Abstract
Conifers are an excellent group in which to explore how changing ecological interactions may have influenced the allocation of reproductive tissues in seed plants over long time scales, because of their extensive fossil record and their important role in terrestrial ecosystems since the Palaeozoic. Measurements of individual conifer pollen-producing and seed-producing cones from the Pennsylvanian to the Recent show that the relative amount of tissue invested in pollen cones has remained constant through time, while seed cones show a sharp increase in proportional tissue investment in the Jurassic that has continued to intensify to the present day. Since seed size in conifers has remained similar through time, this increase reflects greater investment in protective cone tissues such as robust, tightly packed scales. This shift in morphology and tissue allocation is broadly concurrent with the appearance of new vertebrate groups capable of browsing in tree canopies, as well as a diversification of insect-feeding strategies, suggesting that an important change in plant–animal interactions occurred over the Mesozoic that favoured an increase in seed cone protective tissues.
Keywords: reproductive allocation, conifer, cone, predation
1. Introduction
The allocation of reproductive resources in seed plants can be influenced by a wide variety of ecological interactions, including processes as diverse as investment in attractants and rewards for pollinators [1–5], and trade-offs in seed size associated with different dispersal strategies and life histories [6–9]. While an extensive body of research has explored such relationships among living seed plants, and in living flowering plants in particular, relatively few studies have attempted to directly investigate patterns of reproductive resource allocation in extinct seed plants (e.g. [10,11]). However, a deep historical perspective is important because ancient seed plants, especially Palaeozoic and Early Mesozoic species, lived in ecosystems with no modern analogues and undoubtedly experienced certain kinds of ecological interaction that were very different from those experienced by modern species [12,13]. Identifying major changes in plant reproductive allocation through time may therefore aid in a broader understanding of the evolution of terrestrial ecosystems.
Conifers are an excellent group in which to study changes in reproductive allocation because they have an extensive and well-documented fossil history dating back to the Pennsylvanian [14,15], they are major components of many Palaeozoic, Mesozoic, Cenozoic and modern ecosystems, and they have significant living diversity (approx. 600 living species in six families [16,17]). Reproduction in conifers is also relatively simple; ancient and living conifers bear pollen-producing (or pre-pollen-producing in the earliest conifers [18]) and seed-producing organs in separate, compact structures (pollen cones and seed cones, respectively, although bisexual cones can occur within some populations [19]) and these structures are often preserved in the fossil record with cellular-level details (see [20,21]). However, the amount of tissue allocated to conifer reproductive structures is likely to have changed over time because many key interactions that influence tissue investment in modern conifer reproductive organs were almost certainly absent in earlier ecosystems. For example, pressure from vertebrate seed predators can increase the amount of cone tissue devoted to protection and armament in living conifers [22–24], but the groups responsible for these interactions, such as passerine birds and placental mammals, did not diversify until the Late Cretaceous or Early Cenozoic [25–27].
This study investigates changes in reproductive allocation in conifers over their evolutionary history in relation to shifting ecological contexts, and in particular to potential changes in the interactions between conifers and animal groups. Specifically, I quantify tissue allocation in fossil and living conifer cones by measuring their size and shape, as well as the size of their constituent units and reproductive organs (seeds in seed cones and microsporangia in pollen cones). I then compare patterns and temporal shifts in tissue allocation in both types of cone to major changes in terrestrial ecosystems.
