial adiposity was measured at the end of the second day using Nile Red (NR) staining and fluorescence microscopy or triacylglycerol content (see below). Each group of animals consisted of 10 larvae of similar size and initial adiposity. They were incubated individually in glass containers containing 25 ml medium and remained in the fasting state under static conditions during exposure to selected compounds or vehicle alone (0.1% DMSO) for one additional day. No mortality was observed at the concentrations used in either control or 24 h exposed animals. Final adiposity in exposed and control animals was recorded after 24 h using NR staining and fluorescence microscopy. Apart from changes in adiposity levels, there was no apparent qualitative behavioral or morphological phenotype associated with these dietary and pharmacological treatments.
Fig.1.
Diagram of the ZO test protocol used to prepare larva exposure and measure adiposity. Zebrafish larvae were nourished with SD during their growth and then divided into two groups. The first one received SD for one day and the second HFD for one day. This was followed by a one-day starvation for both groups. Larvae were then exposed to the selected compounds or to vehicle alone for one additional day in a fasting state. Whole-body adiposity was measured, using Nile Red staining and fluorescence signal quantification, before and after exposure to the compounds. Evaluations were carried out on control animals at the same times.
Nile Red staining
A 500 µg/ml stock NR (N3013) (Sigma-Aldrich) solution was prepared in acetone. Just before use, the working solution was obtained by 1/100 dilution of the stock solution in fish water. Live fish were then exposed to NR working solution in the dark for 30 min at 28°C. Under these conditions, adipocyte NR staining was saturated, as the intensity of the fluorescent signal did not increase after longer exposure. The fish were then rinsed twice with fish water for 5 min, anesthetized in 2-phenoxyethanol (P1126) (Sigma-Aldrich) diluted 1/2000 in fish water for 5 min, and observed under a fluorescence microscope. NR staining was performed twice, once before the one-day exposure period, to measure initial adiposity, and again afterwards, to measure final adiposity. The initial staining dissipated to background levels before the second staining (data not shown).
Quantitative analysis of whole-mount fluorescence signals
Image acquisition quality is a critical point for further analyses, and the presence of food inside the intestinal lumen may interfere by quenching signals from NR-labeled perivisceral WAT. As 24 h starvation is sufficient to empty the zebrafish larva digestive tract, all fish processed for image analyses were starved for that period prior to NR staining. Differential interference contrast and fluorescence images were obtained on anesthetized larvae using a Nikon Eclipse E1000 (Nikon, Champigny sur Marne, France) microscope fitted with Nomarski optics and a Nikon Intensilight C-HGFI unit. Larvae were imaged in water and returned to the medium within approximately 1.5 min. A TRITC filter (EX 540/25, BA 605/55) was used for differential interference contrast and fluorescence image superposition (Fig. 2A) and an HQ-FITC-BP filter (Ex 460-500, BA 510-560) for fluorescence quantification (Fig. 2B). Images were acquired with a Nikon DXM1200 camera and LUCIA G software (version 4.81) and saved in high-resolution (3,840 × 3,005 pixels) tagged image file format (TIFF). All image series for quantification were obtained at the same settings. Quantitative analysis of NR fluorescence signals was performed using free-processing ImageJ software (National Institutes of Health, http://rsb.info.nih.gov/ij/), according to the protocol previously described (12). Three images (head, trunk, and tail) were recorded per larva at the magnification used. The images were combined to produce an overall view of the larva for fluorescence quantitation (Fig. 2B). Background fluorescence was estimated by analyzing average pixel intensity (API) values in image areas that did not contain any WAT, i.e., the background threshold, and this background was calculated individually for each image. To calculate the background levels, the images were first converted to 8-bit grayscales, inverted, and thresholded. WAT and individual adipocytes were then selected using the “wand tool” function of the computer program. Selected areas were transferred to the original three-channel image (red, green, and blue), the inverse selection was created, and the API for the background was calculated. This background threshold was then removed by subtracting the background API from each pixel in the image. Selected WAT areas, including individual adipocytes, were transferred to the subtracted image, and finally, the API of the selected areas was calculated (see ref. 12 for more details). Signal area and intensity were measured and expressed in arbitrary units. FocalCheckTM fluorescence microscope test slide 1 (Invitrogen) was used to evaluate microscope excitation-source stability by plotting the area and signal intensity of the beads over time. Periodic imaging of microbeads using the same acquisition parameters did not reveal any differences (P > 0.05) over at least a two-day period, demonstrating the stability of the excitation source (ref. 12, data not shown). The analytical precision of the quantification method was determined from blind duplicate pairs of NR fluorescence signal areas and was defined as the mean absolute difference between duplicates divided by the mean of the duplicates times 100. The precision value was 0.49%.
Fig.2.
