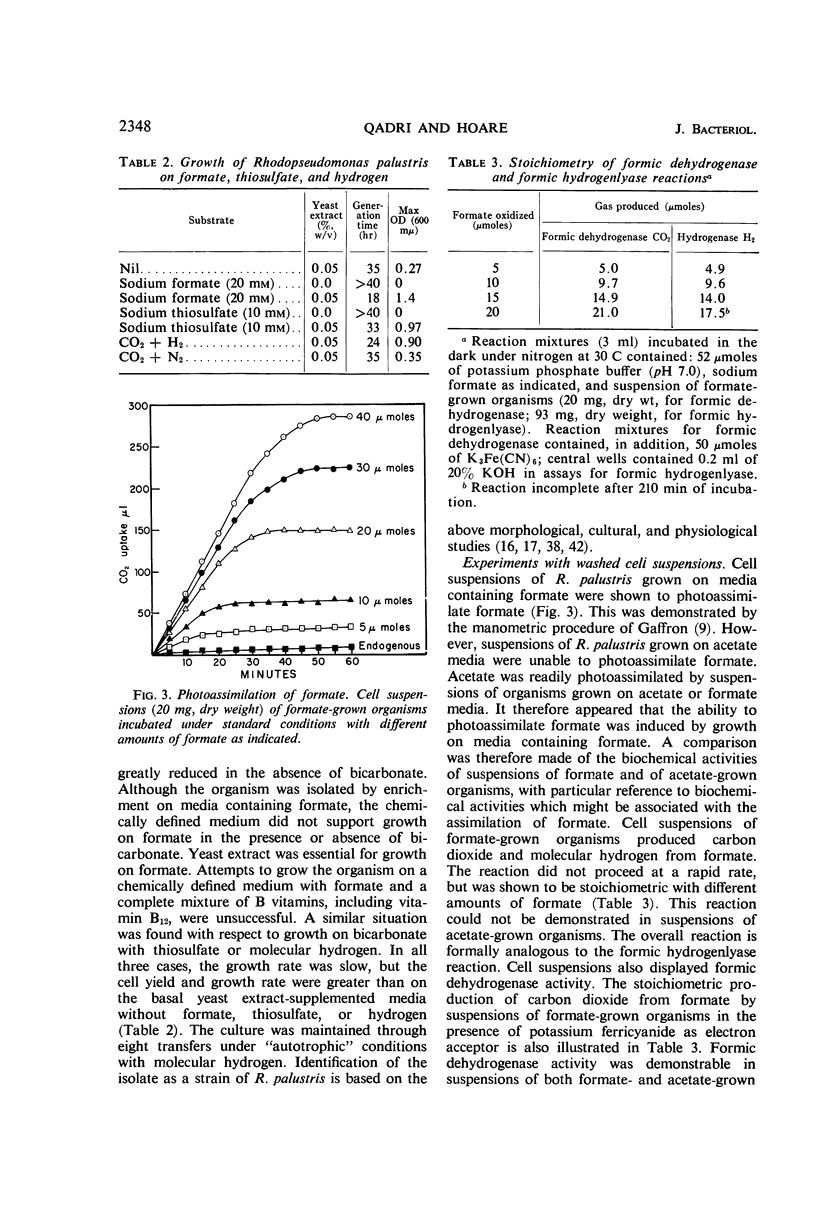
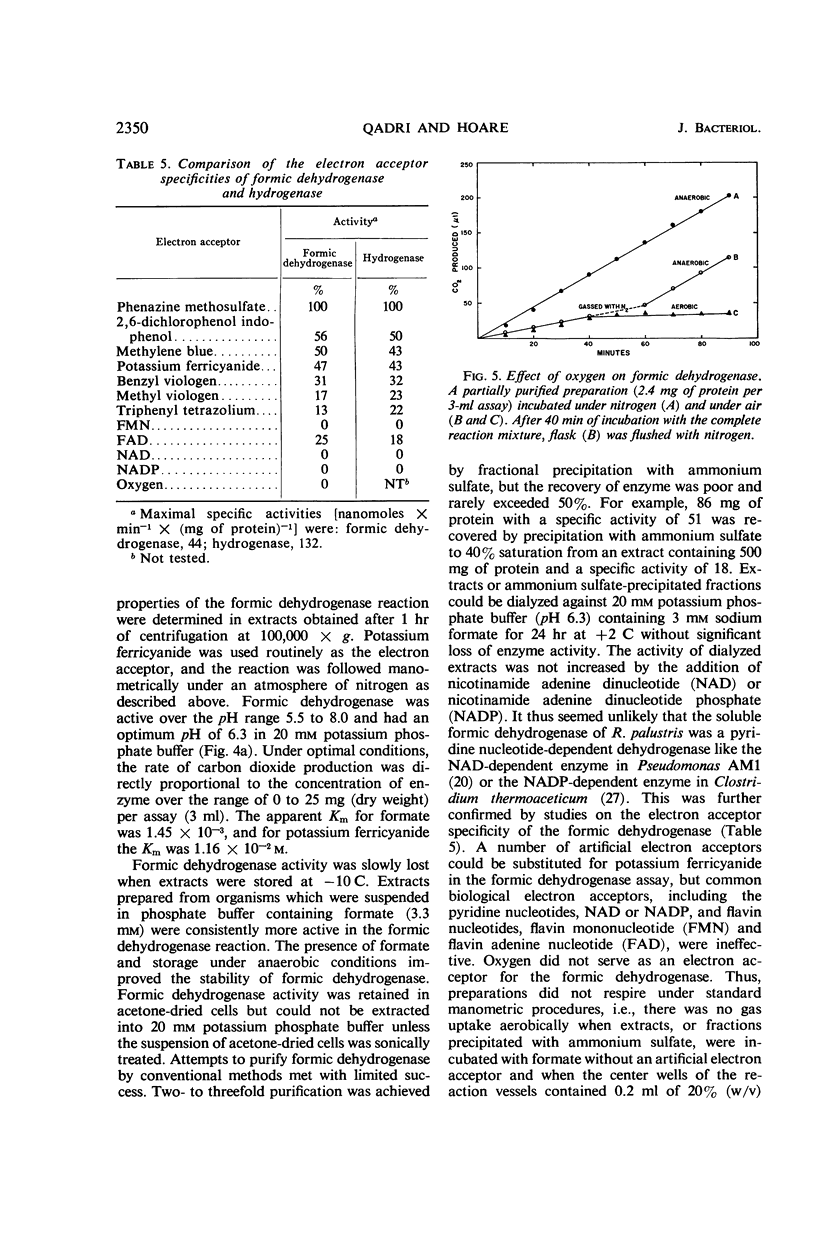
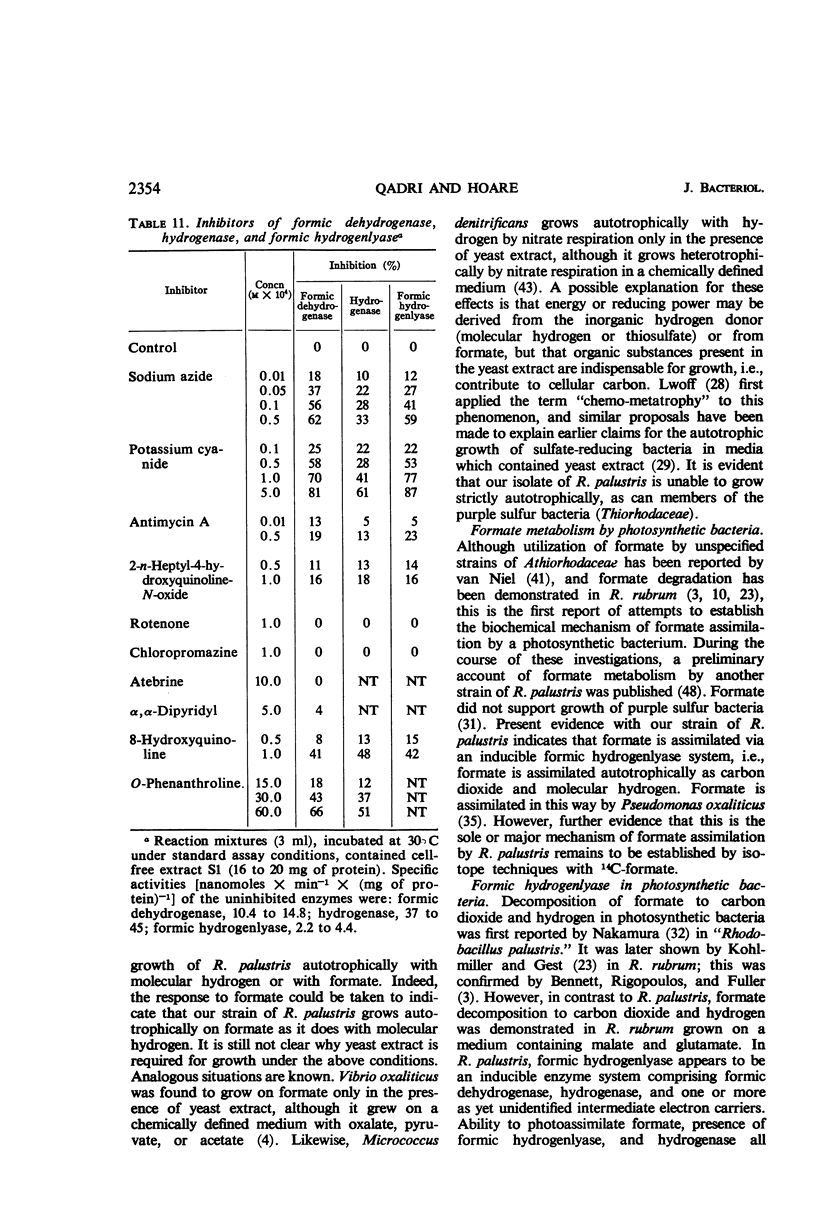
Abstract
A photosynthetic bacterium isolated by enrichment on media containing formate as major source of cell carbon was identified as a strain of Rhodopseudomonas palustris. It grew on a wide range of simple organic compounds including alcohols, fatty acids, and hydroxyacids, on a chemically defined medium with biotin and p-aminobenzoic acid as essential growth factors. The organism grew on formate or photoautotrophically with molecular hydrogen or thiosulfate only in the presence of yeast extract. Ability to photoassimilate formate could be shown only in organisms grown in the presence of formate. The organism contained an inducible formic hydrogenlyase consisting of a soluble formic dehydrogenase, a particulate hydrogenase, and one or more intermediate, but as yet unidentified, electron carriers. The formic hydrogenlyase could be reconstituted from a particulate hydrogenase and a partially purified soluble formic dehydrogenase. Some properties of the formic dehydrogenase and hydrogenase have been compared with that of the formic hydrogenlyase system.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The prosthetic group of the alcohol dehydrogenase of Pseudomonas sp. M27: a new oxidoreductase prosthetic group. Biochem J. 1967 Sep;104(3):960–969. doi: 10.1042/bj1040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT R., RIGOPOULOS N., FULLER R. C. THE PYRUVATE PHOSPHOROCLASTIC REACTION AND LIGHT-DEPENDENT NITROGEN FIXATION IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:762–768. doi: 10.1073/pnas.52.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSE S. K., GEST H. Hydrogenase and light-stimulated electron transfer reactions in photosynthetic bacteria. Nature. 1962 Sep 22;195:1168–1171. doi: 10.1038/1951168a0. [DOI] [PubMed] [Google Scholar]
