Abstract
Whether dominance drives species loss can depend on the power of conspecific self-limitation as dominant populations expand; these limitations can stabilize competitive imbalances that might otherwise cause displacement. We quantify the relative strength of conspecific and heterospecific soil feedbacks in an exotic-dominated savannah, using greenhouse trials and field surveys to test whether dominants are less self-suppressed, highly suppressive of others or both. Soil feedbacks can impact plant abundance, including invasion, but their implications for coexistence in invader-dominated systems are unclear. We found that conspecific feedbacks were significantly more negative than heterospecific ones for all species including the dominant invaders; even the rarest natives performed significantly better in the soils of other species. The strength of these negative feedbacks, however, was approximately 50 per cent stronger for natives and matched their field abundance—the most self-limited natives were rare and narrowly distributed. These results suggest that exotics dominate by interacting with natives carrying heavier conspecific feedback burdens, without cultivating either negative heterospecific effects that suppress natives or positive ones that accelerate their own expansion. These feedbacks, however, could contribute to coexistence because all species were self-limited in their own soils. Although the net impact of this feedback stabilization will probably interact with other factors (e.g. herbivory), soil feedbacks may thus contribute to invader dominance without necessarily being detrimental to species richness.
Keywords: conspecific interactions, heterospecific interactions, soil feedbacks, coexistence, plant invasion, oak savannah
1. Introduction
Interactions within and among species (‘conspecific’ and ‘heterospecific’, respectively) strongly influence the demographic performance of plant populations, with the net outcome potentially determining community-level parameters of abundance, distribution and coexistence. Conspecific interactions can affect rates of population expansion, with larger populations more probably limited by intraspecific competition or enemy attack [1,2]. Heterospecific interactions are influenced strongly by the relative abilities to acquire limiting resources, and to maintain or increase fitness as such limitations intensify [1,3,4]. The two processes can interact when low-frequency populations occur among more abundant populations with heavier conspecific burdens, making it advantageous to grow among other species [5]. When all populations in a community are regulated this way, the end result should be coexistence [6,7]. When conspecific constraints are weak, then coexistence may break down as interspecific differences in rates of population expansion will allow some species to displace others [8–10].
This dynamic balance between conspecific and heterospecific interactions can also influence invasion, by affecting establishment, impacts or both [11–13]. Invader establishment, for example, can be facilitated by low-frequency founder populations with weak conspecific constraints occurring among larger native populations with heavier conspecific burdens [14]. Whether such invaders also exert impacts on the resident community depends on the strength of conspecific limitations as their populations expand [11,15–17]. If conspecific limitations intensify, this may help to stabilize coexistence even if invader populations reach relatively large sizes [18]. If conspecific suppression for invaders is relatively weak owing to factors such as enemy escape, population expansion may continue because positive fitness levels can be maintained even at higher densities. A consequence of these weaker conspecific constraints is that fitness levels of the invading population may greatly exceed those of other populations, thereby driving displacement [15]. In both situations described above, invading populations reach high abundances. Their implications for coexistence, however, differ substantially. Without untangling the relative strengths of conspecific and heterospecific limitations, it may be difficult to tell the difference in exotic-dominated systems.
Here, we examine this issue by quantifying conspecific and heterospecific soil feedbacks for plant species of varying abundance in a heavily invaded but species-rich oak savannah. Soil feedbacks have host-specific and potentially self-limiting effects on plant abundance, where plants influence the community structure of soil biota, which in turn alters their performance and that of their neighbours [16,19–26]. Soil feedbacks can also have important consequences for exotic species [13,27–31]. Invasion can be facilitated by escaping soil-based enemies [30,32], by inhibiting symbiotic mutualists required by native plants [33] or by cultivating positive soil feedbacks that maintain population expansion even at higher frequencies [28,34]. Each of these mechanisms has been associated with invasion success, but can have different implications for coexistence that can only be distinguished by decoupling the soil-based effects of the invaders on themselves versus their heterospecific neighbours [13,16,26]. To do this, we quantify the inhibitory strength of both factors using greenhouse trials, testing whether more abundant exotic species of this system are less affected by negative conspecific soil feedbacks, whether they cultivate positive conspecific soil feedbacks that favour their own expansion or whether they produce negative heterospecific feedbacks that suppress their native competitors. We then test the correspondence between these results and patterns of relative abundance in the field.
2. Methods
(a). Study area
The study system is a species-rich and invaded oak (Quercus garryana) savannah of southwestern British Columbia, Canada [35,36]. This ecosystem reaches its northern distributional limit in British Columbia, extending from California in the rain shadow of the Coast Mountains. The climate is sub-Mediterranean, with wet cool winters and a summer drought period usually extending from June to October. Annual precipitation ranges from 800 to 1100 mm. The savannah occurs on moderately fertile soils (average available n = 150.5 mg kg−1 soil (±61 s.e.)) overlaying fractured shale bedrock, with soil depths ranging from less than 5 to greater than 100 cm. Currently, remnant savannah is dominated by exotic grasses, forbs and shrubs, with the regional exotic pool exceeding 150 species [37,38].