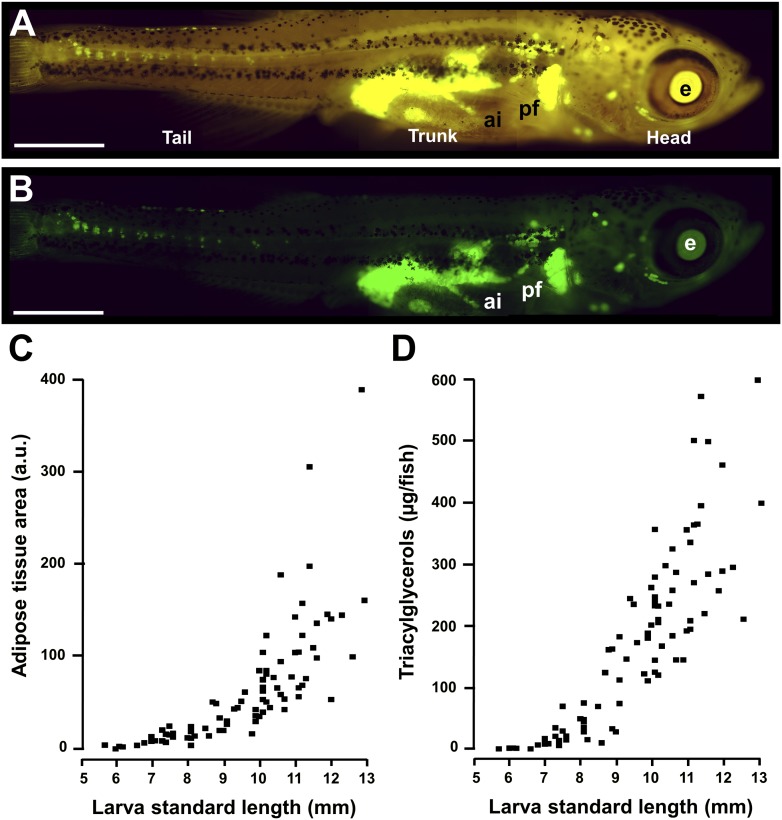
In vivo quantitative assessment of WAT in zebrafish larvae. A: Lateral view under a fluorescence microscope after NR staining, using a TRITC filter. Anterior part to the right and dorsal part to the top. SL of the larva was 8.2 mm. B: Lateral view of the same animal under an HQ-FITC-BP filter with adipocytes stained green. C: Relationship between WAT fluorescence area after NR staining and larva SL. D: Relationship between triacylglycerol content per larva and larva SL. A total of 88 larvae were selected, with a broad distribution of SL, from 5 to 13 mm. Larvae, on a SD background, were starved for one day before sampling. Scale bar, 1 mm. ai, anterior intestine; e, eye; pf, pectoral fin.
Triacylglycerol assay
The area and intensity of NR fluorescence signals were recorded for each larva and were then processed to assay the whole-body triacylglycerol content using a previously described protocol (13) with modifications. Animals were starved for 24 h before NR staining and euthanasia to avoid any contamination from food triacylglycerols in the digestive tract. Glass tubes were used throughout triacylglycerol extraction. Briefly, whole animals were homogenized mechanically in 400 µl homogenizing buffer (PBS, pH 7.4, containing 10 mM EDTA). Homogenates were transferred to tubes containing 2 ml isopropanol:hexane (4:1) solution. After shaking, samples were left in the dark for 30 min. Hexane:dietheylether (500 µl,1:1) solution was then added. Samples were mixed and left in the dark for 10 min. One milliliter water was then added, samples were mixed and left standing until the two phases separated (20 to 30 min). Eight hundred microliters of the supernatant were transferred to new tubes and processed until complete evaporation. Two hundred and fifty microliters of colorimetric reagent were added per tube, and the triacylglycerol content was evaluated by microassay, using a commercially available kit (Biolabo S.A., Maizy, France). In parallel, a standard curve was plotted using 0, 10, 20, 30, 40, and 50 µg triolein in 400 µl homogenizing buffer. Added triolein was extracted, processed, and treated identically to the other samples. The reaction was allowed to develop at 37°C for 1.5 h, with shaking at 220 rpm. Only samples within the standard curve were taken into account for data analysis.
Statistics
All statistical analyses were conducted using SPSS 17.0 microcomputer software (SPSS, Chicago, IL). At least three independent experiments were performed per condition with 10 larvae per group. To offset the variability among independent experiments in terms of the decrease in adiposity in control groups after a one-day starvation period and to have a view of negative or positive quantitative variations from initial adiposities, values presented in graphs are mean ± SEM of representative experiments. This procedure did not preclude highly reproducible absolute differences in adiposity between control and chemically treated groups in independent similar experiments. Normality of the distribution was assessed using the Shapiro-Wilk test (0.01% risk). Levene's test was used to verify the equality of variances. In experiments comparing a control and a chemically treated group with animals that received the same background diet, the statistical significance of difference in mean values was determined by Student's t-test. In experiments combining PPARγ agonist and antagonist and their controls, the statistical significance was determined by single-factor ANOVA followed by the post hoc Dunnett's test. A P value of 0.05 or less was considered significant. In the experiment combining rosiglitazone or TBT treatment with HFD, we used the univariate general linear model to check the individual effect of each factor and to determine whether there was any interaction between factors.