- BRILL W. J., WOLIN E. A., WOLFE R. S. ANAEROBIC FORMATE OXIDATION: A FERREDOXIN-DEPENDENT REACTION. Science. 1964 Apr 17;144(3616):297–298. doi: 10.1126/science.144.3616.297. [DOI] [PubMed] [Google Scholar]
- Bennett R., Fuller R. C. The pyruvate phosphoroclastic reaction in Chromatium. A probable role for ferredoxin in a photosynthetic bacterium. Biochem Biophys Res Commun. 1964 Jul 1;16(4):300–307. doi: 10.1016/0006-291x(64)90030-0. [DOI] [PubMed] [Google Scholar]
- Bhat J. V., Barker H. A. Studies on a New Oxalate-Decomposing Bacterium, Vibrio oxaliticus. J Bacteriol. 1948 Mar;55(3):359–368. doi: 10.1128/jb.55.3.359-368.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKLERK H., BARTSCH R. G., KAMEN M. D. ATYPICAL SOLUBLE HAEM PROTEINS FROM A STRAIN OF RHODOPSEUDOMONAS PALUSTRIS SP. Biochim Biophys Acta. 1965 Feb 15;97:275–280. doi: 10.1016/0304-4165(65)90092-9. [DOI] [PubMed] [Google Scholar]
- ELSDEN S. R., ORMEROD J. G. The effect of monofluoroacetate on the metabolism of Rhodospirillum rubrum. Biochem J. 1956 Aug;63(4):691–701. doi: 10.1042/bj0630691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H. Metabolic patterns in photosynthetic bacteria. Bacteriol Rev. 1951 Dec;15(4):183–210. doi: 10.1128/br.15.4.183-210.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEST H., PECK H. D., Jr A study of the hydrogenlyase reaction with systems derived from normal and anaerogenic coli-aerogenes bacteria. J Bacteriol. 1955 Sep;70(3):326–334. doi: 10.1128/jb.70.3.326-334.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., WIMPENNY J. W., HUGHES D. E., RANLETT M. A soluble c-type cytochrome from anaerobically grown Escherichia coli and various Enterobacteriaceae. Biochim Biophys Acta. 1963 Jan 8;67:157–160. doi: 10.1016/0006-3002(63)91809-2. [DOI] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- HUTNER S. H. Anaerobic and aerobic growth of purple bacteria (Athiorhodaceae) in chemically defined media. J Gen Microbiol. 1950 Sep;4(3):286–293. doi: 10.1099/00221287-4-3-286. [DOI] [PubMed] [Google Scholar]