Seeds from 14 native and exotic species, representing a range of abundances (table 1), were collected in the summer of 2007 from the 10.8 ha Cowichan Garry Oak preserve on southeastern Vancouver Island [35]. Abundances were percentage cover estimates to 1 per cent from 160 plots (area 1 m2 per plot) located across the reserve. Cover estimates were determined by placing a 25-cell 1 m2 sampling frame over each plot, and tallying cover values for each species one cell at a time [35]. Frequencies were numbers of the 160 plots within which each species occurred, ranging potentially from 1 to 100 per cent. Seeds were collected from across the study area for each species, pooled and air-dried for two months, and then cold-stratified at 4°C for an additional two months. Soil from the reserve was collected in June 2007 by pooling ten soil cores (each of 10 cm diameter) in areas containing grasses and forbs to depths of less than 20 cm. Soil was air-dried and refrigerated for five weeks prior to use.
Table 1.
Relative abundance and frequency, biogeographic origin and life form of the 14 species used in this study. Abundance refers to the average percentage cover in 160 plots (area 1 m2 per plot) across the study area, not including plots where they are absent. Frequency refers to the number of the 160 plots occupied by each species.
| species | origin | life form | frequency (per 160 plots) | abundance (1 s.d.) |
|
|---|---|---|---|---|---|
| Sanicula crassicaulis | native | perennial forb | 159 | 9.4 | 11.8 |
| Vicia sativa | exotic | perennial forb | 152 | 7.8 | 5.3 |
| Poa pratensis | exotic | perennial grass | 121 | 46.2 | 23.1 |
| Cytisus scoparius | exotic | perennial shrub | 108 | 3.2 | 2.3 |
| Dactlyis glomerta | exotic | perennial grass | 103 | 19.4 | 12.2 |
| Camassia quamash | native | perennial forb | 99 | 5.4 | 4.1 |
| Bromus cariantus | native | perennial grass | 76 | 10.8 | 12.4 |
| Dodecatheon hendersonii | native | perennial forb | 66 | 4.4 | 3.2 |
| Lomatium utriculatum | native | perennial forb | 48 | 5.6 | 4.7 |
| Lapsana communis | exotic | perennial forb | 11 | 2.7 | 2.5 |
| Elymus glaucus | native | perennial grass | 6 | 3.5 | 1.8 |
| Lactuca biennis | exotic | perennial forb | 2 | 3.5 | 2.5 |
| Hypochaeris radicata | exotic | perennial forb | 2 | 1 | 1 |
| Lupinus bicolor | native | annual forb | 1 | 1 | 1 |
To isolate the effects of the soil community on plant performance, we sterilized the air-dried soils with gamma irradiation. Air-drying prior to irradiation is assumed to reduce physical and chemical changes that can sometimes occur, including nutrient pulses following the death of the microbial community [39–41]. This appeared to be effective, as we observed no detectable response differences between conspecific and heterospecific soil feedbacks on the irradiated soils (F1,13 = 1.529, p = 0.227) [25]. Also, biomass production by many species was significantly higher on live than sterilized soil (see §3), further suggesting the absence of irradiation-derived nutrient pulses.
(b). Experimental work
We determined the strength of soil feedbacks using a two-step plant–soil feedback greenhouse experiment.
First, we trained the soils with the different plant species by growing individual plants in 1 l pots for 16 weeks, with 10 replicate pots per species. The soil medium in those pots was the field soil mixed with silica sand at a ratio of 1 : 1. Plants were placed on a greenhouse bench in a completely randomized design. Plants were not fertilized during this stage, but were watered on a daily basis during the first two weeks of growth, followed by watering every 3 days after that. At harvest, all shoots were removed and the remaining bulk soil mixture, including all roots (subsequently referred to as trained soil), was combined and stored at 4°C for one week prior to being used in the main feedback experiment.
Second, we used the trained soils in the following factorial design: 14 plant species (table 1) × 4 soil treatments (conspecific live, conspecific sterile, heterospecific live, heterospecific sterile). Recent studies have calculated feedback intensities by averaging performance differences across all species (e.g. [24], using six rainforest tree species). This was not logistically possible for our study, because we needed a larger pool of species that included a range of abundances (from common to rare) for both exotics and natives. As a compromise, we randomly selected 20 seeds from the 13 possible species to calculate the heterospecific component of our feedback work, with 20 replicates per treatment combination for a total of 1120 experimental units. These units were each placed randomly on two greenhouse benches. Each experimental unit consisted of a 1 l pot containing a mixture of sterile silica sand and 10 g of trained soil (from one of the 4 soil trainings described above). To each unit, we added two pre-germinated seeds from one of the 14 plant species. We grew the plants for 10 days, after which we thinned each pot to a single healthy plant. Plants were grown for a total of 12 weeks before harvest. As they grew, they were watered on a daily basis for the first two weeks and then watered every 3 days after that. Plants began showing signs of nutrient deficiency after 3 weeks; from then on we fertilized them weekly with 40 ml half-strength Hoaglands solution. There was no visible difference in nutrient deficiency between live and irradiated soil. After 12 weeks, plants were harvested, dried at 60°C for 48 h and weighed to determine total biomass (shoots and roots).