RESULTS AND DISCUSSION
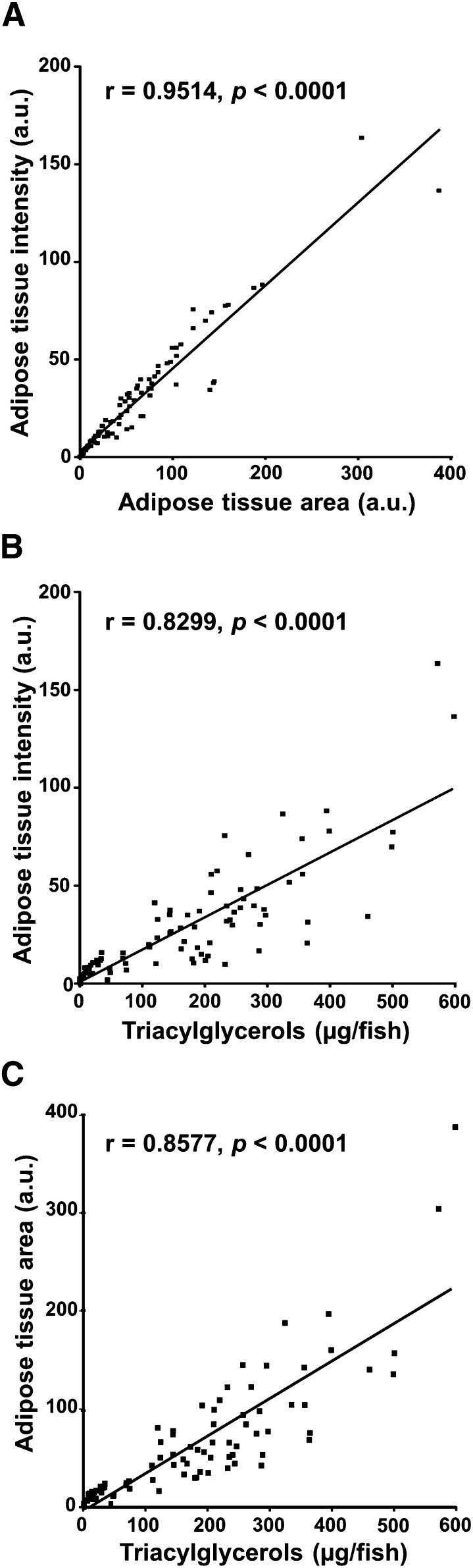
The first step was to perform in vivo staining of zebrafish adipocytes with vital NR using a rigorous feeding protocol (Fig. 1). As previously described, NR was found to be a selective fluorescent vital dye for adipocyte intracellular lipid droplets (6, 14). Relationships between WAT fluorescence area and SL and between triacylglycerol content and SL were established (Fig. 2C, D). The adipose tissue signal area was strongly correlated with intensity in larvae with an SL of 5 to 13 mm (Fig. 3A). There was also a very good correlation between whole-mount NR fluorescence adipose tissue signal intensity and area and whole-larva triacylglycerol content (Fig. 3B, C). During postembryonic zebrafish development, WAT appearance is correlated with size rather than age (7). We found that larvae suitable to the ZO test had to have an SL between 7.5 and 9 mm to obtain an adipose tissue fluorescence area ranging from 10 to 60 arbitrary units after NR staining. These values were within the linear range between adipose tissue fluorescent area and triacylglycerol content of individual larvae (Fig. 3C). In this SL range, the main anatomic locations of WAT were established (Fig. 2B). The main perivisceral WAT mass spreads from the anterior dorsal limits of the general/visceral cavity to the rectum, above and around the two swim bladder chambers, closely associated with the first loop of the anterior intestine, as well as the posterior intestine, close to the rectum. Other main locations are at the base of the pectoral fins, surrounding the eyes (individual adipocytes or clusters), and in the dermis of the tail.
Fig.3.
Relationship between WAT fluorescence intensity and area after NR staining and triacylglycerol content per larva. A total of 88 larvae were selected, with a broad distribution of SL, from 5 to 13 mm. Larvae, on a SD background, were starved for one day before sampling. Adipose tissue intensity and area values were expressed in arbitrary units (a.u.). A: Relationship between adipose tissue fluorescence intensity and area after NR staining of live zebrafish larvae. B: Triacylglycerol content per larva was plotted against WAT fluorescence intensity. C: Triacylglycerol content per larva was plotted against WAT area values. The slopes of the calculated linear regressions were significantly different from zero at P < 0.0001, and the variables were significantly correlated, with nonparametric Spearman correlation (P, two-tailed) as shown on the graph.