- Henderson R. W., Nankiville D. D. Electrophoretic and other studies on haem pigments from Rhodopseudomonas palustris: cytochrome 552 and cytochromoid c. Biochem J. 1966 Feb;98(2):587–593. doi: 10.1042/bj0980587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. H. Organic Growth Essentials of the Aerobic Nonsulfur Photosynthetic Bacteria. J Bacteriol. 1946 Aug;52(2):213–221. doi: 10.1128/jb.52.2.213-221.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS N. J., WOLIN M. J. Electron-transport system of Vibrio succinogenes. I. Enzymes and cytochromes of electron-transport system. Biochim Biophys Acta. 1963 Jan 1;69:18–28. doi: 10.1016/0006-3002(63)91221-6. [DOI] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., LASCELLES J. The formation of isocitratase by the Athiorhodaceae. J Gen Microbiol. 1960 Dec;23:511–517. doi: 10.1099/00221287-23-3-511. [DOI] [PubMed] [Google Scholar]
- LEIFSON E. Staining, shape and arrangement of bacterial flagella. J Bacteriol. 1951 Oct;62(4):377–389. doi: 10.1128/jb.62.4.377-389.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. F., Ljungdahl L., Wood H. G. Properties of Nicotinamide Adenine Dinucleotide Phosphate-Dependent Formate Dehydrogenase from Clostridium thermoaceticum. J Bacteriol. 1966 Aug;92(2):405–412. doi: 10.1128/jb.92.2.405-412.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECHALAS B. J., RITTENBERG S. C. Energy coupling in Desulfovibrio desulfuricans. J Bacteriol. 1960 Oct;80:501–507. doi: 10.1128/jb.80.4.501-507.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., GEST H. Symposium on metabolism of inorganic compounds. IV. Hydrogen photosynthesis and alternative metabolic pathways in photosynthetic bacteria. Bacteriol Rev. 1962 Mar;26:51–66. doi: 10.1128/br.26.1.51-66.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J. R., KEECH D. B. Carbon assimilation by Pseudomonas oxalaticus (OX 1). 