Abstract
Sex-allocation theories generally assume differential fitness costs of raising sons and daughters. Yet, experimental confirmation of such costs is scarce and potential mechanisms are rarely addressed. While the most universal measure of physiological costs is energy expenditure, only one study has related the maternal energy budget to experimentally controlled offspring sex. Here, we experimentally test this in the bank vole (Myodes glareolus) by simultaneously manipulating the litter's size and sex ratio immediately after birth. Two weeks after manipulation, when mothers were at the peak of lactation and were pregnant with concurrent litters, we assessed their energy budget. We found that maternal food consumption and daily energy expenditure increased with the size of the litters being lactated. Importantly, the effects of offspring sex on energy budget depended on the characteristics of the simultaneously gestating litters. Specifically, the mothers nursing all-male litters and concurrently pregnant with male-biased litters had the highest energy expenditure. These had consequences for the next generation, as size of female offspring from the concurrent pregnancy of these mothers was compromised. Our study attests a higher cost of sons, consequently leading to a lower investment in them, and reveals the significance of offspring sex in moulding the trade-off between current and future maternal investment.
Keywords: bank vole, costly sons, daily energy expenditure, doubly labelled water, food consumption, costs of reproduction
1. Introduction
Sex allocation is one of the central topics in evolutionary biology [1–4]. Sex allocation may arise both as manipulation of offspring sex (reviewed by [5] and [6]) and as sex-specific resource allocation (e.g. [7]). The expected relationship between maternal allocation and offspring sex is closely tied to sex-specific requirements. These different investments result from different fitness returns expected from each sex owing to, e.g. mating systems, relative parental attractiveness and environmental factors [8]. Increased maternal investment, as any life-history trait, inevitably comes at a cost. Generally, costs of reproduction are manifested in reduction of subsequent physiological performance, decreased future fecundity and compromised survival (e.g. [9–11]). Any mechanism responsible for the costs of reproduction would probably be involved in mediating fitness consequences of differential investment into sons and daughters (e.g. [12–15]).
One of the most universal measures of maternal effort is energy expenditure directly and indirectly related to raising offspring [11,16]. Indeed, although there is a great interest in energy requirements of provisioning male versus female offspring, earlier investigations have usually involved correlative studies in mammals (e.g. [17–19]). Recently, however, Robert et al. [20] carried out an experimental study on tammar wallabies (Macropus eugenii derbianus) which shows that the capacity of maternal investment is a significant predictor of offspring sex. The only previous study we are aware of, which compared maternal energy expenditure when raising sons and daughters in an experimental setting, was performed on a sexually dimorphic bird: the brown songlark (Cinclorhamphus cruralis). Brown songlark mothers rearing all-male broods ate more food and expended 27 per cent more energy than those provisioning all-female broods [21]. Unfortunately, the authors were not able to assess potential negative consequence of this increased reproductive effort on future performance of the mothers.
Previous studies have suggested that raising offspring of a given sex might have a significant impact on future reproductive performance, especially in mammals (e.g. [13]). They also suggest that in mammals, maternal energy budget may depend on offspring sex, but this has yet to be demonstrated in experimental conditions. Furthermore, no study so far has been able to demonstrate whether and how maternal energy allocation to offspring of a given sex affects her future reproductive performance. The current study contributes by filling these gaps in our understanding. It uses experimental manipulation to answer the questions (i) how maternal energy budget relates to offspring sex, and (ii) how energy allocation to male and female offspring affects mothers' subsequent reproductive output. To answer these questions, we capitalized on an experiment that simultaneously manipulated litter size and sex ratio in the bank vole (Myodes glareolus) [22]. In this previous study, maternal effort was significantly elevated when raising enlarged litters. More importantly, the study revealed that bank vole mothers invested (postnatally) more into their daughters than into sons, which was apparent in greater milk production (estimated using the weigh-suckle-weigh method), more vigorous nest defence and led to a faster growth rate of female compared with male offspring. This allocation tactic was suggested beneficial as a larger size predicts higher reproductive success in female, but not in male bank voles [22]. As our experimental set-up was successful in manipulating various aspects of maternal care, here we expected to find significant effects of litter size and sex treatment on the maternal energy budgets (food consumption and daily energy expenditure (DEE)), corresponding to the observed behavioural effects. We also predicted that females simultaneously pregnant with larger litters in late lactation would have elevated energetic needs, and this effect would be related to the relative value of male and female offspring of the suckling and gestating litters. This is the first study, to our knowledge, that has examined maternal energy budgets in relation to sex ratio of the two generations of offspring.