2. Material and methods
Cone length, maximum cone width and cone scale length were measured in pollen cones from 22 Palaeozoic species, 17 Triassic species, 18 Jurassic species, 17 Cretaceous species and 296 extant species. This dataset includes living representatives from all extant families and fossil representatives from four extant families (Araucariaceae, Cupressaceae sensu lato, Pinaceae and Podocarpaceae), and several extinct conifer groups. Parameters for seed cones were measured from 33 Palaeozoic species, 23 Triassic species, 27 Jurassic species, 43 Cretaceous species and 213 extant species. This dataset includes living representatives from the four extant families that produce cones (Araucariaceae, Cupressaceae s.l., Pinaceae and Sciadopityaceae), fossil representatives belonging to these families and to extant families that once produced cones (Podocarpaceae), as well as several extinct conifer groups. In this study, the cone scale refers to the basic iterated unit that composes the reproductive strobilus, regardless of the homologies of those structures. The number and length of the seeds borne on each cone scale were recorded when available, and cones were also scored for whether the shape of the scales was influenced by the growth of adjacent scales (every measurement could not be made on all species owing to preservation; see the electronic supplementary material for full data). Where possible, measurements were based on mature specimens collected or preserved after or during the process of pollen or seed dispersal, and immature cones were not included in the dataset. In order to derive a relationship between linear cone measurements and tissue allocation, the dry mass of mature seed cones from 34 extant conifer species (from the families Cupressaceae and Pinaceae) and mature pollen cones from 49 extant species (from the families Araucariaceae, Cupressaceae, Pinaceae and Sciadopityaceae) were recorded in addition to cone length and width.
Fossil data are based on specimens housed in collections at the Paleobotanical Herbarium at Ohio University (Athens, OH, USA) and the Laboratory of Palaeobotany and Palynology at Utrecht University (Utrecht, Netherlands), as well as published descriptions of taxa. Data from extant conifers are based on herbarium specimens housed in the collections of the Royal Botanic Gardens, Kew (London, UK) and the John G. Searle Herbarium at the Field Museum of Natural History (Chicago, IL, USA). Cone mass data are based on specimens collected from living trees at the Chicago Botanic Garden (Glencoe, IL, USA) and the Morton Arboretum (Lisle, IL, USA). In order to create a consistent dataset, each species is represented by measurements from a single exemplar pollen and/or seed cone, since herbarium collections typically do not contain a broad sample of cones and fossil species are usually known from only a few cones. All statistical analyses were performed using the open-source statistical software R (v. 2.8.1).
3. Results
(a). Extant conifer cones
Pollen and seed cone length and width, taken together to approximate cone volume, are good predictors of cone biomass (R2 values 0.93 and 0.92 for seed cones and pollen cones, respectively; see electronic supplementary material, figure S1) and are therefore useful measures of the total amount of tissue (including cone scale and cone axis tissue) allocated to cones in extant conifers. Pollen cones are proportionally narrower than seed cones in all families of living conifers (figure 1a), resulting in much less tissue investment in individual cones on average (0.057 g cone−1 compared with 6.8 g cone−1).
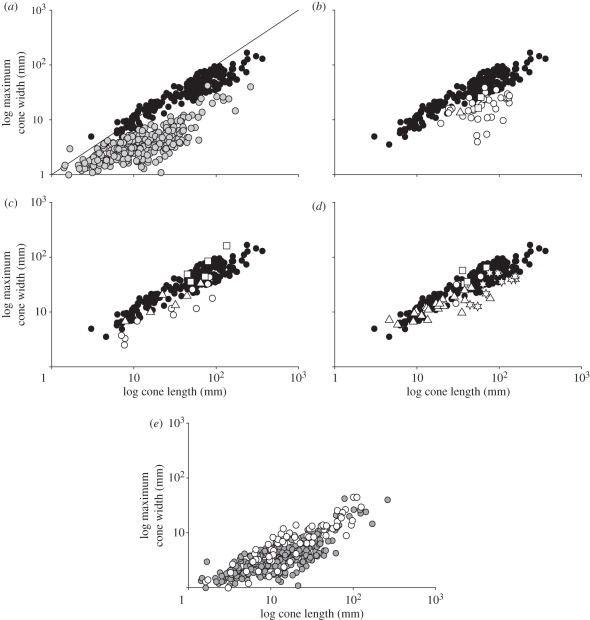
Figure 1.