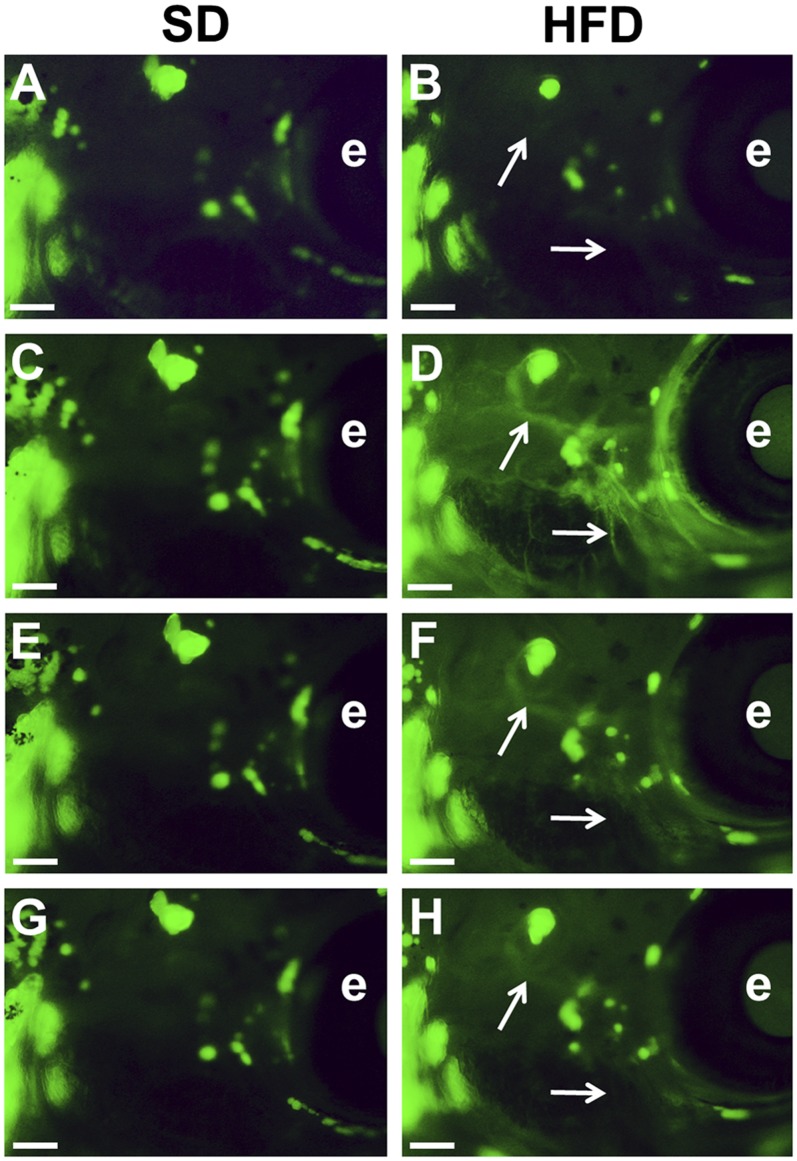
We then investigated the effect of starvation and initial diet lipid content on the adiposity of live animals. Under the experimental conditions used, individual adipocytes were easily identified under the microscope, and their size was rapidly sensitive to the conditions applied. Zebrafish larvae were fed SD or HFD for one day, starved for one day, and then exposed or not to the chemicals for one additional day in starved condition (Fig. 1). HFD induced an increase in NR lipid staining in the blood vessels of the larvae after feeding (Fig. 4D), which gradually returned to basal levels during fasting (Fig. 4B, F, H). These variations in NR staining in the circulatory system were not observed in SD larvae (Fig. 4A, C, E, G). A one-day starvation in the presence of 0.1% DMSO as a vehicle control induced a decrease in whole-body adiposity, as evaluated by NR fluorescent staining (−19.77 ± 1.87% in SD versus −11.83 ± 0.93% in HFD background, P < 0.005, n = 10 independent experiments analyzed).
Fig.4.
The effect of lipid content of the diet on Nile Red fluorescent signal in the circulatory system of live larvae. Lateral view under a fluorescence microscope after NR staining. Anterior part to the right and dorsal part to the top. Images of the head region of representative larvae enrolled in ZO test represent animals initially nourished for one day on SD (A, C, E, G) or HFD (B, D, F, H) diet. A, B: Before feeding. C, D: At the end of the one-day feeding period (day 1). E, F: At the end of the one-day fast (day 2). G, H: After two days of fasting (day 3). See Fig. 1 for details of ZO test diagram. Initial SL of the larva was 8.9 mm in (A) and 8.8 mm in (B). Adipocytes are stained green, and the positions of selected blood vessels that may be labeled on HFD are indicated by white arrows. Scale bar, 200 µm. e, eye.