(c). Data analysis
We defined our soil feedbacks as the difference in growth in soils trained by conspecific individuals (‘conspecific effects’) versus soils trained by other species (‘heterospecific effects’). This incorporated both the effects of individual species on themselves and the effects of their competitors [13,16,25]. The conspecific and heterospecific effects values were first calculated as follows:
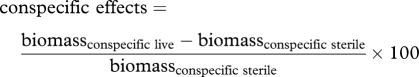 |
and
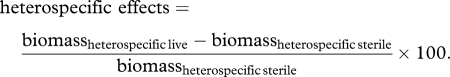 |
We then calculated the magnitude and direction (negative or positive) of the soil feedback by the difference between the two effects values (soil feedback = conspecific effects − heterospecific effects). Negative values thus indicate conspecific suppression, because biomass production would be less compared with performance in soils trained by other species.
We used multi-factor ANOVA to determine the relationship between feedback strength versus abundance in the field, treatment (conspecific or heterospecific interactions) and biogeographic origin (native or exotic). Post hoc comparisons followed Fisher's protected least significant difference procedure, with post hoc Tukey's tests restricted to significant higher order interactions. All analyses were conducted in JMP 8 [42] (table 2).
Table 2.
Relationship between feedback strength and the individual and higher order effects of treatment (conspecific versus heterospecific feedbacks), origin (native versus exotic) and relative abundance in the savannah community (based on 160 field plots, area 1 m2 per plot).
| source | d.f. | F ratio | probability > F |
|---|---|---|---|
| treatment | 1 | 69.36 | <0.0001 |
| origin | 1 | 21.54 | <0.0001 |
| treatment × origin | 1 | 16.13 | <0.0001 |
| abundance × treatment | 1 | 6.51 | 0.0110 |
| abundance × treatment × origin | 1 | 4.74 | 0.0300 |
| abundance × origin | 1 | 2.21 | 0.14 |
| abundance | 1 | 0.96 | 0.33 |
3. Results
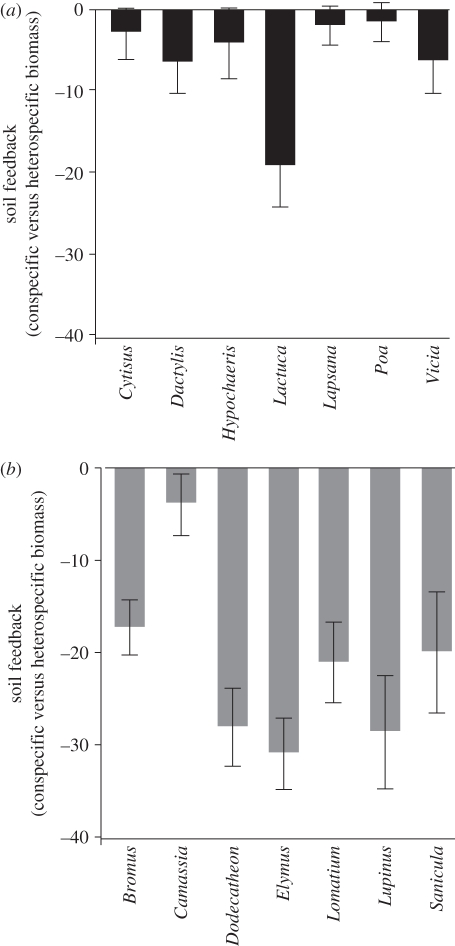
All species had more limiting conspecific biotic soil feedbacks than heterospecific ones (figure 1). This effect was significantly more pronounced in native species (F1,2 = 6.82, p < 0.0001), with native grasses in particular showing the greatest decreases in biomass when grown in their own soils, compared with other native forbs and legumes (F1,2 = 49.8, p = 0.0003; Tukey's test). The average reduction in biomass production in conspecific versus heterospecific soils for natives was −21.31 per cent (s.e. = 1.87; range: −17.5 to −25.01%), compared with −6.09 per cent for exotic species (s.e. = 1.42; range: −3.27 to −8.91).
Figure 1.
Strength of conspecific soil feedbacks, with negative values indicating that all species were self-limited, and thus performed better, in soils conditioned by other species. The magnitude of this effect was weaker for (a) exotics than (b) natives, with the former having producing significantly more biomass in their own soils (F1,13 = 41.7, p < 0.0001).
