Abstract
Decline in landscape complexity owing to agricultural intensification may affect biodiversity, food web complexity and associated ecological processes such as biological control, but such relationships are poorly understood. Here, we analysed food webs of cereal aphids, their primary parasitoids and hyperparasitoids in 18 agricultural landscapes differing in structural complexity (42–93% arable land). Despite little variation in the richness of each trophic group, we found considerable changes in trophic link properties across the landscape complexity gradient. Unexpectedly, aphid–parasitoid food webs exhibited a lower complexity (lower linkage density, interaction diversity and generality) in structurally complex landscapes, which was related to the dominance of one aphid species in complex landscapes. Nevertheless, primary parasitism, as well as hyperparasitism, was higher in complex landscapes, with primary parasitism reaching levels for potentially successful biological control. In conclusion, landscape complexity appeared to foster higher parasitism rates, but simpler food webs, thereby casting doubt on the general importance of food web complexity for ecosystem functioning.
Keywords: food webs, biological control, landscape complexity, parasitoids, hyperparasitoids
1. Introduction
In agricultural landscapes, the loss of semi-natural habitats and the fragmentation and degradation of remaining habitat remnants may reduce biodiversity and associated ecosystem processes [1–5], but can also promote species groups via higher productivity or specific resources provided by agriculture [5,6]. Higher trophic level organisms can be expected to be at a disadvantage in anthropogenically fragmented habitats when they exhibit traits such as a small body size and low dispersal ability, high resource specialization or high population size variability [7,8]. Furthermore, even when species richness is unaffected by agricultural intensification, the structure of the food web interactions may change [9], and this may affect biological control. However, the relationship of food web structure and ecological processes, such as biological control is poorly understood and has been so far largely ignored. Moreover, it is even less clear how these relationships change across landscapes differing in structure and community composition [10,11]. There is experimental evidence for pest suppression in agricultural systems by diverse enemy communities [12–15], but this is also documented in simplified habitats and by less species-rich enemy communities [16–18]. For example, Rodriguez & Hawkins [19] found no effect of parasitoid richness on pest suppression, probably owing to a low-resource complementarity and/or strong bottom-up control. By contrast, species richness and parasitism rates are often positively related [20], but such relationships may not be causal as the dynamics of systems are often driven by one or few species [21].
Biological control of aphids is an important ecosystem service as aphids are one of the major pests in cereal fields in Europe [22–24]. Naturally occurring parasitoids have been shown to be important in suppressing aphid abundances [14,23]. Their populations are enhanced in agricultural landscapes with a high percentage of semi-natural habitats providing shelter from agricultural practices, alternative hosts and flower resources [23,25,26]. However, hyperparasitoids may disrupt biological control of aphids mediated by primary parasitoids [27], and the effects of landscape complexity on this fourth trophic level remain largely unexplored. Hence, it is necessary to analyse biological aphid pest control in a multi-trophic context [11,13], and more specifically, to assess the impact of the fourth trophic level on the third trophic level in changing landscapes, and whether and how these effects cascade down within food webs.
Here, we examined food webs of cereal aphids, their primary parasitoids and hyperparasitoids in 18 winter wheat fields in Germany across landscapes differing in structural complexity (42–93% arable land). We used recently developed quantitative, weighted descriptors of food web complexity [28] that are more accurate, more robust to differences in sampling effort and less sensitive to among system differences, compared with their qualitative counterparts [29,30]. They account for variation in link magnitude and energetic importance of each species in a community. Increasingly used in the last decade, these methods have been shown to provide a powerful tool with which to explore the structure of ecological communities and their responses to environmental factors that may not be revealed by analyses of species richness per se [9,31–34]. Here, we analysed four of these quantitative metrics (generality, vulnerability, interaction diversity, linkage density) as well as the mortality rates of primary and hyperparasitoids to test the functional significance of these descriptors and their response to decline in landscape complexity. We expected that: (i) a decline in landscape complexity would lead to lower species richness, with stronger effect on higher trophic levels; (ii) food web complexity would decrease as species richness decreases in simple landscapes; and (iii) the simpler the food web, the lower parasitism rates would be.
2. Methods
(a). The organisms
The most dominant aphids (Hemiptera: Sternorrhyncha) in winter wheat in Germany are Sitobion avenae (Fabricius), Metopolophium dirhodum (Walker) and Rhopalosiphum padi (Linnaeus). Cereal aphids are attacked by primary parasitoids in the subfamily Aphidiinae (Braconidae, Ichneumonidea) and family Aphelinidae (Chalcidoidea). Larvae of each species of the primary parasitoids that are commonly found in winter wheat can develop by feeding internally in all three aphid species [35], subsequently killing them and forming a cocoon (referred to as a ‘mummy’). Primary parasitoids are attacked by secondary parasitoids including Alloxystinae (Cynipoidea, Charipidae) that feed internally on a primary larval host within the living aphid (true hyperparasitoids), as well as Pteromalidae (Chalcidoidea) and Megaspilidae (Ceraphronoidea, namely Dendrocerus sp.) that feed externally on the primary or secondary larval parasitoid inside the mummy (mummy parasitoids) [36]. For simplicity, we will refer to both true hyperparasitoids and mummy parasitoids as hyperparasitoids in this paper.