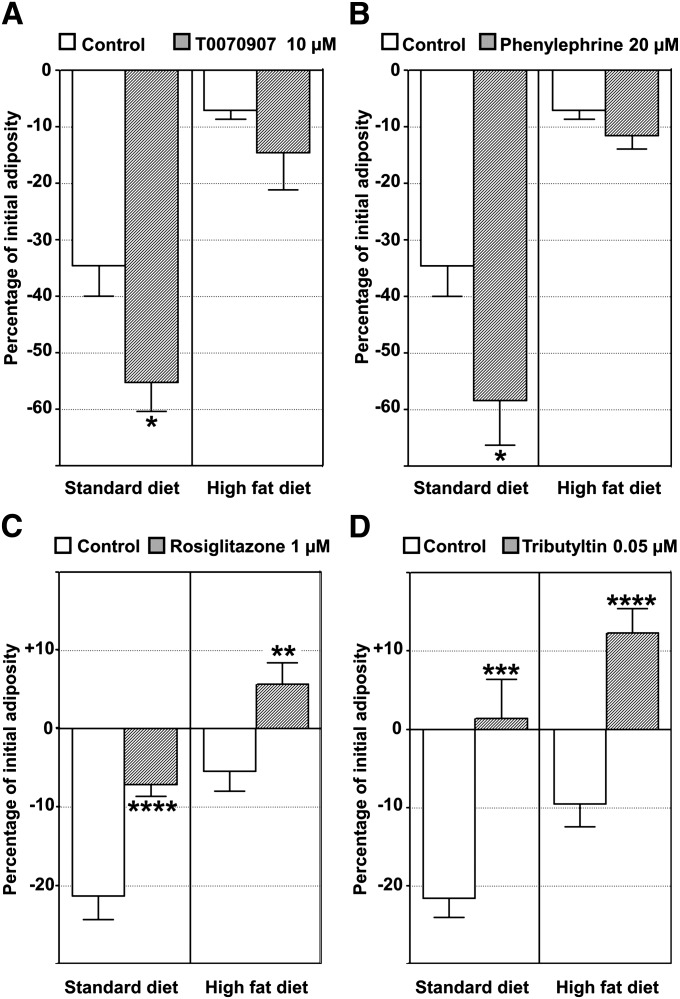
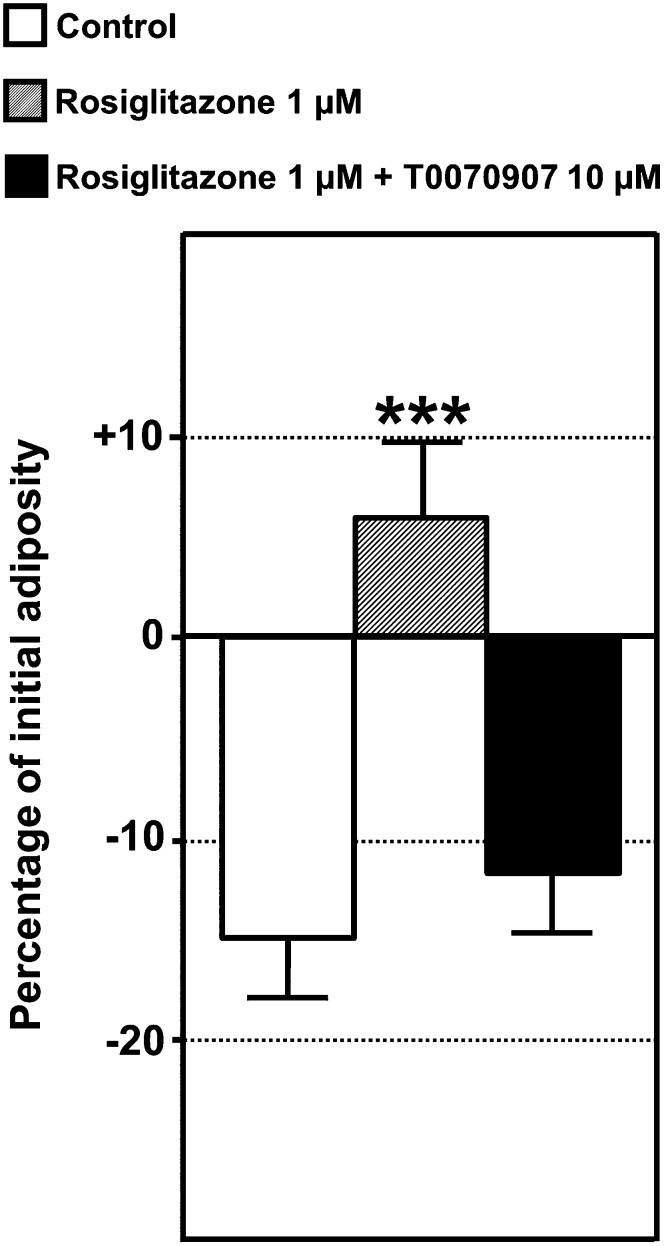
Given these findings, the ZO test was used to study WAT dynamics after exposure to pharmaceuticals and environmental pollutants in interaction with the initial diet lipid content. Food intake ability and/or nutrient absorption at the intestinal level may be altered during exposure to exogenous molecules. In addition, the presence of food inside the intestinal lumen may interfere by quenching signals from NR-labeled perivisceral WAT. We found that starting starvation one day before exposure to compounds and extending it throughout the exposure period avoided any interference by these confounding factors, thus focusing on the exogenous compound's effect on adiposity regulation. However, it was still possible to study the interaction between the initial diet composition and the chemicals used, as nutritional history has proved to be a significant factor in the effects of these molecules (see below). The following compounds were selected: i) T0070907 used as a PPARγ antagonist (15, 16), ii) phenylephrine used as an α1-adrenergic receptor agonist capable of eliciting an increase in lipolytic activity of human WAT (17), iii) rosiglitazone as a member of the thiazolidinedione family used for type II diabetes treatment and a well-known potent PPARγ agonist (16), and iv) TBT, a biocide found in antifouling paints capable of binding to PPARγ but also to its heterodimeric partner retinoid X receptor (18, 19). The whole-body adiposity dynamics of each larva were expressed as percentage decrease or increase in NR fluorescence signal areas after the one-day exposure period. The results of representative experiments are depicted in Fig. 5. Exposure to T0070907 (Fig. 5A) and phenylephrine (Fig. 5B) caused a decrease in adiposity compared with controls on an SD nutrient background. These exogenous compounds favored a decrease in adiposity in the fasting state and were classified as anti-obesogenic. In SD fish, rosiglitazone demonstrated the ability to prevent adiposity loss in the unfed condition (Fig. 5C). Adiposity even increased after this treatment on an HFD background (Fig. 5C). Compared with controls, Rosiglitazone-treated larvae had 16.09% ± 2.17 more adiposity than controls on an HFD background (n = 5 independent experiments). In addition, the ZO test made it possible to monitor individual adipocytes in vivo at higher magnification (Fig. 6). As in other vertebrates, zebrafish-differentiated adipocytes were unilocular (6, 7). Whereas control larvae exhibited a decrease or disappearance of NR-stained lipid droplets (Fig. 6A, C), rosiglitazone induced an increase in droplet size (Fig. 6B, D). The effect of rosiglitazone was completely abolished by PPARγ antagonist T0070907, indicating that rosiglitazone had a specific effect on adipocyte hypertrophy (Fig. 7).
Fig.5.