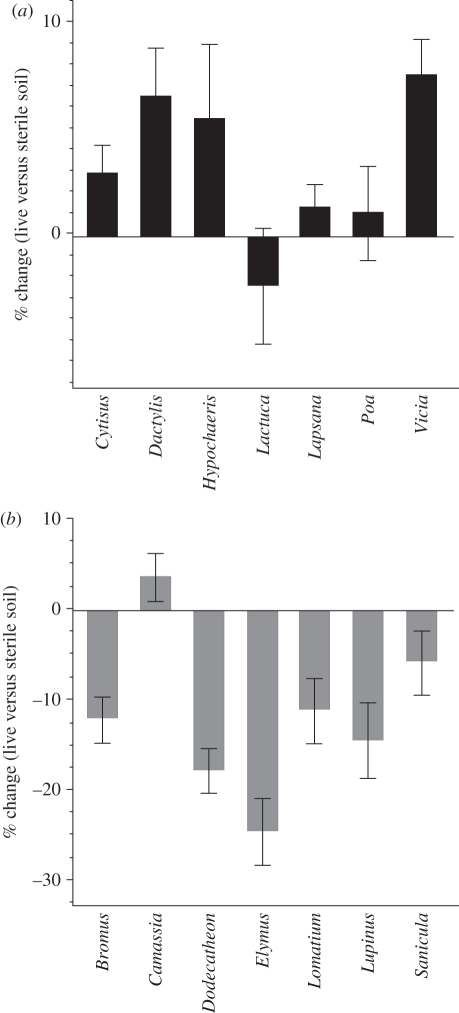
The soil feedback differences are further illustrated by the differences in plant performance in sterilized versus live soil for native and exotic species (figure 2). For the exotics, average biomass production per species was almost always higher in the live soils, for both conspecific and heterospecific treatments (conspecific: +3.27% (s.e. = 0.87); heterospecific: +9.04 per cent (s.e. = 1.42)). That is to say, exotic plants were smaller in their own soils compared with the soils of others (a negative soil feedback—see above paragraph), but conspecific growth on live soils was, on average, always greater than conspecific growth on sterilized soils. For the natives, in contrast, average biomass per species was only higher in live soils when they were conditioned by other species (heterospecific effects: +9.46% (s.e. = 1.2)). In soils conditioned by conspecific individuals, performance was always less in live than in irradiated soils (conspecific effects: −11.85% (s.e. = 0.99)).
Figure 2.
Difference in biomass production between sterilized and live soils conditioned by conspecific individuals. Positive values indicate better performance in live soils, for (a) exotic and (b) native species. Error bars are 1 s.e.
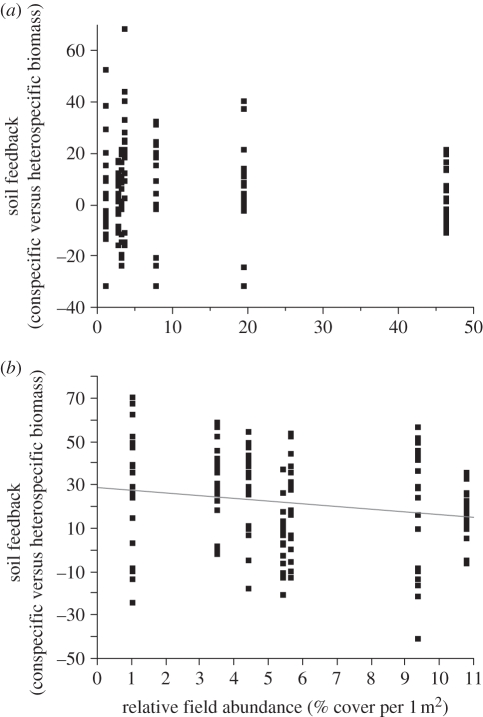
There was a significant negative correlation between the strength of the soil feedbacks and both abundance and frequency of native species within the study area (abundance: F1,138 = 4.42, p = 0.037; frequency: F1,138 = 7.62, p = 0.0065; figure 3). The least abundant and most narrowly distributed native taxa in the field had the strongest conspecific soil feedbacks in the greenhouse trials. In contrast, these relationships were both insignificant for exotic species (abundance: F1,138 = 1.39, p = 0.24; frequency: F1,138 = 2.04, p = 0.15). The only exotic species with greater biomass production in sterilized soils than live soils, Lactuca biennis, was one of the rarest on the study area (table 1).
Figure 3.
Relationship between strength of conspecific soil feedbacks and abundance within the oak savannah community, for (a) exotic and (b) native species. Only the native species show a relationship with abundance (F1,13 = 4.42, p = 0.037).
For the heterospecific soil treatments, there was no relationship between the strength of heterospecific growth and the abundance of the competitor in the field (F1,12 = 0.12, p = 0.73). That is to say, the rarest native species grew significantly larger in the soils of other species than their own soils, even if those other species were highly abundant exotics.
4. Discussion
The outcome of species interactions, and ultimately coexistence, depends on the relative balance between limitations within and among species [1,15]. We found strong self-limiting soil feedbacks for all species, with the intensity of these effects associated with relative abundance of the native species in the community. Further, this relationship was closely aligned with biogeographic origin, with non-native species having the weakest conspecific limitations and the highest abundances. Both findings have been observed previously in other systems [21,27,43–45], such that our results support the importance of soil-based processes for community structure, including invasion. What has been less clear is the connection between feedback differences and coexistence [13]. The presence of these conspecific effects for highly abundant invasives, for example, may be insufficient to promote coexistence if the negative heterospecific feedbacks of these species are even stronger, as has been observed with some invaders [33]. Here, we detected no such heterospecific effects, as even the rarest native species grew significantly larger in the soils conditioned by other species, including abundant invaders. This combination of self-limiting soil feedbacks and no detectable heterospecific effects could potentially contribute to species persistence, as has been recently reported in other systems (e.g. [2,46]). At the least, it indicates that soil feedbacks may contribute to differences in abundance in invaded systems without necessarily being detrimental to diversity.