(b). Study design
We analysed a dataset partly used and described in detail by Thies et al. [23], in which the focus was on the effect of landscape complexity on aphid–parasitoid population densities and parasitism rates across different spatial scales. Our study was carried out in 18 conventionally managed winter wheat fields in the surroundings of Göttingen, Lower Saxony, Germany. The most common habitats in the region are intensively used arable fields and patchily distributed semi-natural habitats, such as forest fragments, fallows and grasslands. Proportions of the habitat types were measured in the surrounding of each field. Percentage of arable land in a landscape sector has been shown to be a good indicator of landscape complexity owing to its close correlation with other landscape metrics, such as habitat type diversity [2,37,38]. We used a circle with 1 km diameter around each study field to measure landscape complexity (i.e. the percentage of arable land), as this scale has been found to be appropriate given the low dispersal abilities of cereal aphid parasitoids [23]. Structural complexity of landscapes in this dataset ranged from 42 (structurally complex landscapes) up to 93 per cent arable land (structurally simple landscapes). Land-use intensity (i.e. the amount of nitrogen fertilizers and pesticides used) was not related to landscape complexity (see [23]). The average temperature (°C) and total rainfall (millimetres) during the study period from June to July 2001 were 13.9°C, 59.9 mm in June and 18.4°C, 68.8 mm in July (data from the meteorological station in Göttingen). Sampling was conducted in each field after the main period of aphid reproduction in July (wheat milk-ripening) in an insecticide-free area of 800 m2, reaching 40 m along the field edge and 20 m into the fields. Aphids and mummies (parasitized aphids) were visually quantified on 100 wheat shoots per field. Additionally, aphid mummies were collected for 2 h per field during the milk-ripening period and reared in the laboratory to identify primary and hyperparasitoid genera. Hyperparasitoid–primary parasitoid genera relationships were identified using typical mummy morphologies induced by primary parasitoids [35]. Thus, links between food web members were fully quantified, which makes this economically important system a good ecological model system for investigating multi-trophic interactions [39].
Quantitatively weighted food web metrics (linkage density, generality, vulnerability, interaction diversity) were calculated following Bersier et al. [28] (for details refer to the electronic supplementary material, methods S1). Quantitative vulnerability is the mean number of consumers per host species and quantitative generality is the mean number of host species per consumer species. Quantitative linkage density is the mean number of links per species and quantitative interaction diversity is a measure of Shannon diversity of interactions taking the number as well as the evenness of interactions into account. These metrics are often used to represent measures of food web complexity [30,40,41]. Parasitism rates were calculated as the proportion of mummies from all aphids (including mummies) and the proportion of hyperparasitoid mummies from all mummies (including primary and hyperparasitoids).
(c). Statistical analysis
We used general linear models to test the effect of landscape complexity on food web metrics as well as primary parasitism and hyperparasitism rates, while controlling for genera richness of hosts and consumers by including them in the models before arable land (the measure of landscape complexity) following Tylianakis et al. [9]. Thus, we accounted for the effect of variation in genera richness across different landscapes on food web metrics and parasitism rates. Overall variance in the response variables was quantified by using type I sum of squares. Additionally, we tested the influence of food web topologies on parasitism rates for primary and hyperparasitoids. Residuals of the models were tested for normality of errors and homogeneity of variance. (log + 1)-transformations or reciprocal transformations were used for genera richness and food web metrics, and arcsine square-root transformation for percentages (when necessary), to meet assumptions of the approach. To account for nonlinearity, models were also tested by including quadratic terms of explanatory variables. The best-fit models were chosen according to the Akaike information criterion (AIC). We found no hyperparasitoids in two fields, thus we excluded these fields from primary-hyperparasitoid food web analysis. All models were tested for spatial autocorrelation in the residuals using Moran's I statistic, and marginally significant (p = 0.049) spatial autocorrelation was present in only one model (for the effect of generality on parasitism rates). We used a generalized least squares model with exponential spatial correlation structure (which was the best-fit choice among other correlation structures according to AIC) to successfully account for spatial autocorrelation in this model, and the model results remained very similar.
We used path analysis (a form of structural equation modelling (SEM)) to evaluate pathways of direct and indirect effects of landscape structural complexity on parasitism and hyperparasitism rates (see the electronic supplementary material, methods S2). Indirect effects mediated by genera richness and food web structure on parasitism rate were tested in separate models for primary and hyperparasitoids. We report these results with caution because our sample size was relatively small. In addition, we used bootstrapping methods to estimate standard errors and to avoid the large sample assumptions [42].
Statistical analyses were performed using the statistical software R V. 2.8.0 [43], and the packages ‘bipartite’ (for food web analysis, [44,45]) and ‘SEM’ [46].
3. Results
Genera abundance and food web metrics varied considerably across the landscape complexity gradient (for an overview, see table 1 and electronic supplementary material, table S1). Aphid communities were dominated by S. avenae, whose relative abundance decreased with increasing percentage of arable land (Spearman's rank correlation, rs = −0.57, p = 0.01), while that of M. dirhodum increased (Spearman's rank correlation, rs = 0.48, p = 0.04; figure 1). In total, 845 aphids were recorded in all fields, of which 67.7 per cent were S. avenae, 29.6 per cent M. dirhodum and 2.8 per cent R. padi. Absolute aphid abundance did not differ across the landscape gradient. The dominant primary parasitoid genus in the fields was Aphidius with 78.7 per cent of all rearings (emerged parasitoids from mummies) dominant in all landscape types, and among hyperparasitoids, Dendrocerus with 51.7 per cent and Asaphes with 42.7 per cent of all rearings. Relative abundances of primary parasitoid genera did not change, while relative abundance of the hyperparasitoid genus Dendrocerus decreased with increasing percentage of arable land (Spearman's rank correlation, rs = −0.64, p = 0.01). Within the guild of primary parasitoids, only absolute abundance of Ephedrus decreased significantly with percentage of arable land (rs = −0.515, p = 0.029) and in the guild of hyperparasitoids, only Dendrocerus (rs = −0.658, p = 0.006).