2. Methods
(a). Study species and experimental design
The bank vole is one of the most common small rodents in Europe. In central Finland, the breeding season lasts from May to mid-September, during which time up to four litters ranging from two to 10 pups (5.3 ± 1.3, mean ± s.e.) can be born [23]. The bank vole mating system is polygynandrous in which males provide no material resources to the female or offspring [24] and females have not been found to adjust the amount of their maternal effort according to the characteristics of males [25]. Females remate in post-partum oestrus and offspring are weaned at around 20 days of age when a subsequent litter is born. The females used in this experiment originated from the third and fourth generations of a captive colony, originally stocked from Konnevesi, central Finland. All females had given birth previously and were of similar age, i.e. on average 9.5 months old. The voles were housed individually in mouse cages (43 × 26 × 15 cm) and maintained on a 16 L : 8 D photoperiod at 20 ± 2°C. Wood shavings and hay were provided as bedding, and food (Labfor 36; Lactamin AB, Stockholm, Sweden) and water were provided ad libitum.
Details of the manipulation of litter size and sex ratio were described in our previous study [22]. In short, after parturition, newborn pups were immediately sexed, individually marked with toe codes and manipulations carried out. At the same time, females were mated in postpartum oestrus with randomly chosen (proven stud) males originating from the first or second generation of the captive colony. The maternal energy budget in late lactation was determined for 50 mothers but the current study concerns 44 females that were later confirmed to be simultaneously pregnant. There was no relationship between experimental treatment and likelihood of pregnancy. Simultaneous lactation and pregnancy is a natural state for females in the wild, exerting high metabolic demands on mothers [26–28]. Experimental litters were created by cross-fostering pups within 1 day of parturition, and they consisted of the same-aged pups, each pup originating from different mothers. Mothers were randomly assigned to two groups of litter sex ratio manipulation and they reared either all-male or all-female litters. Moreover, original litter size was manipulated by either reducing or enlarging by two pups. Using all-male and all-female litters was chosen as the most powerful design when testing for an effect, although the occurrence of single sex litters is relatively rare in natural populations (10% in [23]). In the current study, offspring sex ratio prior to manipulation ranged from all-female to all-male litters and the mean (±s.e.) proportion of male offspring was 0.52 ± 0.03. Average litter size before the manipulation ranged from three to eight pups (5.0 ± 0.19 mean ± s.e.) and after manipulation the range was 1–10 pups (5.0 ± 0.39 mean ± s.e.). Manipulation based on reduction or enlargement of the original litter size immediately after birth has been a classic experimental design when studying the costs of reproduction and other reproductive trade-offs, as well as investigating the effects of offspring number upon parental investment decisions and for quantifying the energy budgets during lactation (e.g. [28–32]). This method is not without complications (e.g. [33]), but can serve as a powerful tool to investigate the mother's allocation decisions between offspring and her own body condition. Bank vole females easily accept foreign pups, as the growth and survival do not differ between the pups that are cared by natural versus foster mothers (e.g. [31]). In five different litters, there were single pups that died at the age of a few days. Those litters were not excluded from the analyses. Throughout the text, ‘mother’ refers to the foster mother (nursing mother) of the offspring.
(b). Measurements
In the current study, we focused on maternal energy budgets during the late lactation period, when the offspring are relatively big, but are still fully dependent on maternal milk production. This is also the period of peak energy requirements in small rodents including voles [28,34,35]. We estimated average daily food consumption of the females over the 8 day period (between day 6 and day 15 of lactation) by weighing food (electronic scale, ±0.1 g) on both days 6 and 15 and dividing the difference by number of hours that have passed between the two measurements and multiplying that by 24. Between days 14 and 15, we measured the mother's DEE (see below).
To explore the potential effect of the maternal energy budget on future offspring, we looked at the number of pups, their sex [22] and body mass (electronic scale, ±0.01 g) at birth in second litters. In six out of 44 litters, we lacked complete information on sex and mass of the pups and thus sample size in some analyses is smaller. To assess potential conflict between maternal allocation into the two generations of the offspring, allocation into the simultaneously gestating litter was studied in relation to the mean mass of pups weaned in the manipulated litters (data from [22]).
(c). Daily energy expenditure
DEE was measured using the doubly labelled water (DLW) technique [36]. Individuals were weighed (electronic scale, ±0.01 g) and labelled with an intraperitoneal injection of approximately 0.2 g of water containing enriched deuterium (4.63 atom%) and oxygen-18 (9.44 atom%). The syringe was weighed before and after the injection (electronic scale, ±0.0001 g) to provide an accurate measurement of the amount of isotope injected. An initial 50–100 µl blood sample was taken from each individual's retro-orbital sinus with capillary tubes (Haematocrit tube, Hirschmann Laborgeräte, Germany) approximately 1 h after the injection, which is the time that has generally been assumed to be required for the isotopes to reach equilibrium [37]. Blood samples were immediately flame-sealed into 50 µl pipettes (Vitrex, Camlab Ltd, Cambridge, UK) until analysis. Blood samples were taken from unlabelled animals to evaluate the background isotope levels ([38], method C). A final blood sample was collected 24 h after the initial sample. Blood samples were vacuum-distilled into glass Pasteur pipettes (Volac, John Poulten Ltd, Barking, UK [39]) and the distillates used for mass spectrometric analysis of stable isotopes. Mass spectrometric analysis of deuterium enrichment was performed using H2 gas. The H2 was produced by the pyrolysis reduction method [40]. For analysis of 18O enrichment in the blood samples, the water distilled from the blood was equilibrated with CO2 gas using the small-sample equilibration technique [41]. DEE was calculated using the single-pool intercept method [42], assuming 25 per cent of water loss was evaporative: [43, eqn (7.17)].