The relative weakness of conspecific feedbacks in exotic populations is consistent with other studies (e.g. [27]) and also confirms how indirect interactions mediated across trophic levels can influence invasion (e.g. [8,47]). Plant invasion studies can often emphasize within-trophic-level differences in the ability to acquire limiting resources as a mechanism for success and impact (e.g. [48,49]). Here, exotics appear to reach high abundances because they are less inhibited by negative conspecific feedbacks than are their co-occurring native plants. This would presumably contribute to establishment and expansion in the early stages of invasion, as recruitment by invading individuals would be exclusively in soils conditioned by heterospecifics.
Our study was not designed to detect the exact cause of these weaker conspecific feedbacks for the exotics, as it could reflect relationships with pathogens, pests, root herbivores such as nematodes or even relative differences in the cultivation of symbiotic mutualisms [44]. Given that we observed conspecific effects to always exceed heterospecific ones, however, it appears that these feedbacks must be host-specific [25,50]. It is unclear why exotic species are less affected by their host-specific enemies, although it may reflect a trait-based tolerance or avoidance of pathogen or pest loads that native species do not possess [21]. Regardless of the exact mechanism, the suppressive effects of conspecific feedbacks were significantly greater for native plants, which suffered more substantial biomass reductions.
The absence of feedback-based heterospecific effects of exotics on natives indicates that the former neither cultivate pathogen loads (or at least not pathogens effective on natives) nor produce phytotoxic root exudates that disproportionately harm the below-ground fungal partners of native plant species [33]. We also did not detect significant positive feedbacks, where the exotics cultivate beneficial soil relationships that accelerate their own population expansion [34,51]. Many studies have shown that invaders can succeed by influencing their soil environment to favour their own growth [52]. Here, we see that native species appear to be far more limited by the effect of their own soil feedbacks than by direct feedback-based interactions with the exotic species. This lack of direct interaction has been observed previously in this system, where the experimental removal of the dominant exotic grasses for 3 years had no impact on the percentage cover of almost half of 79 species in the community that already were established in the plots [49]. One explanation for this result, despite a presumed increase in resource availability caused by the removals, could have been the constraining presence of strong conspecific soil feedbacks [13].
Given the host-specific nature of the soil feedback relationships, we could have detected a negative relationship between abundance in the study area and likelihood of encountering host-specific pathogens during recruitment [45,53–57]. That is to say, the rarer the species across the study area, the less likely it would occur within the proximity of 10 soil cores used for our feedback trials, such that its host-specific enemies might be absent. We did not observe this pattern, however, with strong conspecific suppression evident in rarer species even after a planting cycle of only four months. One possible explanation is that the distances within the 10.8 ha study area were not great enough relative to the dispersal capabilities of the soil enemies so that the soil samples captured these organisms even when the host plant was absent. Another possibility is that the species-specific soil enemies, which affect rarer plant species, are able to make a living by other means (e.g. they may live as facultative saprobes), so they are present throughout the site [57]. The end result is that these rarer populations appear to be significantly self-limited, despite their low abundance and relatively restricted distribution in the study area.
Our results are consistent with other studies showing how soil feedbacks could potentially contribute to coexistence by reducing rates of population expansion among more abundant species [25], even for dominant invasives. The strength of this effect will ultimately depend on the relative importance of other limiting factors that can influence abundance and persistence, such as competition, dispersal limitation and herbivory [2,13]. All of these factors have been shown to constrain native plant performance in this system [58–61], such that the net strength of the conspecific feedbacks could be relatively weak. This remains to be tested, although the strong connections we observed between the greenhouse trials and the field-based patterns of abundance for natives suggest that soil-based processes do make substantial and detectable contributions towards community structure. One implication of these results is that rare species are unlikely to ever increase widely in population size or distribution due to the strength of their conspecific feedbacks, but could persist by continuously recruiting in heterospecific soils.
Acknowledgements
Seed collection by Sarah Pinto, Sandra Van Vliet and Andrea Ellis. Funding to A.S.M. and J.K. by the NSERC Discovery Grant programme (Canada).