Table 1.
Arithmetic means ± s.e., minimum and maximum values (n = 18) of cereal aphid, their primary and hyperparasitoid densities (individuals per 100 shoots).
| individuals per 100 shoots |
|||
|---|---|---|---|
| taxa code | mean ± s.e. | min | max |
| aphids | |||
| 1. S. avenae | 31.74 ± 6.19 | 6.25 | 101.25 |
| 2. M. dirhodum | 13.89 ± 2.93 | 0 | 46.25 |
| 3. R. padi | 1.32 ± 0.29 | 0 | 3.75 |
| primary parasitoids | |||
| 4. Aphidius sp. | 6.94 ± 1.26 | 0 | 19.09 |
| 5. Ephedrus sp. | 1.10 ± 0.39 | 0 | 5.68 |
| 6. Praon sp. | 0.63 ± 0.15 | 0 | 2.05 |
| 7. Aphelinus sp. | 0.15 ± 0.08 | 0 | 1.17 |
| 8. Diaeretiella sp.a | <0.01 | ||
| 9. Toxares sp. | 0.68 ± 0.00 | 0 | 0.68 |
| hyperparasitoids | |||
| 10. Alloxysta sp. | 0.06 ± 0.03 | 0 | 0.42 |
| 11. Phaenoglyphis sp. | 0.07 ± 0.05 | 0 | 0.88 |
| 12. Dendrocerus sp. | 1.33 ± 0.38 | 0 | 5.88 |
| 13. Asaphes sp. | 1.10 ± 0.34 | 0 | 4.81 |
| 14. Coruna sp. | 0.02 ± 0.01 | 0 | 0.23 |
aOnly one individual of Diaeretiella sp. was found (mummy collection data).
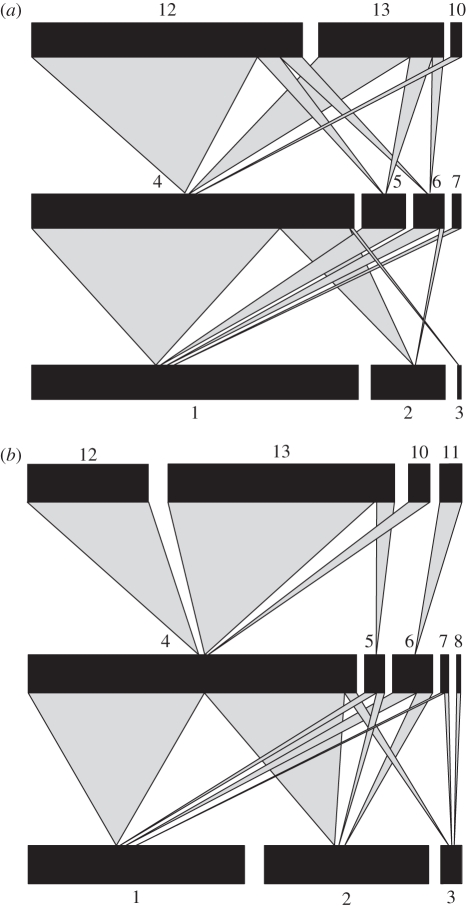
Figure 1.
Parasitoid food webs calculated from pooled data for (a) four landscapes with the lowest (57.6 ± 5.22%; mean ± s.e.) and (b) four landscapes with the highest (90.16 ± 1.23%; mean ± s.e.) percentage arable land. Black bars represent relative abundances of aphids (lower bars), primary parasitoids (middle bars) and hyperparasitoids (upper bars) drawn at different scales. The numbers are genera codes from table 1. Frequency of trophic interactions is indicated by the link width.
We found significant differences in the food web structure across the landscape complexity gradient (figures 1 and 2 and table 2). In aphid–primary parasitoid food webs, linkage density, generality and interaction diversity (figure 2a) increased as the percentage of arable land increased, while vulnerability did not change across the landscape gradient (table 2). Linkage density, interaction diversity and vulnerability were positively influenced by consumer (primary parasitoid) richness, while generality and linkage density were positively influenced by host (aphid) richness.
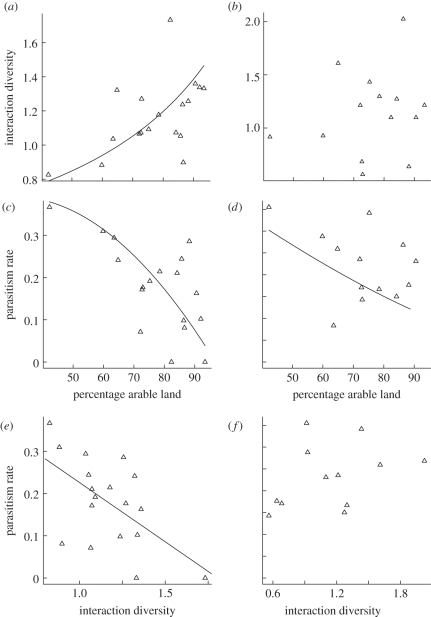
Figure 2.