ZO test as a tool for screening molecules and nutritional factors that target adiposity in living zebrafish. A, B: Molecules with a lipogenic (anti-obesogenic) effect. C, D: Molecules with an adipogenic (obesogenic) effect. Quantitative analysis was performed by recording the image area of NR fluorescence signals. WAT dynamics is expressed as a percentage of initial adiposity value. Representative experiments are presented. Quantification was assayed in the presence of 0.1% DMSO as a vehicle control or 0.1% DMSO plus the molecule to be tested at the indicated concentration on an SD or HFD background. T0070907 (A) and phenylephrine (B) exposure induced a decrease in adiposity compared with control groups on SD, whereas no significant difference was found on HFD. Rosiglitazone exposure induced a smaller decrease in adiposity compared with controls on an SD background, whereas an increase in adiposity was observed on an HFD background (C). TBT induced an increase in adiposity, irrespective of the lipid content of the diet (D). Rosiglitazone-induced adipogenesis was additive to the effect of a high-fat diet (C). TBT, an environmental contaminant, also had an additive effect and was strongly obesogen at this environmentally relevant level (D). Values are mean ± SEM, n = 10 animals per group. *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0005 by comparison of means using the same background diet.
Fig.6.
In vivo adipocyte lipid droplet dynamics using the ZO test. Representative clusters of individual adipocytes from live unfed larvae were selected after NR staining, and images were recorded from the same animal before (A, B) and after (C, D) a 24-h exposure to the compounds. Adipocytes are stained green. C: Control larva recorded in (A) (SL = 8.9 mm) exposed to 0.1% DMSO, used as a vehicle control. D: Rosiglitazone-treated larva recorded in (B) (SL = 8 mm) exposed to 0.1% DMSO plus 1 µM rosiglitazone. Transparent casper line was used to avoid any pigmented cell optical artifact at the magnification used. Whole-body variations in initial versus final adiposity of the two selected animals were −5.6% and +5.6% in control and rosiglitazone-treated animals, respectively. Scale bar, 150 µm.
Fig.7.
ZO test as a tool for studying molecular mechanisms underlying adiposity dynamics in living zebrafish. Quantitative analysis was performed by recording the image area of NR fluorescence signals. WAT dynamics is expressed as a percentage of initial adiposity value. A representative experiment is presented. Quantification was assayed in the presence of 0.1% DMSO as a vehicle control or 0.1% DMSO plus rosiglitazone 1 µM or 0.1% DMSO plus rosiglitazone 1 µM and T0070907 10 µM on an HFD background. Rosiglitazone-induced adipogenesis was abolished by PPARγ antagonist T0070907, indicating that rosiglitazone had a specific effect on adipocyte hypertrophy. Values are mean ± SEM, n = 10 animals per group. ***P < 0.001 by comparison with control group using ANOVA and Dunnett's test.