Relationship between length and maximum width in conifer seed cones and pollen cones through time. Seed cones produced by extinct members of the three major extant cone-producing families are specifically indicated (squares, Araucariaceae; triangles, Cupressaceae sensu lato; stars, Pinaceae). (a) Seed cones (black circles, n = 209) and pollen cones (grey circles, n = 292) of extant species. The line represents a one-to-one relationship between cone length and maximum cone width. (b) Seed cones of Palaeozoic (Pennsylvanian and Permian) and Triassic species (white symbols, n = 39); black circles represent extant seed cones for comparison. (c) Seed cones of Jurassic species (white symbols, n = 21); black circles represent extant seed cones for comparison. (d) Seed cones of Cretaceous species (white symbols, n = 41); black circles represent extant seed cones for comparison. (e) Pollen cones of all fossil species (Pennsylvanian through Cretaceous; white circles, n = 69) and extant species (grey circles).
(b). Fossil conifer cones
Seed cones from ancient conifers show a pronounced temporal shift in the relationship between length and width (figure 1b–d). Most Palaeozoic and Triassic seed cones are similar in shape to extant pollen cones, and are proportionally much narrower than seed cones of extant conifers (figure 1b). However, seed cone shape begins to shift in the Middle Jurassic, especially with the appearance of extremely wide cones produced by members of the Araucariaceae family (figure 1c). Jurassic seed cones are wider than those of earlier conifers on average, although a number of Jurassic taxa produced very narrow cones (figure 1c). There are fewer extremely narrow seed cones in the Cretaceous and most taxa fall within the range of widths seen in modern species, although many early Pinaceae seed cones are narrower than modern representatives (figure 1d). In contrast to seed cones, pollen cones from all time periods are narrow and display a similar relationship between length and width (figure 1e).
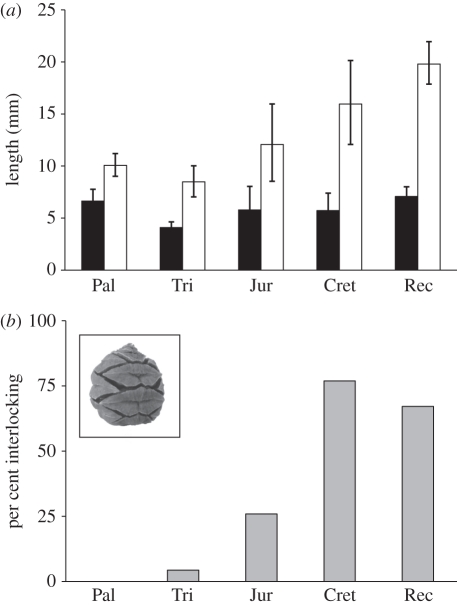
Based on the subset of the data for which both scale length and seed size are known, the shift in average seed cone width in the Jurassic and Cretaceous is associated with an increase in the average length of the cone scales, but not an increase in the average size of the seeds borne by them (figure 2a). These longer cone scales are not associated with an increased number of seeds per scale, either in fossil taxa only or in all taxa considered together (Spearman's ρ = −0.09 and −0.18, respectively). Jurassic and Cretaceous conifers also show a dramatic increase in the number and proportion of species having tightly packed cone scales for which the shape is clearly influenced by the growth of neighbouring scales (figure 2b and inset picture). These species formed compact, tightly sealed structures during the growth and maturation of their cones, in contrast to Palaeozoic and Triassic species, where mature cones were typically elongate or lax with loosely arranged scales. The relative increase in tissue allocation suggested by wider seed cones with longer, more closely packed scales is supported by direct estimates of cone tissue measured from the cross-sectional area of whole cones cut longitudinally (analysed in ImageJ). Although this could only be measured in a few well-preserved specimens, Jurassic and Cretaceous seed cones have more tissue on average per unit cone length (32.0 mm2 mm−1; n = 5 taxa from four families) than Palaeozoic and Triassic seed cones (5.1 mm2 mm−1; n = 6 taxa).