Interaction diversity and parasitism rates across a landscape complexity gradient and relation of parasitism rate to interaction diversity for (a,c,e) primary and (b,d,f) hyperparasitoid webs.
Table 2.
F-values and levels of significance from general linear models relating parasitism rates and food web metrics (linkage density, interaction diversity, vulnerability and generality) for aphid–primary parasitoid webs and primary-hyperparasitoid webs to three predictive factors: (i) percentage of arable land, (ii) aphid species richness, (iii) parasitoid (or hyperparasitoid) genera richness. (Significant codes: *p < 0.05; **p < 0.01; ***p < 0.001; p > 0.05 n.s.)
| F-value | |
|---|---|
| aphid–primary parasitoid food webs | |
| linkage density | |
| no. of aphid species | 4.74* |
| no. of primary parasitoid genera | 10.38** |
| arable land | 5.77* |
| interaction diversity | |
| no. of aphid species | n.s. |
| no. of primary parasitoid genera | 11.81** |
| arable land | 13.89** |
| vulnerability | |
| no. of aphid species | n.s. |
| no. of primary parasitoid genera | 9.47** |
| arable land | n.s. |
| generality | |
| no. of aphid species | 7.26* |
| no. of primary parasitoid genera | n.s. |
| arable land | 7.41* |
| primary parasitism rate | |
| no. of aphid species | n.s. |
| no. of primary parasitoid genera | 8.32* |
| arable land | 17.44** |
| primary-hyperparasitoid food webs | |
| linkage density | |
| no. of primary parasitoid genera | 12.84** |
| no. of hyperparasitoid genera | 12.37** |
| arable land | n.s. |
| interaction diversity | |
| no. of primary parasitoid genera | 21.37** |
| no. of hyperparasitoid genera | 13.75** |
| no. of hyperparasitoid genera | 5.41* |
| arable land | n.s. |
| vulnerability | |
| no. of primary parasitoid genera | n.s. |
| no. of hyperparasitoid genera | 24.93*** |
| arable land | n.s. |
| generality | |
| no. of primary parasitoid genera | 49.09*** |
| no. of hyperparasitoid genera | n.s. |
| arable land | n.s. |
| hyperparasitism rate | |
| no. of primary parasitoid genera | 53.18*** |
| no. of hyperparasitoid genera | 14.75** |
| arable land | 8.01* |
In primary-hyperparasitoid food webs, food web metrics did not significantly respond to percentage of arable land (see figure 2b for correlation among interaction diversity and percentage arable land), but linkage density and interaction diversity were positively influenced by host (primary parasitoid) and consumer (hyperparasitoid) richness, while vulnerability and generality responded positively only to consumer and host richness, respectively. Richness of all three trophic levels was not correlated to landscape complexity (Spearman's rank correlations: aphid richness: rs = 0.29, p = 0.23; primary parasitoid richness rs = 0.002, p = 0.99; hyperparasitoid richness rs = 0.078, p = 0.76; electronic supplementary material, figure S2).
Overall, S. avenae was the most heavily parasitized species by 67.8 per cent, M. dirhodum by 30.0 per cent and R. padi by 2.2 per cent of all parasitoids (463 mummies in total). The most hyperparasitized primary parasitoid genera were Aphidius 76.6 per cent, Ephedrus 15.3 per cent, Praon 6.4 per cent and Aphelinus 1.6 per cent (124 mummies in total). Aphid mortality owing to parasitism, as well as primary parasitoid mortality owing to hyperparasitism, significantly increased as the percentage of arable land decreased (figure 2c,d and table 2). In aphid–parasitoid food webs, parasitism correlated negatively with interaction diversity (F1,16 = 8.14, p = 0.01; figure 2e) and linkage density (F1,16 = 5.77, p = 0.03). By contrast, in the primary parasitoid–hyperparasitoid webs, hyperparasitism correlated positively with linkage density (F1,11 = 6.82, p = 0.02), generality (F1,11 = 7.73, p = 0.02) and vulnerability (F1,11 = 7.13, p = 0.02), but not with interaction diversity (figure 2f).
The most parsimonious, biologically meaningful models in path analysis for the effect of landscape on parasitism and hyperparasitism rates (before and after bootstrapping), indicated that all significant effects were direct (see the electronic supplementary material, figure S1). There were no indirect effects of landscape mediated by host and consumer richness or food web structural properties (linkage density and interaction diversity) on parasitism and hyperparasitism rates.
4. Discussion
The structure of interactions in aphid–parasitoid–hyperparasitoid communities showed distinct changes across the landscape complexity gradient and was related to host and consumer richness. In contrast to our expectations, food webs were more complex (i.e. revealed a higher interaction diversity and linkage density) in structurally simple landscapes characterized by high percentages of arable land, while host and consumer genera richness did not respond to landscape complexity. Moreover, complex food webs were negatively related to primary parasitism rate, thereby calling into question the general importance of food web complexity for ecosystem functioning.