(d). Data analyses
In all analyses, manipulated litter sex was a fixed dichotomous variable (all-male/all-female litters) and manipulated offspring number was a continuous covariate. Treating manipulated litter size as a covariate was based on previous findings that for female energy budget, the manipulated litter size is more important over the original litter size [11]. (Including litter size treatment (−2/+2 pups) in the models does not change any of the results.) Maternal body mass, sex ratio (arcsin transformed) and size of the subsequent litter were treated as covariates. Data were analysed using general linear models (GLMs) and, in case of variation of mass of individual pups, using the general linear mixed model (GLMM), in which mother identity was controlled for as a random factor. The analyses were performed with SAS v. 9.1.
3. Results
At the time of measurement of their energy budgets, maternal body mass did not vary in relation to sex of the current litter (litter sex: F1,37 = 0.26, p = 0.613) but was significantly positively related to litter size (F1,37 = 6.89, p = 0.013) and size of the subsequent litter with which she was simultaneously pregnant (F1,37 = 11.35, p = 0.002). Consequently, we included characteristics of the gestating litter as potential explanatory covariates affecting variation in the maternal energy budget.
(a). Energy intake and expenditure
In general, maternal food consumption was positively related to maternal body mass (F1,41 = 10.31, p = 0.003) and the current litter size (table 1). There was a non-significant difference between litter sex treatments in their response to gestating litter sex ratio shaping variation in food intake (table 1).
Table 1.
GLM analyses of factors explaining variation in maternal food consumption rate between 6 and 15 days after manipulation and daily energy expenditure (DEE) in relation to size and sex ratio of suckling and gestating litters. (All models initially included main effects and all their interactions.)
| source of variation | food consumption |
maternal DEE |
||||
|---|---|---|---|---|---|---|
| estimate ± s.e. | F1,34 | p | estimate ± s.e. | F1,34 | p | |
| intercept | 2.11 ± 1.84 | 29.56 ± 10.10 | ||||
| litter size | 0.98 ± 0.13 | 54.81 | <0.0001 | 2.33 ± 0.72 | 10.31 | 0.003 |
| litter sex | 3.25 ± 1.73 | 3.52 | 0.069 | 19.18 ± 9.52 | 4.06 | 0.052 |
| second litter size | 0.14 ± 0.22 | 0.41 | 0.524 | 3.40 ± 1.23 | 7.59 | 0.009 |
| second litter sex ratio | 2. 82 ± 1.36 | 1.62 | 0.212 | 25.15 ± 7.48 | 4.73 | 0.037 |
| litter sex × second litter sex ratio | −3.45 ± 2.00 | 2.97 | 0.094 | −26.83 ± 10.99 | 5.96 | 0.020 |
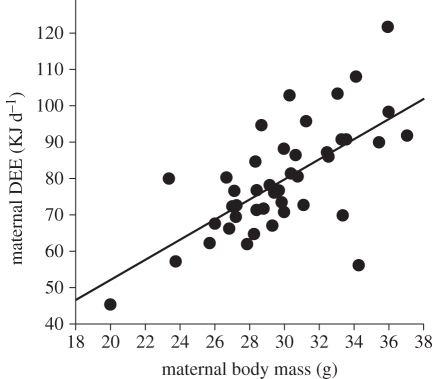
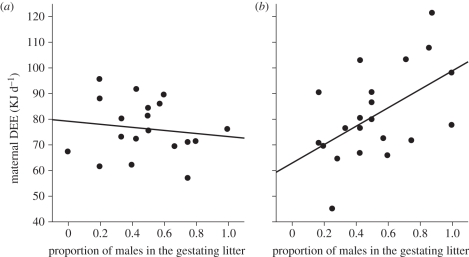
Maternal DEE was significantly positively related to body mass (r = 0.67, F1,42 = 33.89, p < 0.0001; figure 1), food consumption (r = 0.67, F1,42 = 34.57, p < 0.0001) and current and gestating litter sizes (table 1). Litter sex manipulation affected DEE via its significant interaction with sex ratio of the gestating litter (table 1 and figure 2a,b) so that mothers nursing all-male litters and simultaneously gestating male-biased litters had the highest energy expenditure. This was revealed in separate analyses where DEE of mothers nursing all-female litters was related only to the current litter size (F1,25 = 6.54, p = 0.017), but it was not affected by the size and sex ratio of the gestating litter (both p > 0.5). By contrast, DEE of mothers nursing all-male litters was also significantly positively related to litter size (F1,17 = 6.50, p = 0.021), but it additionally increased for male-biased (F1,17 = 9.42, p = 0.007, figure 2b) and larger gestating litters (F1,17 = 6.74, p = 0.019).
Figure 1.