Figure 2.
(a) Mean length of the seed (black bars) and its subtending cone scale (white bars) in conifer species during different time intervals. The number of species per interval is (from left to right) 14, 14, 19, 22 and 94. 95% confidence intervals around the mean were calculated from 10 000 resampled means. (b) Percentage of taxa in each time interval with seed cones composed of compact, tightly interlocking scales. These cones have scales for which the mature shape is determined by the growth of adjacent scales (such as in extant Metasequoia glyptostroboides; see inset photo). Pal, Palaeozoic; Tri, Triassic; Jur, Jurassic; Cret, Cretaceous; Rec, Recent.
4. Discussion
Proportional increases in seed cone tissue allocation appear to have occurred independently within the three major extant cone-producing conifer families (Araucariaceae, Cupressaceae sensu lato and Pinaceae), since even late-diverging groups such as Cupressaceae s.l. [16,17] are known from the Triassic [28,29] and therefore pre-date the shift in tissue allocation. Fossil evidence further suggests that two of the three major cone-producing extant families (Cupressaceae s.l. and Pinaceae) show parallel trends towards increased tissue investment, since fossil members produced narrower seed cones on average than living members (length/width ratios 1.66 to 1.04, respectively, in Cupressaceae and 2.88 to 1.59 in Pinaceae; fossil and living Araucariaceae have similar ratios). Overall, the increase in proportional seed cone tissue investment (figures 1b–d and 2a) and the shift in growth towards more tightly packed cones (figure 2b) suggest that the importance of seed protection in conifers increased over the Mesozoic, with a major shift first occurring between the Early and Middle Jurassic. In contrast, relative tissue allocation in conifer pollen cones has remained similar, most probably because their functional role (releasing pollen into the atmosphere) has remained unchanged over time.
Increasingly strong interactions with animals, especially within the tree canopy, may offer the best explanation for these patterns. The initial shift in tissue investment in the Jurassic is roughly concurrent with the diversification of large sauropod dinosaurs in the Early to Middle Jurassic [30,31] and a consequent increase in vertebrate browsing height [13], although the exact height is debated [32,33]. Intriguingly, the first conifer group to develop truly large cones (Araucariaceae, with Jurassic cones up to 15 cm in diameter) has also been suggested as a primary sauropod food source based on their ubiquity and the relatively high energy content of their foliage [34]. The appearance of early birds and potentially arboreal mammals by the Late Jurassic [26,27], and the subsequent radiation of modern bird groups and small placental mammals in the Early Cenozoic, almost certainly resulted in a further increase in vertebrate seed predation pressures in the canopy, since these groups are effective seed predators in modern ecosystems and are known to cause morphological changes within extant species (such as larger, more robust scales or proportionally more cone tissue relative to seed tissue [22,35–38]) that mirror changes seen in the fossil record on a much broader taxonomic scale. The shift towards increased seed cone tissue allocation also coincides with a significant diversification of insect mouthparts, especially piercing and sucking types, during the Late Triassic to Middle Jurassic [39]. Insects are important conifer seed and seed cone predators in modern ecosystems [40,41], and the evolution of more diverse feeding strategies during this time period, coupled with the appearance of groups such as weevils (which are capable of chewing through tough plant tissues) [39,42], may have further increased the need for more protective tissue in cones.
While the specific environments occupied by conifers have changed over time—especially during the Late Cretaceous and Cenozoic, as they were outcompeted by angiosperms in many tropical and temperate habitats [43,44]—climatic regime does not appear to be the strongest factor influencing the relative amount of tissue in living seed cones, and therefore seems a less likely driver of the pattern in the palaeontologic record. For example, extremely robust cones are produced by species of Araucariaceae living in wet tropical settings as well as species of Pinaceae living in arid temperate regions. The increased frequency of charcoal in many Jurassic and Cretaceous deposits relative to Triassic sediments [45] suggests that an increase in the ecological importance of wildfire could have contributed to the shift towards more heavily armoured conifer cones. However, wildfires were also common in Pennsylvanian and some Permian terrestrial ecosystems [45,46] long before the shift in tissue allocation. Furthermore, while thick cone scales can provide better seed protection in some species of modern fire-adapted conifers [47], resins are the primary means by which cones are sealed [48], and morphological adaptation to fire is not detectable in all fire-adapted species [49].