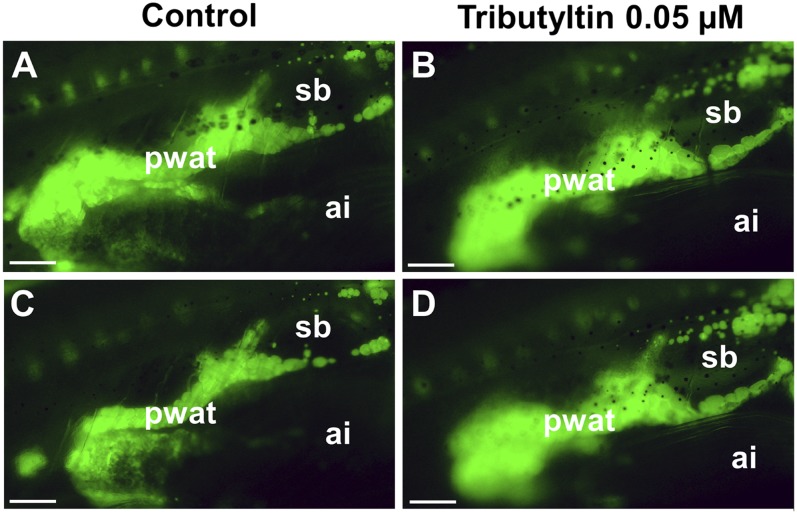
TBT is a recognized environmental obesogen (19–21). Larvae treated at an environmentally relevant concentration (22–24) exhibited a remarkable increase in adiposity, irrespective of the lipid composition of the background diet (Fig. 5D and Fig. 8). Compared with controls, TBT-treated larvae had 15.75% ± 2.45 more adiposity than controls on an HFD background (n = 5 independent experiments). Statistical analysis using a univariate general linear model of the effect of factors, rosiglitazone or TBT treatment, combined with HFD, indicated that each of these two factors had a significant effect (P < 0.0001). However, there was no interaction between the chemical and diet factors that resulted in an additive effect. The observed effect of TBT was limited to WAT, as no significant NR signal was found in the circulatory system, intestines (Fig. 8), or other anatomic locations (e.g., liver and gall bladder; data not shown). There is currently evidence to suggest that prenatal exposure to TBT or rosiglitazone activates the PPARγ, a key adipogenesis regulator (16), thereby altering the fate of multipotent stromal stem cells, predisposing them to become adipocytes (23, 25). However, due to its short-term, one-day window of exposure at larval stage, the ZO test is probably not suitable for evaluating the potential of chemicals and drugs to induce adipocyte hyperplasia. Adipocyte lipid droplet size results from a balance between the actions of various physiological stimuli and factors that promote triacylglycerol lipolysis and those that promote lipogenesis, irrespective of the mechanisms involved. HFD induced an increase in lipid content in the circulatory system of the larvae compared with SD, indicated by an increase in the NR signal after feeding (Fig. 4). Consequently, the fatty acids available for triacylglycerol synthesis may be more abundant, facilitating neutral lipid deposition in adipocytes even in the fasting state. It should be pointed out that, at the time of initial adiposity recording (i.e., after a-one day fast) and at the image magnification and fluorescent microscope settings used (e.g., the background subtracted method used), there was no significant nonadipose NR fluorescence signal likely to interfere with WAT quantitation (Fig. 2B, Fig.4F, Fig.8). The nutritional condition used favored a lipolysis state, as demonstrated by the decrease in lipid droplet size in controls (Fig. 6A, C). However, exposure to obesogenic molecules (e.g., rosiglitazone) induced an increase in lipid droplet size, as evidenced on an HFD background (Fig. 6B, D). Exogenous compounds (e.g., rosiglitazone or TBT) that increased adiposity in the fasting state were classified as obesogenic.
Fig.8.
In vivo adiposity dynamics under the effect of TBT using the ZO test. Representative perivisceral WAT from live unfed larvae were selected after NR staining and images were recorded before (A, B) and after (C, D) a 24-h exposure or not to the compounds. Lateral view and anterior part to the right and dorsal part to the top. Larvae on an HFD background were starved for 24 h before initial adiposity recording (see Fig. 1 for details of ZO test diagram). C: Control larva recorded in (A) (SL = 8.2 mm) exposed to 0.1% DMSO, used as a vehicle control. D: TBT-treated larva recorded in (B) (SL = 8.1 mm) exposed to 0.1% DMSO plus 0.05 µM TBT. Adipocytes are stained green. Perivisceral and whole-body variations in initial versus final adiposity of the two selected animals was −23% and −19.14% in control and +12% and +12.81% in TBT-treated animals, respectively. Scale bar, 500 µm. ai, anterior intestine; pwat, perivisceral white adipose tissue; sb, swim bladder.
In summary, our data demonstrated that zebrafish larvae provided a suitable vertebrate model for screening chemicals and mixtures likely to impair adipocyte fat storage and mobilization in interaction with diet lipid content. The ZO test is an intermediate step in obesity research, between in vitro and rodent assays, and it also may be used to study the effect of environmental toxicants on the adiposity of aquatic species. One major advantage of the described method is that the complex, dynamic, interactive, multi-organ events that occur in vivo remain intact, thus making it easier to characterize potentially obesogenic or anti-obesogenic substances.