Relationship between maternal body mass and daily energy expenditure (DEE) at day 14 of lactation.
Figure 2.
Daily energy expenditure (DEE) at day 14 of lactation of mothers nursing (a) all-female and (b) all-male litters in relation to the sex ratio of their concurrent pregnancy.
(b). Mass of pups in subsequent litters
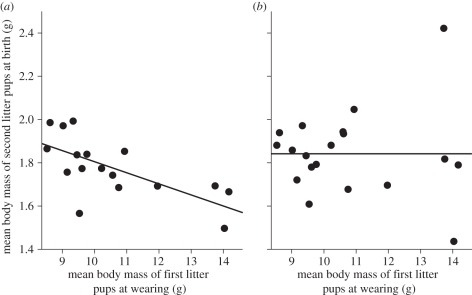
To further explore consequences of elevated maternal energy intake and expenditure in response to male-biased current and gestating litters, we looked at factors shaping characteristics of subsequent litters. We found that offspring body mass at birth in the second litters was not directly affected by litter size and sex manipulation in the first litters (all p > 0.1). However, offspring body mass at birth was significantly related to maternal effort during nursing (measured as DEE and mean size of weaned pups), the effect of which was different for male and female neonates of the subsequent litter (table 2a,b). In mothers nursing all-male litters, body mass of subsequent female neonates was negatively related to the mean mass of weaned pups (mixed model controlling for mothers' identity: F1,37 = 12.17, p = 0.0013; figure 3a). This was not the case for male neonates (p = 0.9; figure 3b). Among mothers nursing all-female litters, there were no significant relationships between maternal effort and offspring body mass at birth for either sex (both p > 0.4).
Table 2.
Results of GLMM in which variation of offspring body mass at birth in subsequent litters was analysed in relation to manipulated sex and size of mothers' suckling litters, neonate's sex and maternal effort estimated as (a) daily energy expenditure (DEE) and (b) mean mass of pups from the manipulated litters at weaning. (Maternal identity was included as a random factor (with the estimates of 0.028 and 0.026, respectively). The analyses performed without the non-significant effects of litter sex manipulation and litter size revealed the same interactions of neonate sex and maternal effort.)
| estimate ± s.e. | d.f. | F | p | |
|---|---|---|---|---|
| (a) source of variation | ||||
| intercept | 1.572 ± 0.195 | |||
| litter size | 0.016 ± 0.013 | 1,174 | 1.44 | 0.232 |
| litter sex | −0.006 ± 0.059 | 1,174 | 0.01 | 0.919 |
| neonate sex | 0.200 ± 0.119 | 1,174 | 2.85 | 0.093 |
| maternal DEE | 0.002 ± 0.002 | 1,174 | 0.10 | 0.755 |
| neonate sex × maternal DEE | −0.003 ± 0.001 | 1,174 | 3.70 | 0.056 |
| (b) source of variation | ||||
| intercept | 1.368 ± 0.300 | |||
| litter size | 0.025 ± 0.014 | 1,174 | 3.47 | 0.064 |
| litter sex | −0.023 ± 0.056 | 1,174 | 0.17 | 0.682 |
| neonate sex | 0.244 ± 0.134 | 1,174 | 3.32 | 0.070 |
| mean mass of weaned pups | 0.031 ± 0.023 | 1,174 | 0.70 | 0.405 |
| neonate sex × mean mass of weaned pups | −0.025 ± 0.012 | 1,174 | 4.14 | 0.043 |
Figure 3.
Mean body mass at birth of (a) female and (b) male pups in second litters in relation to mean body mass of pups in manipulated all-male litters at weaning (a measure of maternal effort during nursing).
4. Discussion
We have previously demonstrated that maternal allocation in terms of offspring defence and milk production in the bank vole is increased for enlarged compared with reduced litters, as well as for daughters compared with sons [22]. Our current results show that maternal energy budget, i.e. energy intake (food consumption) and DEE, were also clearly elevated for larger litter size. Maternal DEE, but not food consumption, was also significantly shaped by litter sex, albeit in relation to characteristics of the simultaneously gestating litter—its size and sex ratio (table 1). Specifically, in mothers nursing all-male litters, DEE was higher when pregnant with male-biased and larger second litters (figure 2). This increased maternal expenditure had a negative effect on size at birth of female offspring in successive litters (table 2 and figure 3). Our study provides evidence for the long-standing expectation that sons exert more maternal energy than daughters (e.g. [17–19,44]). Elevated maternal energy demands of sons together with mothers' fixed intake level constrain the amount of energy that the mothers raising all-male litters can allocate into milk.