Regardless of whether the changes in conifer seed cone tissue allocation were primarily caused by biotic or abiotic factors, they suggest a fundamental shift in the kinds of ecological relationships experienced by trees occurred in Jurassic and Cretaceous ecosystems. Conifer seed cone data can therefore provide important insights not only into the evolution of reproductive allocation in seed plants, but also into broader changes in terrestrial ecosystems through time. This also includes potential coevolutionary interactions between extinct plants and animals that must have been important in ancient terrestrial ecosystems but that often leave little direct evidence in the fossil record.
Acknowledgements
This work was supported by a Geological Society of America Graduate Student Award. I would like to thank Han van Konijnenburg-van Cittert, Gar Rothwell and Ruth Stockey for providing access to specimens and for helpful discussions of fossil material, Aljos Farjon and Christine Niezgoda for providing access to herbarium collections, and Andrew Bell and Kunso Kim for access to living conifers. I would like to thank Jason Hilton and an anonymous reviewer whose comments improved the manuscript. I would also like to thank Kevin Boyce, Peter Crane, Michael LaBarbera, David Jablonski and Fred Ruddat, who also contributed helpful suggestions and comments during the course of this study.
References
- 1.Charlesworth D., Charlesworth B. 1987. The effect of investment in attractive structures on allocation to male and female functions in plants. Evolution 41, 948–968 10.2307/2409184 (doi:10.2307/2409184) [DOI] [PubMed] [Google Scholar]
- 2.Harder L. D., Barrett S. C. H. 1995. Mating cost of large floral displays in hermaphodrite plants. Nature 373, 512–515 10.1038/373512a0 (doi:10.1038/373512a0) [DOI] [Google Scholar]
- 3.Biernaskie J. M., Cartar R. V. 2004. Variation in rate of nectar production depends on floral display size: a pollinator manipulation hypothesis. Funct. Ecol. 18, 125–129 10.1111/j.1365-2435.2004.00815.x (doi:10.1111/j.1365-2435.2004.00815.x) [DOI] [Google Scholar]
- 4.Vamosi J. C., Vamosi S. M., Barrett S. C. H. 2006. Sex in advertising: dioecy alters the net benefits of attractiveness in Sagittaria latifolia (Alismataceae). Proc. R. Soc. B 273, 2401–2407 10.1098/rspb.2006.3599 (doi:10.1098/rspb.2006.3599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L.-L., Huang S.-Q. 2007. Evidence for reductions in floral attractants with increase in selfing rates in two heterandrous species. New Phytol. 175, 588–595 10.1111/j.1469-8137.2007.02115.x (doi:10.1111/j.1469-8137.2007.02115.x) [DOI] [PubMed] [Google Scholar]
- 6.Leishman M. R. 2001. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 93, 294–302 10.1034/j.1600-0706.2001.930212.x (doi:10.1034/j.1600-0706.2001.930212.x) [DOI] [Google Scholar]
- 7.Moles A. T., Falster D. S., Leishman M. R., Westoby M. 2004. Small-seed species produce more seeds per square metre of canopy per year, but not per individual lifetime. J. Ecol. 92, 384–396 10.1111/j.0022-0477.2004.00880.x (doi:10.1111/j.0022-0477.2004.00880.x) [DOI] [Google Scholar]