(a). Species richness
Ecological theory predicts that insect diversity will increase with increasing vegetation diversity and structural complexity [47–49], which may then spill over to adjacent habitats [50]. In contrast to this common theory and our first hypothesis, we found no differences in richness of any trophic level across the landscape complexity gradient. This has been shown for primary parasitoids [51–53], but not for hyperparasitoids. However, parasitoids and hyperparasitoids are known to respond in a similar way to many of the factors that influence their species richness [21]. Hence, as shown for primary parasitoids, our finding suggests that simple landscapes, dominated by cereal crops, provide large amounts of food resources that may support and sustain diverse hyperparasitoid communities.
(b). Food web complexity
Absence of variation in trophic groups' richness leads us to dismiss our second hypothesis that food web complexity would decrease as species richness decreases in simple landscapes. Food web complexity did change across landscape complexity gradient in aphid–parasitoid webs, but contrary to our expectations, interaction diversity decreased as landscape complexity increased, mainly because of a lower number of unique interactions between aphid and parasitoid species. In particular, trophic interaction between the main aphid (Sitobion) and the main parasitoid genus (Aphidius) dominated the food webs in complex landscapes. Host use by the main parasitoid genus Aphidius in simple landscapes included larger proportions of Metopolophium, whose relative abundances increased while those of Sitobion decreased, resulting in more evenly distributed aphid species in simple landscapes. Landscape structural complexity is positively correlated with percentage of grassland (in our region and at the spatial scale we used for analysis, see [37,54]), and habitats such as grassland may provide a good source for colonization of cereals by grass-hibernating aphid species S. avenae [23,55]. Furthermore, the landscape complexity gradient had no influence on the mean number of consumers per host species (vulnerability), partly because of the absence of significant differences in parasitoid richness and in their relative abundances among landscapes. This suggests that parasitoids may be able to adjust average attack rates on each aphid species to changes in aphid relative abundances, by favouring the dominant species, and keeping vulnerability of aphids constant across landscape types. Hence, landscape complexity changes host range of parasitoids and overall food web complexity in cereal aphid–parasitoid food webs, presumably owing to changes in the structure of aphid communities, thereby triggering bottom-up effects that affect interactions with the next trophic level. This is in agreement with Hawkins [56], who argues that parasitoid communities are likely to be bottom-up controlled (see also Scherber et al. [57]).
In contrast to aphid–primary parasitoid food webs, the structure of parasitoid–hyperparasitoid interactions was not influenced by landscape complexity, but by host and consumer richness. This may be related to the lack of response of parasitoid and hyperparasitoid richness to landscape complexity. In addition, relative abundances of primary parasitoids remained similar across landscapes, diminishing bottom-up effects induced by aphids that can propagate to the fourth trophic level.
(c). Parasitism and hyperparasitism rates
The third hypothesis that the simpler the food web the lower the parasitism rates would be, was partly disproved by our results. In spite of lower food web complexity and narrow host range of primary parasitoids in structurally complex landscapes, parasitism rates in these landscapes reached values that can be effective for biological control [23,58]. These findings are consistent with the studies showing that top-down control is often stronger in simplified food webs dominated by a single link [9,16–18]. However, hyperparasitism rates were positively influenced by both landscape and food web complexity (except for interaction diversity), suggesting that hyperparasitoids might benefit from increased availability of alternative resources (similar to primary parasitoids, see [23,26]), but also from increased host range. Increased parasitism and hyperparasitism rates were not the result of higher aphid densities as they did not change across landscape complexity gradient and may be related to the occurrence of the primary parasitoid genus Ephedrus and the most common hyperparasitoid genus Dendrocerus, whose abundances increased across the landscape complexity gradient. Furthermore, high rates of parasitism in structurally complex landscapes may indirectly benefit from higher relative abundances of the ear-colonizing aphid S. avenae, which is more easily accessible to parasitoids than leaf-colonizing aphid species, and frequently associated with the hyperparasitoid genus Dendrocerus [59]. In addition, specific interactions between these particular species may be fostered owing to the closely related colonization time of wheat fields by S. avenae (later in the season with a time lag of two to four weeks compared with M. dirhodum and R. padi, [22,24]) and the main parasitoid and hyperparasitoid genera, Aphidius and Dendrocerus [60]. However, the main effect of landscape structural complexity on parasitism and hyperparasitism rates was direct rather than indirect via host and consumer richness and food web structure, as indicated by our SEMs.
5. Conclusions
Despite the presence of simplified food webs in structurally complex landscapes and similar host and consumer genera richness among landscapes, complex landscapes supported higher parasitoid densities, causing higher levels of aphid biological control. Hence, food web complexity appeared to be a poor predictor of ecological functioning in aphid–primary parasitoid webs. However, aphid–parasitoid systems are typically characterized by strong population dynamics (boom and bust cycles), and changes in community composition in time [23,61], implying dynamic changes in food web structures among years and regions. Our results represent a snap-shot of the interaction structure of this aphid–parasitoid system. More long-term research would contribute to better understanding the response of multi-trophic systems to agricultural landscape changes.
Acknowledgements
We thank C. Scherber, C. Dennis and two anonymous reviewers for helping in statistical analysis and/or insightful comments on the manuscript. This research was founded by the German Ministry of Research and Education (BMBF). C.F.D. acknowledges funding by the Helmholtz Association (VH-NG 247).