Energy expenditure of mothers nursing all-male litters was elevated when they were simultaneously pregnant with larger and male-biased second litters. We suggest that this may have been caused by male offspring exploiting more maternal energy in the uterus. In fact, there is evidence that during pregnancy male foetuses extract more maternal resources. Specifically, in mice, rats and humans, male embryos were found to develop faster than females even before sexual differentiation [45–47], and in 7-day-old bovine embryos glucose metabolism was twofold higher in males compared with females [48]. Furthermore, we presume that male offspring (in utero and during nursing) create an androgenic environment [49] that potentially induces a higher metabolic rate [50,51] in their mothers, even if male offspring do not necessarily grow bigger [22]. However, as producing male offspring increases energy expenditure of mothers, what then sets the limits for female's maximum sustainable level of energy intake? Current understanding suggests that these limits are imposed by the capacity of the animal to dissipate body heat generated as a by-product of processing food and producing milk [40]. This may take place especially during late lactation, and failure to dissipate produced heat that might lead to hyperthermia, which has many negative effects on physiological functions [52]. Thus, heat dissipation limit can be considered as the key physiological mechanism linking current energy expenditure to life history in endothermic animals [40].
The trade-off between investment into current versus future reproduction is one of the fundamental concepts of the evolution of life histories [16]. Species that have overlapping generations provide a model system for direct investigations of such between-cohort competition. Significant fecundity costs of raising enlarged litters in (semi)natural conditions have been previously observed in the bank vole [23,31,53]. However, in ad libitum food conditions, delays in pregnancy have been the only clear fecundity costs found for mothers raising concurrent litters [22,54]. Mice that are pregnant while lactating are usually not found to compromise suckling pups in favour of the gestating litter (e.g. [27,28,55]). However, these kinds of situations are often prone to a conflict between the mother and the offspring, when both aim at maximizing their life-time reproductive success. For mothers, the resource available to devote to offspring through milk is the difference between energy intake (food consumption) and expenditure (DEE). In the current study, energy expenditure of mothers was highest when they were nursing all-male litters and gestating male-biased second litters, revealing higher costs of producing sons. In spite of these elevated physiological costs, energy (food) intake of mothers was not significantly increased when nursing sons versus daughters. Consequently, the reserve (milk) available for investment for mothers was greater when nursing daughters instead of sons. The parent–offspring conflict in this system led to the situation where mothers increased their allocation to daughters by producing them more milk as well as defending them more vigorously than sons [22]. Still, they could not escape physiological costs of reproduction owing to producing sons, as it leads to compromised size of future female offspring. Such an effect could also occur if females were primed by their initial maternal resource allocation. Female mice were shown to invest less in their second litter if the allocation into the first one was experimentally reduced [56]. Our study suggests that such priming effects might be also related to the sex of produced offspring. Females forced to nurse all-male litters differentiated their allocation towards the subsequent pregnancy in a way that favoured future male offspring while mass of female offspring from the subsequent litters was compromised (figure 3).
We used the largest possible manipulation and created single-sex litters that allowed us to observe significant differences in maternal investment. However, such design also has some limitations, as female bank voles usually have mixed-sex litters. It is known that behaviourally, mammalian females might differentiate their care between individual offspring, as in rats (Rattus norvegicus) [57] and ferrets (Mustela putorius furo) [58], male pups receive more licking than female pups. Licking might be one of the mechanisms by which mothers get information on the sex ratio of their offspring. Consequently, although we had no group of mixed sex litters, we can argue that under natural circumstances physiological investment could be proportional to the sex ratio of a litter. One could wonder whether potential differences in milk quality produced for sons and daughters, such as those reported in the rhesus macaques (Macaca mulatta) [59], could alter the interpretation of our current results. However, this is unlikely as the cost of producing milk is driven by the synthesis of the lipids and is independent of how dilute the milk is [60]. In our study, offspring in all-female litters grew bigger [22], indicating that the costs of milk production in that group were indeed higher.
To conclude, our study reveals contrasting strategies of mothers nursing female and male offspring. Those raising daughters direct their behavioural allocation towards them [22] and do not adjust energy expenditure to the needs of gestating young. Mothers nursing sons are constrained by the high DEE that raising sons involves and consequently must adjust their energy budget to the subsequent litter. Thus, offspring sex appears to be highly influential in shaping the trade-off between current and future maternal allocation. These findings highlight the need of incorporating different measures of maternal investment and especially different generations of the offspring to get better insight into sex-allocation strategies.
Acknowledgements
The use of the animals adhered to ethical guidelines for animal research in Finland (permission number 38/13.5.2003) as well as all the institutional guidelines.
We thank Paula Redman and Peter Thomson for assistance with DLW measurements and Tuuli Niskanen for help with handling the voles. Mikael Mokkonen and two referees provided helpful comments on the manuscript. The study was supported financially by the Academy of Finland (project numbers: 100143, 103148, 78777, 115961, 119200 to E.K.; 132190 to T.M.), NERC grant NE/C004159/1 to J.R.S., and Marie Curie training site funding to Centre of Excellence in Evolutionary Ecology in the University of Jyväskylä (allocated to J.R.).