- 8.Moles A. T., Ackerly D. D., Webb C. O., Tweedle J. C., Dickie J. B., Pitman A. J., Westoby M. 2005. Factors that shape seed mass evolution. Proc. Natl Acad. Sci. USA 102, 10 540–10 544 10.1073/pnas.0501473102 (doi:10.1073/pnas.0501473102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolmgren K., Eriksson O. 2010. Seed mass and the evolution of fleshy fruits in angiosperms. Oikos 119, 707–718 10.1111/j.1600-0706.2009.17944.x (doi:10.1111/j.1600-0706.2009.17944.x) [DOI] [Google Scholar]
- 10.Niklas K. J. 1993. The allometry of plant reproductive biomass and stem diameter. Am. J. Bot. 80, 461–467 10.2307/2445392 (doi:10.2307/2445392) [DOI] [Google Scholar]
- 11.Niklas K. J. 1994. Plant allometry: the scaling of form and process. Chicago, IL: University of Chicago Press [Google Scholar]
- 12.DiMichele W. A., Hook R. W. 1992. Paleozoic terrestrial ecosystems. In Terrestrial ecosystems through time (eds Behrensmeyer A. K., et al.), pp. 205–325 Chicago, IL: University of Chicago Press [Google Scholar]
- 13.Wing S. L., Sues D. 1992. Mesozoic and Early Cenozoic terrestrial ecosystems. In Terrestrial ecosystems through time (eds Behrensmeyer A. K., et al.), pp. 327–416 Chicago, IL: University of Chicago Press [Google Scholar]
- 14.Scott A. C., Chaloner W. G. 1983. The earliest fossil conifer from the Westphalian B of Yorkshire. Proc. R. Soc. Lond. B 220, 163–182 10.1098/rspb.1983.0094 (doi:10.1098/rspb.1983.0094) [DOI] [Google Scholar]
- 15.Plotnik R. E., Kenig F., Scott A. C., Glasspool I. J., Eble C. F., Lang W. J. 2009. Pennsylvanian paleokarst and cave fills from Northern Illinois, USA: a window into Late Carboniferous environments and landscapes. Palaios 24, 627–637 10.2110/palo.2009.p09-012r (doi:10.2110/palo.2009.p09-012r) [DOI] [Google Scholar]
- 16.Quinn C. J., Price R. A., Gadek P. A. 2002. Familial concepts and relationships in the conifers based on rbcL and matK sequence comparisons. Kew Bull. 57, 513–531 10.2307/4110984 (doi:10.2307/4110984) [DOI] [Google Scholar]
- 17.Rai H. S., Reeves P. A., Peakall R., Olmstead R. G., Graham S. W. 2008. Inference of higher-order conifer relationships from a multi-locus plastid data set. Botany 86, 658–669 10.1139/B08-062 (doi:10.1139/B08-062) [DOI] [Google Scholar]
- 18.Poort R. J., Visscher H., Dilcher D. L. 1996. Zoidogamy in fossil gymnosperms: the centenary of a concept, with special reference to prepollen of late Paleozoic conifers. Proc. Natl Acad. Sci. USA 93, 11 713–11 717 10.1073/pnas.93.21.11713 (doi:10.1073/pnas.93.21.11713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores-Rentería L., Vázquez-Lobo A., Whipple A. V., Piñero D., Márquez-Guzmán J., Domínguez C. A. 2010. Functional bisporangiate cones in Pinus johannis (Pinaceae): implications for the evolution of bisexuality in seed plants. Am. J. Bot. 98, 130–139 10.3732/ajb.1000275 (doi:10.3732/ajb.1000275) [DOI] [PubMed] [Google Scholar]
- 20.Stockey R. A. 1975. Seeds and embryos of Araucaria mirabilis. Am. J. Bot. 62, 856–868 10.2307/2441898 (doi:10.2307/2441898) [DOI] [Google Scholar]
- 21.Schwendemann A. B., Taylor T. N., Taylor E. L., Krings M. 2010. Organization, anatomy, and fungal endophytes of a Triassic conifer embryo. Am. J. Bot. 97, 1873–1883 10.3732/ajb.1000194 (doi:10.3732/ajb.1000194) [DOI] [PubMed] [Google Scholar]