References
- 1.Robinson R. A., Sutherland W. J. 2002. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 39, 157–176 10.1046/j.1365-2664.2002.00695.x (doi:10.1046/j.1365-2664.2002.00695.x) [DOI] [Google Scholar]
- 2.Thies C., Tscharntke T. 1999. Landscape structure and biological control in agroecosystems. Science 285, 893–895 10.1126/science.285.5429.893 (doi:10.1126/science.285.5429.893) [DOI] [PubMed] [Google Scholar]
- 3.Benton T. G., Vickery J. A., Wilson J. D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188 10.1016/S0169-5347(03)00011-9 (doi:10.1016/S0169-5347(03)00011-9) [DOI] [Google Scholar]
- 4.Bianchi F. J. J. A., Booij C. J. H., Tscharntke T. 2006. Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B 273, 1715–1727 10.1098/rspb.2006.3530 (doi:10.1098/rspb.2006.3530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tscharntke T., Klein A. M., Kruess A., Steffan-Dewenter I., Thies C. 2005. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 8, 857–874 10.1111/j.1461-0248.2005.00782.x (doi:10.1111/j.1461-0248.2005.00782.x) [DOI] [Google Scholar]
- 6.Rand T. A., Tylianakis J. M., Tscharntke T. 2006. Spillover edge effects: the dispersal of agriculturally subsidized insect natural enemies into adjacent natural habitats. Ecol. Lett. 9, 603–614 10.1111/j.1461-0248.2006.00911.x (doi:10.1111/j.1461-0248.2006.00911.x) [DOI] [PubMed] [Google Scholar]
- 7.Holt R. D., Lawton J. H., Polis G. A., Martinez N. D. 1999. Trophic rank and the species–area relationship. Ecology 80, 1495–1504 10.1890/0012-9658(1999)080[1495:TRATSA]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[1495:TRATSA]2.0.CO;2) [DOI] [Google Scholar]
- 8.Kruess A., Tscharntke T. 1994. Habitat fragmentation, species loss, and biological control. Science 264, 1581–1584 10.1126/science.264.5165.1581 (doi:10.1126/science.264.5165.1581) [DOI] [PubMed] [Google Scholar]
- 9.Tylianakis J. M., Tscharntke T., Lewis O. T. 2007. Habitat modification alters the structure of tropical host–parasitoid food webs. Nature 445, 202–205 10.1038/nature05429 (doi:10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 10.Loreau M., et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808 10.1126/science.1064088 (doi:10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 11.Memmott J., Alonso D., Berlow E., Dobson A. P., Dunne J. A., Sole R., Weitz J. S. 2006. Biodiversity loss and ecological network structure. In Ecological networks: linking structure to dynamics in food webs (eds Pascual M., Dunne J. A.), pp. 325–347 New York, NY: Oxford University Press [Google Scholar]
- 12.Cardinale B. J., Harvey C. T., Gross K., Ives A. R. 2003. Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 6, 857–865 10.1046/j.1461-0248.2003.00508.x (doi:10.1046/j.1461-0248.2003.00508.x) [DOI] [Google Scholar]
- 13.Letourneau D. K., Jedlicka J. A., Bothwell S. G., Moreno C. R. 2009. Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 573–592 10.1146/annurev.ecolsys.110308.120320 (doi:10.1146/annurev.ecolsys.110308.120320) [DOI] [Google Scholar]
- 14.Schmidt M. H., Lauer A., Purtauf T., Thies C., Schaefer M., Tscharntke T. 2003. Relative importance of predators and parasitoids for cereal aphid control. Proc. R. Soc. Lond. B 270, 1905–1909 10.1098/rspb.2003.2469 (doi:10.1098/rspb.2003.2469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder W. E., Ives A. R. 2003. Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84, 91–107 10.1890/0012-9658(2003)084[0091:IBSAGN]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0091:IBSAGN]2.0.CO;2) [DOI] [Google Scholar]
- 16.Finke D. L., Denno R. F. 2004. Predator diversity dampens trophic cascades. Nature 429, 407–410 10.1038/nature02554 (doi:10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- 17.Hawkins B. A., Mills N. J., Jervis M. A., Price P. W. 1999. Is the biological control of insects a natural phenomenon? Oikos 86, 493–506 10.2307/3546654 (doi:10.2307/3546654) [DOI] [Google Scholar]
- 18.Montoya J. M., Rodriguez M. A., Hawkins B. A. 2003. Food web complexity and higher-level ecosystem services. Ecol. Lett. 6, 587–593 10.1046/j.1461-0248.2003.00469.x (doi:10.1046/j.1461-0248.2003.00469.x) [DOI] [Google Scholar]
- 19.Rodriguez M. A., Hawkins B. A. 2000. Diversity, function and stability in parasitoid communities. Ecol. Lett. 3, 35–40 10.1046/j.1461-0248.2000.00115.x (doi:10.1046/j.1461-0248.2000.00115.x) [DOI] [Google Scholar]
- 20.Hawkins B. A., Gagné R. J. 1989. Determinants of assemblage size for the parasitoids of Cecidomyiidae (Diptera). Oecologia 81, 75–88 10.1007/BF00377013 (doi:10.1007/BF00377013) [DOI] [PubMed] [Google Scholar]