References
- 1.Hardy I. 2002. Sex ratios: concepts and research methods. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Krackow S. 1995. Potential mechanisms for sex-ratio adjustment in mammals and birds. Biol. Rev. Camb. Phil. Soc. 70, 225–241 10.1111/j.1469-185X.1995.tb01066.x (doi:10.1111/j.1469-185X.1995.tb01066.x) [DOI] [PubMed] [Google Scholar]
- 3.Sheldon B. C., West S. A. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54 10.1086/381003 (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- 4.Trivers R. L., Willard D. E. 1973. Natural selection of parental ability to vary sex-ratio of offspring. Science 179, 90–92 10.1126/science.179.4068.90 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Alvarez C. 2006. Manipulation of primary sex-ratio: an updated review. Avian Poultry Biol. Rev. 17, 1–20 10.3184/147020606783437930 (doi:10.3184/147020606783437930) [DOI] [Google Scholar]
- 6.Navara K. J., Nelson R. J. 2009. Prenatal environmental influences on the production of sex-specific traits in mammals. Semin. Cell Dev. Biol. 20, 313–319 10.1016/j.semcdb.2008.12.004 (doi:10.1016/j.semcdb.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 7.Magrath M. J. L., Van Lieshout E., Visser G. H., Komdeur J. 2004. Nutritional bias as a new mode of adjusting sex allocation. Proc. R. Soc. Lond. B 271, S347–S349 10.1098/rsbl.2004.0187 (doi:10.1098/rsbl.2004.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charnov E. I. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 9.Golet G. H., Irons D. B., Estes J. A. 1998. Survival costs of chick rearing in black-legged kittiwakes. J. Anim. Ecol. 67, 827–841 10.1046/j.1365-2656.1998.00233.x (doi:10.1046/j.1365-2656.1998.00233.x) [DOI] [Google Scholar]
- 10.Harshman L. G., Zera A. J. 2007. The cost of reproduction: the devil in the details. Trends Ecol. Evol. 22, 80–86 10.1016/j.tree.2006.10.008 (doi:10.1016/j.tree.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 11.Speakman J. R. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398 10.1098/rstb.2007.2145 (doi:10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clutton-Brock T. H. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Gomendio M., Clutton-Brock T. H., Albon S. D., Guinness F. E., Simpson M. J. 1990. Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343, 261–263 10.1038/343261a0 (doi:10.1038/343261a0) [DOI] [PubMed] [Google Scholar]
- 14.Helle S., Lummaa V., Jokela J. 2002. Sons reduced maternal longevity in preindustrial humans. Science 296, 1085. 10.1126/science.1070106 (doi:10.1126/science.1070106) [DOI] [PubMed] [Google Scholar]
- 15.Hewison A. J. M., Gaillard J. M. 1999. Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol. Evol. 14, 229–234 10.1016/S0169-5347(99)01592-X (doi:10.1016/S0169-5347(99)01592-X) [DOI] [PubMed] [Google Scholar]
- 16.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 17.Bercovitch F. B., Widdig A., Nürnberg P. 2000. Maternal investment in rhesus macaques (Macaca mulatta): reproductive costs and consequences of raising sons. Behav. Ecol. Sociobiol. 48, 1–11 10.1007/s002650000204 (doi:10.1007/s002650000204) [DOI] [Google Scholar]
- 18.Fernández-Llario P., Carranza J., Mateos-Quesada P. 1999. Sex allocation in a polygynous mammal with large litters: the wild boar. Anim. Behav. 58, 1079–1084 10.1006/anbe.1999.1234 (doi:10.1006/anbe.1999.1234) [DOI] [PubMed] [Google Scholar]
- 19.Ono K. A., Boness D. J. 1996. Sexual dimorphism in sea lion pups: differential maternal investment, or sex-specific differences in energy allocation? Behav. Ecol. Sociobiol. 38, 31–41 10.1007/s002650050214 (doi:10.1007/s002650050214) [DOI] [Google Scholar]
- 20.Robert K. A., Schwanz L. E., Mills H. R. 2010. Offspring sex varies with maternal investment ability: empirical demonstration based on cross-fostering. Biol. Lett. 6, 242–245 10.1098/rsbl.2009.0774 (doi:10.1098/rsbl.2009.0774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magrath M. J. L., Van Lieshout E., Pen I., Visser G. H., Komdeur J. 2007. Estimating expenditure on male and female offspring in a sexually size-dimorphic bird: a comparison of different methods. J. Anim. Ecol. 76, 1169–1180 10.1111/j.1365-2656.2007.01292.x (doi:10.1111/j.1365-2656.2007.01292.x) [DOI] [PubMed] [Google Scholar]