References
- 1.Chesson P. 2000. Mechanisms of maintenance of species diversity. Ann. Rev. Ecol. Syst. 31, 343–366 10.1146/annurev.ecolsys.31.1.343 (doi:10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 2.Comita L. S., Muller-Landau H. C., Aguilar S., Hubbell S. P. 2010. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 329, 330–332 10.1126/science.1190772 (doi:10.1126/science.1190772) [DOI] [PubMed] [Google Scholar]
- 3.Goldberg D. E., Barton A. M. 1992. Patterns and consequences of interspecific competition in natural communities: a review of field experiments with plants. Am. Nat. 139, 771–801 10.1086/285357 (doi:10.1086/285357) [DOI] [Google Scholar]
- 4.Tilman D. 1988. Plant strategies and the dynamics and structure of plant communities. Monographs in Population Biology. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Harms K. E., Wright S. J., Calderón O., Hernández A., Herre E. A. 2000. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404, 493–495 10.1038/35006630 (doi:10.1038/35006630) [DOI] [PubMed] [Google Scholar]
- 6.Chase J. A., Leibold M. A. 2003. Ecological niches. Chicago, IL: University of Chicago Press [Google Scholar]
- 7.Connell J. H. 1978. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 10.1126/science.199.4335.1302 (doi:10.1126/science.199.4335.1302) [DOI] [PubMed] [Google Scholar]
- 8.Chesson P., Kuang J. J. 2008. The interaction between predation and competition. Nature 456, 235–238 10.1038/nature07248 (doi:10.1038/nature07248) [DOI] [PubMed] [Google Scholar]
- 9.Harpole W. S., Suding K. N. 2007. Frequency-dependence stabilizes competitive interactions among four annual plants. Ecol. Lett. 10, 1164–1169 10.1111/j.1461-0248.2007.01115.x (doi:10.1111/j.1461-0248.2007.01115.x) [DOI] [PubMed] [Google Scholar]
- 10.Levine J. L., HilleRisLambers J. 2009. The importance of niches for the maintenance of species diversity. Nature 461, 254–257 10.1038/nature08251 (doi:10.1038/nature08251) [DOI] [PubMed] [Google Scholar]
- 11.MacDougall A. S., Gilbert B., Levine J. M. 2009. Plant invasion and the niche. J. Ecol. 97, 609–615 10.1111/j.1365-2745.2009.01514.x (doi:10.1111/j.1365-2745.2009.01514.x) [DOI] [Google Scholar]
- 12.Pachepsky E., Levine J. M. 2011. Density dependence slows invader spread in fragmented landscapes. Am. Nat. 177, 18–28 10.1086/657438 (doi:10.1086/657438) [DOI] [PubMed] [Google Scholar]
- 13.Yelenik S. G., Levine J. M. In press The role of plant–soil feedbacks in driving native species recovery. Ecology. [DOI] [PubMed] [Google Scholar]
- 14.Keane R. M., Crawley M. J. 2002. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17, 164–170 10.1016/S0169-5347(02)02499-0 (doi:10.1016/S0169-5347(02)02499-0) [DOI] [Google Scholar]
- 15.Adler P., HilleRisLambers J., Levine J. M. 2007. A niche for neutrality. Ecol. Lett. 10, 95–104 10.1111/j.1461-0248.2006.00996.x (doi:10.1111/j.1461-0248.2006.00996.x) [DOI] [PubMed] [Google Scholar]
- 16.Bever J. D., Westover K. M., Antonovics J. 1997. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 85, 561–573 10.2307/2960528 (doi:10.2307/2960528) [DOI] [Google Scholar]
- 17.Epstein M. J., Molofsky J. 2007. Invasiveness in plant communities with feedbacks. Ecol. Lett. 10, 253–263 10.1111/j.1461-0248.2007.01017.x (doi:10.1111/j.1461-0248.2007.01017.x) [DOI] [PubMed] [Google Scholar]
- 18.Jäger H., Kowarik I., Tye A. 2009. Destruction without extinction: long-term impacts of an invasive tree species on Galápagos highland vegetation. J. Ecol. 97, 1252–1263 10.1111/j.1365-2745.2009.01578.x (doi:10.1111/j.1365-2745.2009.01578.x) [DOI] [Google Scholar]
- 19.Bever J. D., et al. 2010. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 25, 468–478 10.1016/j.tree.2010.05.004 (doi:10.1016/j.tree.2010.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casper B. B., Castelli J. P. 2007. Evaluating plant–soil feedback together with competition in a serpentine grassland. Ecol. Lett. 10, 394–400 10.1111/j.1461-0248.2007.01030.x (doi:10.1111/j.1461-0248.2007.01030.x) [DOI] [PubMed] [Google Scholar]
- 21.Klironomos J. N. 2002. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417, 67–70 10.1038/417067a (doi:10.1038/417067a) [DOI] [PubMed] [Google Scholar]
- 22.Moora M., Zobel M. 1996. Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108, 79–84 10.1007/BF00333217 (doi:10.1007/BF00333217) [DOI] [PubMed] [Google Scholar]
- 23.Van der Putten W. H., Van Dijk C., Peters B. A. M. 1993. Plant-specific soil-borne diseases contribute to succession in foredune vegetation. Nature 362, 53–56 10.1038/362053a0 (doi:10.1038/362053a0) [DOI] [Google Scholar]