- 21.Hawkins B. A. 1994. Pattern and process in host–parasitoid interactions. London, UK: Cambridge University Press [Google Scholar]
- 22.Ankersmit G. W., Carter N. 1981. Comparison of the epidemiology of Methopolophium dirhodum and Sitobion avenae on winter wheat. Neth. J. Plant Pathol. 87, 71–81 10.1007/BF01976641 (doi:10.1007/BF01976641) [DOI] [Google Scholar]
- 23.Thies C., Roschewitz I., Tscharntke T. 2005. The landscape context of cereal aphid–parasitoid interactions. Proc. R. Soc. B 272, 203–210 10.1098/rspb.2004.2902 (doi:10.1098/rspb.2004.2902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vickerman G. P., Wratten S. D. 1979. The biology and pest status of cereal aphids (Hemiptera: Aphididae) in Europe: a review. B. Entomol. Res. 69, 1–32 10.1017/S0007485300017855 (doi:10.1017/S0007485300017855) [DOI] [Google Scholar]
- 25.Landis D. A., Wratten S. D., Gurr G. M. 2000. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 45, 175–201 10.1146/annurev.ento.45.1.175 (doi:10.1146/annurev.ento.45.1.175) [DOI] [PubMed] [Google Scholar]
- 26.Roschewitz I., Hücker M., Tscharntke T., Thies C. 2005. The influence of landscape context and farming practices of cereal aphids. Agricult. Ecosyst. Environ. 108, 218–227 10.1016/j.agee.2005.02.005 (doi:10.1016/j.agee.2005.02.005) [DOI] [Google Scholar]
- 27.Rosenheim J. A. 1998. Higher-order predators and the regulation of insect herbivore populations. A. Rev. Entomol. 43, 421–447 10.1146/annurev.ento.43.1.421 (doi:10.1146/annurev.ento.43.1.421) [DOI] [PubMed] [Google Scholar]
- 28.Bersier L. F., Banašek-Richter C., Cattin M. F. 2002. Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407 10.1890/0012-9658(2002)083[2394:QDOFWM]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[2394:QDOFWM]2.0.CO;2) [DOI] [Google Scholar]
- 29.Banašek-Richter C., Cattin M. F., Bersier L. F. 2004. Sampling effects and the robustness of quantitative and qualitative food-web descriptors. J. Theor. Biol. 226, 23–32 10.1016/S0022-5193(03)00305-9 (doi:10.1016/S0022-5193(03)00305-9) [DOI] [PubMed] [Google Scholar]
- 30.Banašek-Richter C., et al. 2009. Complexity in quantitative food webs. Ecology 90, 1470–1477 10.1890/08-2207.1 (doi:10.1890/08-2207.1) [DOI] [PubMed] [Google Scholar]
- 31.Albrecht M., Duelli P., Schmid B., Müller C. B. 2007. Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. J. Anim. Ecol. 76, 1015–1025 10.1111/j.1365-2656.2007.01264.x (doi:10.1111/j.1365-2656.2007.01264.x) [DOI] [PubMed] [Google Scholar]
- 32.Bukovinszky T., Van Veen F. J. F., Jongema Y., Dicke M. 2008. Direct and indirect effects of resource quality on food web structure. Science 319, 804–807 10.1126/science.1148310 (doi:10.1126/science.1148310) [DOI] [PubMed] [Google Scholar]
- 33.Murakami M., Hirao T., Kasei A. 2008. Effects of habitat configuration on host–parasitoid food web structure. Ecol. Res. 23, 1039–1049 10.1007/s11284-008-0478-0 (doi:10.1007/s11284-008-0478-0) [DOI] [Google Scholar]
- 34.Van Veen F. J. F., Müller C. B., Pell J. K., Godfray H. C. J. 2008. Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphids. J. Anim. Ecol. 77, 91–200 10.1111/j.1365-2656.2007.01325.x (doi:10.1111/j.1365-2656.2007.01325.x) [DOI] [PubMed] [Google Scholar]
- 35.Powell W. 1982. The identification of hymenopterous parasitoids attacking cereal aphids in Britain. Syst. Entomol. 7, 465–473 10.1111/j.1365-3113.1982.tb00457.x (doi:10.1111/j.1365-3113.1982.tb00457.x) [DOI] [Google Scholar]
- 36.Sullivan D. J. 1988. Hyperparasites. In Aphids, their biology, natural enemies and control, vol. B (eds Minks A. K., Harrewijn P.), pp. 189–203 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 37.Schmidt M. H., Thies C., Tscharntke T. 2004. Landscape context of arthropod biological control. In Ecological engineering for pest management: advances in habitat manipulation for arthropods (eds Gurr G. M., Wratten S. D., Altieri M. A.), pp. 55–63 Melbourne, Australia: CSIRO Publications [Google Scholar]
- 38.Steffan-Dewenter I., Münzenberg U., Bürger C., Thies C., Tscharntke T. 2002. Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83, 1421–1432 10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[1421:SDEOLC]2.0.CO;2) [DOI] [Google Scholar]
- 39.Müller C. B., Godfray H. C. J. 1999. Indirect interactions in aphid–parasitoid communities. Res. Popul. Ecol. 41, 93–106 10.1007/PL00011986 (doi:10.1007/PL00011986) [DOI] [Google Scholar]