- 22.Benkman C. W. 1995. The impact of tree squirrels (Tamiasciurus) on Limber Pine seed dispersal adaptations. Evolution 49, 585–592 10.2307/2410312 (doi:10.2307/2410312) [DOI] [PubMed] [Google Scholar]
- 23.Coffey K., Benkman C. W., Milligan B. G. 1999. The adaptive significance of spines on pine cones. Ecology 80, 1221–1229 10.1890/0012-9658(1999)080[1221:TASOSO]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[1221:TASOSO]2.0.CO;2) [DOI] [Google Scholar]
- 24.Benkman C. W., Holimon W. C., Smith J. W. 2001. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55, 282–294 [DOI] [PubMed] [Google Scholar]
- 25.Ericson P. G. P., Christidis L., Cooper A., Irestedt M., Jackson J., Johansson U. S., Norman J. A. 2002. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. Lond. B 269, 235–241 10.1098/rspb.2001.1877 (doi:10.1098/rspb.2001.1877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiappe L. M., Dyke G. J. 2002. The Mesozoic radiation of birds. Annu. Rev. Ecol. Syst. 33, 91–124 10.1146/annurev.ecolsys.33.010802.150517 (doi:10.1146/annurev.ecolsys.33.010802.150517) [DOI] [Google Scholar]
- 27.Luo X. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 10.1038/nature06277 (doi:10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 28.Yao X., Taylor T. N., Taylor E. L. 1997. A taxodiaceous seed cone from the Triassic of Antarctica. Am. J. Bot. 84, 343–354 10.2307/2446008 (doi:10.2307/2446008) [DOI] [PubMed] [Google Scholar]
- 29.Taylor T. N., Taylor E. L., Krings M. 2009. Paleobotany: the biology and evolution of fossil plants. Burlington, MA: Academic Press [Google Scholar]
- 30.Jain S. L., Kutty R. S., Roy Chowdhury T., Chatterjee S. 1975. The sauropod dinosaur from the Lower Jurassic Kota Formation of India. Proc. R. Soc. Lond. B 188, 221–228 10.1098/rspb.1975.0014 (doi:10.1098/rspb.1975.0014) [DOI] [Google Scholar]
- 31.Brusatte S. L., Nesbitt S. J., Irmis R. B., Butler R. J., Benton M. J., Norell M. A. 2010. The origin and early radiation of dinosaurs. Earth Sci. Rev. 101, 68–100 10.1016/j.earscirev.2010.04.001 (doi:10.1016/j.earscirev.2010.04.001) [DOI] [Google Scholar]
- 32.Stevens K. A., Parrish J. M. 1999. Neck posture and feeding habits of two Jurassic sauropod dinosaurs. Science 284, 798–800 10.1126/science.284.5415.798 (doi:10.1126/science.284.5415.798) [DOI] [PubMed] [Google Scholar]
- 33.Seymour R. S., Lillywhite H. B. 2000. Hearts, neck posture and metabolic intensity of sauropod dinosaurs. Proc. R. Soc. Lond. B 267, 1183–1887 10.1098/rspb.2000.1225 (doi:10.1098/rspb.2000.1225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummel J., Gee C. T., Südekum K.-H., Sander P. M., Nogge G., Clauss M. 2008. In vitro digestibility of fern and gymnosperm foliage: implications for sauropod feeding ecology and diet selection. Proc. R. Soc. B 275, 1015–1021 10.1098/rspb.2007.1728 (doi:10.1098/rspb.2007.1728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parchman T. L., Benkman C. W. 2002. Diversifying coevolution between crossbills and black spruce on Newfoundland. Evolution 56, 1663–1672 10.1111/j.0014-3820.2002.tb01478.x (doi:10.1111/j.0014-3820.2002.tb01478.x) [DOI] [PubMed] [Google Scholar]