Footnotes
- API
- average pixel intensity
- EDC
- endocrine disrupting chemical
- HFD
- high-fat diet
- NR
- Nile Red
- PPARγ
- peroxisome proliferator-activated receptor-γ
- SD
- standard diet
- SL
- standard length
- TBT
- tributyltin chloride
- WAT
- white adipose tissue
- ZO
- zebrafish obesogenic
This work was supported by Conseil Régional d'Aquitaine (CRA) Project 200881301031/TOD (P.J.B.) and postdoctoral fellowship (A.T-S.); by French Ministry of Research and Education predoctoral fellowship (N.O.); and by Université Bordeaux 1 BQR 2009 (P.J.B.).
REFERENCES
- 1.Newbold R. R., Padilla-Banks E., Snyder R. J., Phillips T. M., Jefferson W. N. 2007. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod. Toxicol. 23: 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grün F., Blumberg B. 2009. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 304: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lea-Currie Y. R., Duffin D. J., Buehrer B. M. 2011. Use of adipose-derived stem cells in high-throughput screening to identify modulators of lipogenesis. Methods Mol. Biol. 702: 359–368. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux G. A., Liu J., Mayer N., Bainton R. J., Ashrafi K., Werb Z. 2011. A whole-organism screen identifies new regulators of fat storage. Nat. Chem. Biol. 7: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath P., Li C. Q. 2008. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov. Today. 13: 394–401. [DOI] [PubMed] [Google Scholar]
- 6.Flynn E. J., III, Trent C. M., Rawls J. F. 2009. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J. Lipid Res. 50: 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imrie D., Sadler K. C. 2010. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev. Dyn. 239: 3013–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones K. S., Alimov A. P., Rilo H. L., Jandacek R. J., Woollett L. A., Penberthy W. T. 2008. A high throughput live transparent animal bioassay to identify non-toxic small molecules or genes that regulate vertebrate fat metabolism for obesity drug development. Nutr. Metab. (Lond.). 5: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y., Cone R. D. 2007. Creation of a genetic model of obesity in a teleost. FASEB J. 21: 2042–2049. [DOI] [PubMed] [Google Scholar]
- 10.Oka T., Nishimura Y., Zang L., Hirano M., Shimada Y., Wang Z., Umemoto N., Kuroyanagi J., Nishimura N., Tanaka T. 2010. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White R. M., Sessa A., Burke C., Bowman T., LeBlanc J., Ceol C., Bourque C., Dovey M., Goessling W., Burns C. E., et al. 2008. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raldua D., Babin P. J. 2009. Simple, rapid zebrafish larva bioassay for assessing the potential of chemical pollutants and drugs to disrupt thyroid gland function. Environ. Sci. Technol. 43: 6844–6850. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz D. M., Wolins N. E. 2007. A simple and rapid method to assay triacylglycerol in cells and tissues. J. Lipid Res. 48: 2514–2520. [DOI] [PubMed] [Google Scholar]
- 14.Greenspan P., Fowler S. D. 1985. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 26: 781–789. [PubMed] [Google Scholar]
- 15.Lee G., Elwood F., McNally J., Weiszmann J., Lindstrom M., Amaral K., Nakamura M., Miao S., Cao P., Learned R. M., et al. 2002. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J. Biol. Chem. 277: 19649–19657. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P., Spiegelman B. M. 2008. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 77: 289–312. [DOI] [PubMed] [Google Scholar]
- 17.Boschmann M., Krupp G., Luft F. C., Klaus S., Jordan J. 2002. In vivo response to alpha(1)-adrenoreceptor stimulation in human white adipose tissue. Obes. Res. 10: 555–558. [DOI] [PubMed] [Google Scholar]
- 18.Le Maire A., Grimaldi M., Roecklin D., Dagnino S., Vivat-Hannah V., Balaguer P., Bourguet W. 2009. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 10: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Ycaza J., Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor gamma to induce adipogenesis in murine 3T3-L1 preadipocytes. J. Steroid Biochem. Mol. Biol. Epub ahead of print. March 21, 2011; doi:10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inadera H., Shimomura A. 2005. Environmental chemical tributyltin augments adipocyte differentiation. Toxicol. Lett. 159: 226–234. [DOI] [PubMed] [Google Scholar]
- 21.Zuo Z., Chen S., Wu T., Zhang J., Su Y., Chen Y., Wang C. 2011. Tributyltin causes obesity and hepatic steatosis in male mice. Environ. Toxicol. 26: 79–85. [DOI] [PubMed] [Google Scholar]
- 22.Guo S., Qian L., Shi H., Barry T., Cao Q., Liu J. 2010. Effects of tributyltin (TBT) on Xenopus tropicalis embryos at environmentally relevant concentrations. Chemosphere. 79: 529–533. [DOI] [PubMed] [Google Scholar]
- 23.Grün F., Blumberg B. 2006. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Mol. Endocrinol. 20: 2141–2155. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Zuo Z., Wang Y., Yu A., Chen Y., Wang C. 2011. Tributyltin chloride results in dorsal curvature in embryo development of Sebastiscus marmoratus via apoptosis pathway. Chemosphere. 82: 437–442. [DOI] [PubMed] [Google Scholar]
- 25.Kirchner S., Kieu T., Chow C., Casey S., Blumberg B. 2010. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 24: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]