- 40.Blüthgen N. 2010. Why network analysis is often disconnected from community ecology: a critique and an ecologist's guide. Basic Appl. Ecol. 11, 185–195 10.1016/j.baae.2010.01.001 (doi:10.1016/j.baae.2010.01.001) [DOI] [Google Scholar]
- 41.Neutel A. M., Heesterbeek J. A. P., Van de Koppel J., Hoenderboom G., Vos A., Kaldeway C., Berendse F., de Ruiter P. C. 2007. Reconciling complexity with stability in naturally assembling food webs. Nature 449, 599–602 10.1038/nature06154 (doi:10.1038/nature06154) [DOI] [PubMed] [Google Scholar]
- 42.Grace J. B. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press [Google Scholar]
- 43.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r-project.org [Google Scholar]
- 44.Dormann C., Gruber B., Fründ J. 2008. Introducing the bipartite package: analysing ecological networks. R News 8, 8–11 [Google Scholar]
- 45.Dormann C., Fründ J., Blüthgen N., Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24 10.2174/1874213000902010007 (doi:10.2174/1874213000902010007) [DOI] [Google Scholar]
- 46.Fox J. 2006. Structural equation modeling with the SEM package in R. Struct. Equ. Modeling 13, 465–486 10.1207/s15328007sem1303_7 (doi:10.1207/s15328007sem1303_7) [DOI] [Google Scholar]
- 47.Lawton J. H. 1983. Plant architecture and the diversity of phytophagous insects. Annu. Rev. Entomol. 28, 23–39 10.1146/annurev.en.28.010183.000323 (doi:10.1146/annurev.en.28.010183.000323) [DOI] [Google Scholar]
- 48.Murdoch W. W., Evans F. C., Peterson C. H. 1972. Diversity and pattern in plants and insects. Ecology 53, 819–829 10.2307/1934297 (doi:10.2307/1934297) [DOI] [Google Scholar]
- 49.Stinson C. S. A., Brown V. K. 1983. Seasonal changes in the architecture of natural plant communities and its relevance to insect herbivores. Oecologia (Berlin) 56, 67–69 10.1007/BF00378218 (doi:10.1007/BF00378218) [DOI] [PubMed] [Google Scholar]
- 50.Tscharntke T., Rand T. A., Bianchi F. J. J. A. 2005. The landscape context of trophic interactions: insect spillover across the crop–non-crop interface. Ann. Zool. Fenn. 42, 421–432 [Google Scholar]
- 51.Marino P. C., Landis D. A. 1996. Effect of landscape structure on parasitoid diversity and parasitism in agroecosystems. Ecol. Appl. 6, 276–284 10.2307/2269571 (doi:10.2307/2269571) [DOI] [Google Scholar]
- 52.Menalled F. D., Marino P. C., Gage S. H., Landis D. A. 1999. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Appl. 9, 634–641 10.1890/1051-0761(1999)009[0634:DALSAP]2.0.CO;2 (doi:10.1890/1051-0761(1999)009[0634:DALSAP]2.0.CO;2) [DOI] [Google Scholar]
- 53.Vollhardt I. M. G., Tscharntke T., Wäckers F. L., Bianchi F. J. J. A., Thies C. 2008. Diversity of cereal aphid parasitoids in simple and complex landscapes. Agr. Ecosyst. Environ. 126, 289–292 10.1016/j.agee.2008.01.024 (doi:10.1016/j.agee.2008.01.024) [DOI] [Google Scholar]
- 54.Purtauf T., Thies C., Ekschmitt K., Wolters V., Dauber J. 2005. Scaling properties of multivariate landscape structure. Ecol. Indic. 5, 295–304 10.1016/j.ecolind.2005.03.016 (doi:10.1016/j.ecolind.2005.03.016) [DOI] [Google Scholar]
- 55.Leather S. R. 1993. Overwintering in six arable aphid pests: a review with particular relevance to pest management. J. Appl. Entomol. 116, 217–233 10.1111/j.1439-0418.1993.tb01192.x (doi:10.1111/j.1439-0418.1993.tb01192.x) [DOI] [Google Scholar]
- 56.Hawkins B. A. 1992. Parasitoid-host food webs and donor control. Oikos 65, 159–162 10.2307/3544898 (doi:10.2307/3544898) [DOI] [Google Scholar]
- 57.Scherber C., et al. 2010. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468, 553–556 10.1038/nature09492 (doi:10.1038/nature09492) [DOI] [PubMed] [Google Scholar]
- 58.Hawkins B. A., Cornell H. V. 1994. Maximum parasitism rates and successful biological control. Science 266, 1886. 10.1126/science.266.5192.1886 (doi:10.1126/science.266.5192.1886) [DOI] [PubMed] [Google Scholar]
- 59.Höller C., Borgemeister C., Haardt H., Powell W. 1993. The relationship between primary parasitoids and hyperparasitoids of cereal aphids: an analysis of field data. J. Anim. Ecol. 62, 12–21 10.2307/5478 (doi:10.2307/5478) [DOI] [Google Scholar]
- 60.Höller C., Christiansen-Weniger P., Micha S. G., Siri N., Borgemeister C. 1991. Hyperparasitoid-aphid and hyperparasitoid–primary parasitoid relationships. Redia 74, 153–161 [Google Scholar]
- 61.Leslie T. W., Van Der Werf W., Bianchi F. J. J. A., Honěk A. 2009. Population dynamics of cereal aphids: influence of a shared predator and weather. Agric. Forest Entomol. 11, 73–82 10.1111/j.1461-9563.2008.00405.x (doi:10.1111/j.1461-9563.2008.00405.x) [DOI] [Google Scholar]