- 36.Benkman C. W., Parchman T. L., Favis A., Siepielski A. M. 2003. Reciprocal selection causes a coevolutionary arms race between crossbills and lodgepole pine. Am. Nat. 162, 182–194 10.1086/376580 (doi:10.1086/376580) [DOI] [PubMed] [Google Scholar]
- 37.Smith C. C. 1970. The coevolution of Pine Squirrels (Tamiasciurus) and conifers. Ecol. Monogr. 40, 349–371 10.2307/1942287 (doi:10.2307/1942287) [DOI] [Google Scholar]
- 38.Siepielski A. M., Benkman C. W. 2007. Convergent patterns in the selection mosaic for two North American bird-dispersed pines. Ecol. Monogr. 77, 203–220 10.1890/06-0929 (doi:10.1890/06-0929) [DOI] [Google Scholar]
- 39.Labandeira C. C. 1997. Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Syst. 28, 153–193 10.1146/annurev.ecolsys.28.1.153 (doi:10.1146/annurev.ecolsys.28.1.153) [DOI] [Google Scholar]
- 40.Smith C. C., Balda R. P. 1979. Competition among insects, birds, and mammals for conifer seeds. Am. Zool. 19, 1065–1083 [Google Scholar]
- 41.Turgeon J. J., Roques A., de Groot P. 1994. Insect fauna of coniferous seed cones: diversity, host plant interactions, and management. Annu. Rev. Entomol. 39, 179–212 10.1146/annurev.en.39.010194.001143 (doi:10.1146/annurev.en.39.010194.001143) [DOI] [Google Scholar]
- 42.Zherikhin V. V., Gratshev V. G. 1993. Obrieniidae, fam. nov., the oldest Mesozoic weevils (Coleoptera, Curculionoidea). Paleontol. J. 27, 50–69 [Google Scholar]
- 43.Becker P. 2000. Competition in the regeneration niche between conifers and angiosperms: Bond's slow seedling hypothesis. Funct. Ecol. 14, 401–412 10.1046/j.1365-2435.2000.00455.x (doi:10.1046/j.1365-2435.2000.00455.x) [DOI] [Google Scholar]
- 44.Lusk C. H., Wright I., Reich P. B. 2003. Photosynthetic differences contribute to competitive advantage of evergreen angiosperm trees over evergreen conifers in productive habitats. New Phytol. 160, 329–336 10.1046/j.1469-8137.2003.00879.x (doi:10.1046/j.1469-8137.2003.00879.x) [DOI] [PubMed] [Google Scholar]
- 45.Scott A. C. 2000. The pre-Quaternary history of fire. Palaeogeogr. Palaeoclimatol. Palaeoecol. 164, 281–329 10.1016/S0031-0182(00)00192-9 (doi:10.1016/S0031-0182(00)00192-9) [DOI] [Google Scholar]
- 46.Scott A. C., Glasspool I. J. 2006. The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentration. Proc. Natl Acad. Sci. USA 103, 10 861–10 865 10.1073/pnas.0604090103 (doi:10.1073/pnas.0604090103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linhart Y. B. 1978. Maintenance of variation in cone morphology in California closed-cone pines: the roles of fire, squirrels, and seed output. Southwest. Nat. 23, 29–40 10.2307/3669977 (doi:10.2307/3669977) [DOI] [Google Scholar]
- 48.Johnson E. A., Gutsell S. L. 1993. Heat budget and fire behavior associated with the opening of serotinous cones in two Pinus species. J. Veg. Sci. 4, 745–750 10.2307/3235610 (doi:10.2307/3235610) [DOI] [Google Scholar]
- 49.Gil L., Climent J., Nanos N., Mutke S., Ortiz I., Schiller G. 2002. Cone morphology variation in Pinus canariensis Sm. Plant Syst. Evol. 235, 35–51 10.1007/s00606-002-0218-9 (doi:10.1007/s00606-002-0218-9) [DOI] [Google Scholar]