- 24.Mangan S. A., Schnitzer S. A., Herre E. A., Mack K. M., Valencia M. C., Sanchez E. I., Bever J. D. 2010. Negative plant–soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755 10.1038/nature09273 (doi:10.1038/nature09273) [DOI] [PubMed] [Google Scholar]
- 25.Petermann J. S., Fergus A. J. F., Turnbull L. A., Schmid B. 2008. Janzen–Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89, 2399–2406 10.1890/07-2056.1 (doi:10.1890/07-2056.1) [DOI] [PubMed] [Google Scholar]
- 26.Kulmatiski A., Beard K. H., Stevens J. R., Cobbold S. M. 2008. Plant–soil feedbacks: a meta-analytical review. Ecol. Lett. 11, 980–992 10.1111/j.1461-0248.2008.01209.x (doi:10.1111/j.1461-0248.2008.01209.x) [DOI] [PubMed] [Google Scholar]
- 27.Engelkes T., Morriën E., Verhoeven K. J. F., Bezemer T. M., Biere A., Harvey J. A., McIntyre L. M., Tamis W. L. M., van der Putten W. H. 2008. Successful range-expanding plants experience less above-ground and below-ground enemy impact. Nature 456, 946–948 10.1038/nature07474 (doi:10.1038/nature07474) [DOI] [PubMed] [Google Scholar]
- 28.Levine J. L., Pachepsky E., Kendall B. E., Yelenik S. G., HilleRisLambers J. 2006. Plant soil feedbacks and invasive spread. Ecol. Lett. 9, 1005–1014 10.1111/j.1461-0248.2006.00949.x (doi:10.1111/j.1461-0248.2006.00949.x) [DOI] [PubMed] [Google Scholar]
- 29.te Beest M., Stevens N., Olff H., van der Putten W. H. 2009. Plant–soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. J. Ecol. 97, 1281–1290 10.1111/j.1365-2745.2009.01574.x (doi:10.1111/j.1365-2745.2009.01574.x) [DOI] [Google Scholar]
- 30.Van Grunsven R. H. A., Van Der Putten W. H., Bezemer T. M., Tamis W. L., Berendse F., Veenendaal E. M. 2007. Reduced plant–soil feedback of plant species expanding their range as compared to natives. J. Ecol. 95, 1050–1057 10.1111/j.1365-2745.2007.01282.x (doi:10.1111/j.1365-2745.2007.01282.x) [DOI] [Google Scholar]
- 31.Vogelsang K. M., Bever J. D. 2009. Mycorrhizal densities decline in association with non-native plants and contribute to plant invasion. Ecology 90, 399–407 10.1890/07-2144.1 (doi:10.1890/07-2144.1) [DOI] [PubMed] [Google Scholar]
- 32.Hierro J. L., Maron J. L., Callaway R. M. 2005. A biogeographic approach to plant invasions: the importance of studying exotics in their introduced and native range. J. Ecol. 93, 5–15 10.1111/j.0022-0477.2004.00953.x (doi:10.1111/j.0022-0477.2004.00953.x) [DOI] [Google Scholar]
- 33.Stinson K. A., Campbell S. A., Powell J. R., Wolfe B. E., Callaway R. M., Thelen G. C., Hallett S. G., Prati D., Klironomos J. N. 2006. Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol. 4, e140. 10.1371/journal.pbio.0040140 (doi:10.1371/journal.pbio.0040140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaway R. M., Thelen G., Rodriguez A., Holben W. E. 2004. Release from inhibitory soil biota in Europe and positive plant–soil feedbacks in North America promote invasion. Nature 427, 731–733 10.1038/nature02322 (doi:10.1038/nature02322) [DOI] [PubMed] [Google Scholar]
- 35.MacDougall A. S. 2005. Responses of diversity and invasibility to burning in a northern oak savannah. Ecology 86, 3354–3363 10.1890/04-1733 (doi:10.1890/04-1733) [DOI] [Google Scholar]
- 36.Vellend M., Bjorkman A. D., McConchie A. 2008. Environmentally biased fragmentation of oak savannah habitat on southern Vancouver Island, British Columbia, Canada. Biol. Conserv. 141, 2576–2584 10.1016/j.biocon.2008.07.019 (doi:10.1016/j.biocon.2008.07.019) [DOI] [Google Scholar]
- 37.Lilley P., Vellend M. 2009. Negative native–exotic diversity relationship in oak savannahs explained by human influence and climate. Oikos 118, 1373–1382 10.1111/j.1600-0706.2009.17503.x (doi:10.1111/j.1600-0706.2009.17503.x) [DOI] [Google Scholar]
- 38.MacDougall A. S., Turkington R. 2006. Dispersal, competition, and shifting patterns of diversity in a degraded oak savannah. Ecology 87, 1831–1843 10.1890/0012-9658(2006)87[1831:DCASPO]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1831:DCASPO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 39.Salonius P. O., Robinson J. B., Chase F. E. 1967. A comparison of autoclaved and gamma-irradiated soils as media for microbial colonization experiments. Plant Soil 27, 239–248 10.1007/BF01373392 (doi:10.1007/BF01373392) [DOI] [Google Scholar]
- 40.McNamara N. P., Black H. I. J., Beresford N. A., Parekh N. R. 2003. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 24, 117–132 10.1016/S0929-1393(03)00073-8 (doi:10.1016/S0929-1393(03)00073-8) [DOI] [Google Scholar]