- 22.Koskela E., Mappes T., Niskanen T., Rutkowska J. 2009. Maternal investment in relation to sex ratio and offspring number in a small mammal: a case for Trivers and Willard theory? J. Anim. Ecol. 78, 1007–1014 10.1111/j.1365-2656.2009.01574.x (doi:10.1111/j.1365-2656.2009.01574.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koivula M., Koskela E., Mappes T., Oksanen T. A. 2003. Cost of reproduction in the wild: manipulation of reproductive effort in the bank vole. Ecology 84, 398–405 10.1890/0012-9658(2003)084[0398:CORITW]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0398:CORITW]2.0.CO;2) [DOI] [Google Scholar]
- 24.Mills S. C., Grapputo A., Koskela E., Mappes T. 2007. Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc. R. Soc. B 274, 143–150 10.1098/rspb.2006.3639 (doi:10.1098/rspb.2006.3639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oksanen T. A., Alatalo R. V., Horne T. J., Koskela E., Mappes J., Mappes T. 1999. Maternal effort and male quality in the bank vole. Proc. R. Soc. Lond. B 266, 1495–1499 10.1098/rspb.1999.0806 (doi:10.1098/rspb.1999.0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronson F. H. 1989. Mammalian reproductive biology. Chicago, IL: University of Chicago Press [Google Scholar]
- 27.Johnson M., Thomson S., Speakman J. R. 2001. Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 204, 1925–1935 [DOI] [PubMed] [Google Scholar]
- 28.Johnson M. S., Thomson S. C., Speakman J. R. 2001. Limits to sustained energy intake. III. Effects of concurrent pregnancy and lactation in Mus musculus. J. Exp. Biol. 204, 1947–1956 [DOI] [PubMed] [Google Scholar]
- 29.Humphries M. M., Boutin S. 2000. The determinants of optimal litter size in free-ranging red squirrels. Ecology 81, 2867–2877 10.1890/0012-9658(2000)081[2867:TDOOLS]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[2867:TDOOLS]2.0.CO;2) [DOI] [Google Scholar]
- 30.Koskela E., Juutistenaho P., Mappes T., Oksanen T. A. 2000. Offspring defence in relation to litter size and age: experiment in the bank vole Clethrionomys glareolus. Evol. Ecol. 14, 99–109 10.1023/A:1011051426666 (doi:10.1023/A:1011051426666) [DOI] [Google Scholar]
- 31.Mappes T., Koskela E., Ylönen H. 1995. Reproductive costs and litter size in the bank vole. Proc. R. Soc. Lond. B 261, 19–24 10.1098/rspb.1995.0111 (doi:10.1098/rspb.1995.0111) [DOI] [PubMed] [Google Scholar]
- 32.Oksanen T. A., Jokinen I., Koskela E., Mappes T., Vilpas H. 2003. Manipulation of offspring number and size. Benefits of large body size at birth depend upon the rearing environment. J. Anim. Ecol. 72, 321–330 10.1046/j.1365-2656.2003.00703.x (doi:10.1046/j.1365-2656.2003.00703.x) [DOI] [Google Scholar]
- 33.McGuire B., Bemis W. E. 2007. Litter size influences maternal but not paternal care in three species of voles, as measured by nest attendance. J. Mamm. 88, 1420–1426 10.1644/06-MAMM-A-451R.1 (doi:10.1644/06-MAMM-A-451R.1) [DOI] [Google Scholar]
- 34.Speakman J. R., McQueenie J. 1996. Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol. Zool. 69, 746–769 [Google Scholar]
- 35.Wu S.-H., Zhang L.-N., Speakman J. R., Wang H. 2009. Limits to sustained energy intake. XI. A test of the heat dissipation limitation hypothesis in lactating Brandt's voles (Lasiopodomys brandtii). J. Exp. Biol. 212, 3455–3465 10.1242/jeb.030338 (doi:10.1242/jeb.030338) [DOI] [PubMed] [Google Scholar]
- 36.Butler P., Green J., Boyd I., Speakman J. R. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Funct. Ecol. 18, 168–183 10.1111/j.0269-8463.2004.00821.x (doi:10.1111/j.0269-8463.2004.00821.x) [DOI] [Google Scholar]
- 37.Król E., Speakman J. R. 1999. Isotope dilution spaces of mice injected simultaneously with deuterium, tritium and oxygen-18. J. Exp. Biol. 202, 2839–2849 [DOI] [PubMed] [Google Scholar]
- 38.Speakman J. R., Racey P. 1987. The equilibrium concentration of O-18 in body-water: implications for the accuracy of the doubly-labeled water technique and a potential new method of measuring RQ in free-living animals. J. Theor. Biol. 127, 79–95 10.1016/S0022-5193(87)80162-5 (doi:10.1016/S0022-5193(87)80162-5) [DOI] [Google Scholar]
- 39.Nagy K. A. 1983. The doubly labelled water 3HH18O method: a guide to its use. Los Angeles, CA: University of California [Google Scholar]
- 40.Król E., Murphy M., Speakman J. R. 2007. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 210, 4233–4243 10.1242/jeb.009779 (doi:10.1242/jeb.009779) [DOI] [PubMed] [Google Scholar]