1. Formate and carbon dioxide utilization during growth on formate. Biochem J. 1959 Aug;72:623–630. doi: 10.1042/bj0720623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls J. P., Lindstrom E. S. Effect of thiosulfate on the photosynthetic growth of Rhodopseudomonas palustris. J Bacteriol. 1967 Oct;94(4):860–869. doi: 10.1128/jb.94.4.860-866.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls J. P., Lindstrom E. S. Induction of a thiosulfate-oxidizing enzyme in Rhodopseudomonas palustris. J Bacteriol. 1967 Sep;94(3):784–785. doi: 10.1128/jb.94.3.784-785.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenlyases: Bacterial enzymes liberating molecular hydrogen. Biochem J. 1932;26(3):712–724. doi: 10.1042/bj0260712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERHOEVEN W., KOSTER A. L., VAN NIEVELT M. C. Studies on true dissimilatory nitrate reduction. III. Micrococcus denitrificans Beijerinck, a bacterium capable of using molecular hydrogen in denitrification. Antonie Van Leeuwenhoek. 1954;20(3):273–284. doi: 10.1007/BF02543730. [DOI] [PubMed] [Google Scholar]
- Wertlieb D., Vishniac W. Methane utilization by a strain of Rhodopseudomonas gelatinosa. J Bacteriol. 1967 May;93(5):1722–1724. doi: 10.1128/jb.93.5.1722-1724.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., McLee A. G. Rhodopseudomonas palustris and Rh. viridis--photosynthetic budding bacteria. Arch Mikrobiol. 1967;59(1):324–334. doi: 10.1007/BF00406346. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., KAMEN M. D. PURIFICATION OF AN NADP-REDUCTASE AND OF FERREDOXIN DERIVED FROM THE FACULTATIVE PHOTOHETEROTROPH, RHODOPSEUDOMONAS PALUSTRIS. Biochem Biophys Res Commun. 1965 Feb 17;18:611–616. doi: 10.1016/0006-291x(65)90799-0. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Lindstrom E. S. Photosynthetic conversion of formate and CO2 to glutamate by rhodopseudomonas palustris. Biochem Biophys Res Commun. 1967 Jul 10;28(1):65–69. doi: 10.1016/0006-291x(67)90407-x. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]