- 41.Berns A. E., Philipp H., Narres H. D., Burauel P., Vereecken H., Tappe W. 2008. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Euro. J. Soil Sci. 59, 540–550 10.1111/j.1365-2389.2008.01016.x (doi:10.1111/j.1365-2389.2008.01016.x) [DOI] [Google Scholar]
- 42.SAS 2009. JMP 8 user guide, 2nd edn Cary, NC: SAS Institute [Google Scholar]
- 43.Bever J. D. 1994. Feedback between plants and their soil communities in an old field community. Ecology 75, 1965–1977 10.2307/1941601 (doi:10.2307/1941601) [DOI] [Google Scholar]
- 44.Bever J. D. 2003. Soil community dynamics and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 157, 465–473 10.1046/j.1469-8137.2003.00714.x (doi:10.1046/j.1469-8137.2003.00714.x) [DOI] [PubMed] [Google Scholar]
- 45.Packer A., Clay K. 2000. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 478–481 10.1038/35005072 (doi:10.1038/35005072) [DOI] [PubMed] [Google Scholar]
- 46.Volkov I., Banavar J. R., Hubbell S. P., Maritan A. 2009. Inferring species interactions in tropical forests. Proc. Natl Acad. Sci. USA 106, 13 854–13 859 10.1073/pnas.0903244106 (doi:10.1073/pnas.0903244106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seifert E. K., Bever J. D., Maron J. L. 2009. Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90, 1055–1062 10.1890/08-0419.1 (doi:10.1890/08-0419.1) [DOI] [PubMed] [Google Scholar]
- 48.Bakker J. P., Wilson S. D. 2001. Competitive abilities of introduced and native grasses. Plant Ecol. 157, 117–125 10.1023/A:1013972403293 (doi:10.1023/A:1013972403293) [DOI] [Google Scholar]
- 49.MacDougall A. S., Turkington R. 2005. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology 86, 42–55 10.1890/04-0669 (doi:10.1890/04-0669) [DOI] [Google Scholar]
- 50.Freckleton R. P., Lewis O. T. 2006. Pathogens, density dependence and the coexistence of tropical trees. Proc. R. Soc. B 273, 2909–2916 10.1098/rspb.2006.3660 (doi:10.1098/rspb.2006.3660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bais H. P., Vepachedu R., Gilroy S., Callaway R. M., Vivanco J. M. 2003. Allelopathy and exotic plants: from genes to invasion. Science 301, 1377–1380 10.1126/science.1083245 (doi:10.1126/science.1083245) [DOI] [PubMed] [Google Scholar]
- 52.Callaway R. M. 2007. Positive interactions and interdependence in plant communities. Dordrecht, The Netherlands: Springer [Google Scholar]
- 53.Augspurger C. K. 1992. Experimental studies of seedling recruitment from contrasting seed distributions. Ecology 73, 1270–1284 10.2307/1940675 (doi:10.2307/1940675) [DOI] [Google Scholar]
- 54.Gilbert G. S., Hubbell S. P., Foster R. B. 1994. Density and distance-to-adult effects of a canker disease of trees in a moist tropical forest. Oecologia 98, 100–108 10.1007/BF00326095 (doi:10.1007/BF00326095) [DOI] [PubMed] [Google Scholar]
- 55.Zadocks J. C., van den Bosch F. 1994. On the spread of plant disease: a theory of foci. Ann. Rev. Phytopath. 32, 503–521 10.1146/annurev.py.32.090194.002443 (doi:10.1146/annurev.py.32.090194.002443) [DOI] [PubMed] [Google Scholar]
- 56.McCormick M. K., Whigham D. F., O'Neill J. P., Becker J. J., Werner S., Rasmussen H. N., Bruns T. D., Taylor D. L. 2009. Abundance and distribution of Corallorhiza odontorhiza reflect variations in climate and ectomycorrhizae. Ecol. Monogr. 79, 619–635 10.1890/08-0729.1 (doi:10.1890/08-0729.1) [DOI] [Google Scholar]
- 57.Reinhart K. O., Clay K. 2009. Spatial variation in soil-borne disease dynamics of a temperate tree, Prunus serotina. Ecology 90, 2984–2993 10.1890/08-1380.1 (doi:10.1890/08-1380.1) [DOI] [PubMed] [Google Scholar]
- 58.Gonzales E. K., Arcese P. 2008. Herbivory more limiting than competition on early and established native plants in an invaded meadow. Ecology 89, 3282–3289 10.1890/08-0435.1 (doi:10.1890/08-0435.1) [DOI] [PubMed] [Google Scholar]
- 59.MacDougall A. S., Duwyn A., Jones N. T. 2010. Consumers drive oak recruitment failure. Ecology 91, 2092–2099 10.1890/09-0204.1 (doi:10.1890/09-0204.1) [DOI] [PubMed] [Google Scholar]
- 60.Marsico T. D., Hellmann J. J. 2009. Dispersal limitation inferred from an experimental translocation of Lomatium (Apiaceae) species outside their geographic ranges. Oikos 118, 1783–1792 10.1111/j.1600-0706.2009.17698.x (doi:10.1111/j.1600-0706.2009.17698.x) [DOI] [Google Scholar]
- 61.Shaben J., Myers J. H. 2010. Relationships between Scotch broom (Cytisus scoparius), soil nutrients, and plant diversity in the Garry oak savannah ecosystem. Plant Ecol. 207, 81–91 10.1007/s11258-009-9655-7 (doi:10.1007/s11258-009-9655-7) [DOI] [Google Scholar]