- 41.Speakman J. R., Nagy K. A., Masman D., Mook W. G., Poppitt S. D., Strathearn G. E., Racey P. A. 1990. Interlaboratory comparison of different analytical techniques for the determination of oxygen-18 abundance. Anal. Chem. 62, 703–708 10.1021/ac00206a011 (doi:10.1021/ac00206a011) [DOI] [Google Scholar]
- 42.Speakman J. R. 1993. How should we calculate CO2 production in doubly labeled water studies of animals? Funct. Ecol. 37, 746–750 [Google Scholar]
- 43.Speakman J. R. 1997. Doubly labelled water: theory and practice. London, UK: Chapman and Hall [Google Scholar]
- 44.Koskela E., Huitu O., Koivula M., Korpimäki E., Mappes T. 2004. Sex-biased maternal investment in voles: importance of environmental conditions. Proc. R. Soc. Lond. B 271, 1385–1391 10.1098/rspb.2004.2711 (doi:10.1098/rspb.2004.2711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgoyne P. S., Thornhill A. R., Boudrean S. K., Darling S. M., Bishop C. E., Evans E. P., Capel B., Mittwoch U. 1995. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Phil. Trans. R. Soc. Lond. B 350, 253–261 10.1098/rstb.1995.0159 (doi:10.1098/rstb.1995.0159) [DOI] [PubMed] [Google Scholar]
- 46.Mittwoch U. 1993. Blastocysts prepare for the race to be male. Hum. Reprod. 8, 1550–1555 [DOI] [PubMed] [Google Scholar]
- 47.Scott W. J., Holson J. F. 1977. Weight differences in rat embryos prior to sexual differentiation. J. Embryol. Exp. Morphol. 40, 259–263 [PubMed] [Google Scholar]
- 48.Tiffin G. J., Rieger D., Betteridge K. J., Yadav B. R., King W. A. 1991. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J. Reprod. Fertil. 93, 125–132 10.1530/jrf.0.0930125 (doi:10.1530/jrf.0.0930125) [DOI] [PubMed] [Google Scholar]
- 49.Moles A., Rizzi R., D'Amato F. R. 2003. The number of male pups within a litter of NMRI mice is associated with the dam's food preferences late in pregnancy. Psychoneuroendocrinology 28, 250–260 10.1016/S0306-4530(02)00018-5 (doi:10.1016/S0306-4530(02)00018-5) [DOI] [PubMed] [Google Scholar]
- 50.Buchanan K. L., Evans M. R., Goldsmith A. R., Bryant D. M., Rowe L. V. 2001. Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc. R. Soc. Lond. B 268, 1337–1344 10.1098/rspb.2001.1669 (doi:10.1098/rspb.2001.1669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobler M., Nilsson J.-Å., Nilsson J. F. 2007. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol. Lett. 3, 408–410 10.1098/rsbl.2007.0127 (doi:10.1098/rsbl.2007.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speakman J. R., Król E. 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 10.1111/j.1365-2656.2010.01689.x (doi:10.1111/j.1365-2656.2010.01689.x) [DOI] [PubMed] [Google Scholar]
- 53.Oksanen T. A., Koskela E., Mappes T. 2002. Hormonal manipulation of offspring number: maternal effort and reproductive costs. Evolution 56, 1530–1537 10.1111/j.0014-3820.2002.tb01463.x (doi:10.1111/j.0014-3820.2002.tb01463.x) [DOI] [PubMed] [Google Scholar]
- 54.Woodside B., Wilson R., Chee P., Leon M. 1981. Resource partitioning during reproduction in the Norway rat. Science 211, 76–77 10.1126/science.7444451 (doi:10.1126/science.7444451) [DOI] [PubMed] [Google Scholar]
- 55.Bateman N. 1957. Some physiological aspects of lactation in mice. J. Agr. Sci. (Camb.) 49, 60. 10.1017/S0021859600034328 (doi:10.1017/S0021859600034328) [DOI] [Google Scholar]
- 56.Charalambous M., Ward A., Hurst L. D. 2003. Evidence for a priming effect on maternal resource allocation: implications for interbrood competition. Proc. R. Soc. Lond. B 270, S100–S103 10.1098/rsbl.2003.0028 (doi:10.1098/rsbl.2003.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore C. L., Morelli G. A. 1979. Mother rats interact differently with male and female offspring. J. Comp. Physiol. Psychol. 93, 677–684 10.1037/h0077599 (doi:10.1037/h0077599) [DOI] [PubMed] [Google Scholar]
- 58.Baum M. J., Bressler S. C., Daum M. C., Veiga C. A., McNamee C. S. 1996. Ferret mothers provide more anogenital licking to male offspring: possible contribution to psychosexual differentiation. Physiol. Behav. 60, 353–359 10.1016/S0031-9384(96)80004-7 (doi:10.1016/S0031-9384(96)80004-7) [DOI] [PubMed] [Google Scholar]
- 59.Hinde K. 2007. First-time macaque mothers bias milk composition in favor of sons. Curr. Biol. 17, R958–R959 10.1016/j.cub.2007.09.029 (doi:10.1016/j.cub.2007.09.029) [DOI] [PubMed] [Google Scholar]
- 60.Król E., Speakman J. R. 2003. Limits to sustained energy intake. VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4267–4281 10.1242/jeb.00675 (doi:10.1242/jeb.00675) [DOI] [PubMed] [Google Scholar]