Abstract
The nest micro-environment is a widely studied area of avian biology, however, the contribution of nest conductance (the inverse of insulation) to the energetics of the incubating adult and offspring has largely been overlooked. Surface-specific thermal conductance (W °C−1 cm−2) has been related to nest dimensions, wall porosity, height above-ground and altitude, but the most relevant measure is total conductance (G, W °C−1). This study is the first to analyse conductance allometrically with adult body mass (M, g), according to the form G = aMb. We propose three alternative hypotheses to explain the scaling of conductance. The exponent may emerge from: heat loss scaling (M0.48) in which G scales with the same exponent as thermal conductance of the adult bird, isometric scaling (M0.33) in which nest shape is held constant as parent mass increases, and structural scaling (M0.25) in which nests are designed to support a given adult mass. Data from 213 cup-shaped nests, from 36 Australian species weighing 8–360 g, show conductance is proportional to M0.25. This allometric exponent is significantly different from those expected for heat loss and isometric scaling and confirms the hypothesis that structural support for the eggs and incubating parent is the primary factor driving nest design.
Keywords: bird nest design, insulation, thermal conductance, scaling, allometry, reproductive energetics
1. Introduction
Birds' nests have evolved into many shapes and sizes, but all function to provide a secure substrate for eggs and hatchlings, camouflage and defence from predators, as well as moderate the micro-environment (temperature, humidity, gas composition) surrounding the eggs and hatchlings [1–3]. Of the variables that influence the nest micro-environment, nest temperature is among one of the most widely studied [4–11], because of its importance for the development and growth of young [1,12]. Maintenance of nest temperature may conflict with the life demands of the parent birds—such as the need to leave the nest to forage and to conserve energy while incubating [1]. Whether the parent's energy requirement increases during incubation of eggs is not clear. Some studies show an increase in metabolic heat production [13–15], while others show that resting metabolism of incubating birds can be lower than that of non-incubating individuals owing to the insulation provided by the nest and appropriate nest-site selection [16–18]. Furthermore, the energy demands of the parent and offspring increase after hatching [19,20]. Therefore, in addition to attenuating changes in egg temperature, well-insulated nests may also provide an environment in which the energy reserves of the parents and hatchlings may be conserved at low ambient temperatures.
It is reasonable that heat loss can be minimized by optimizing the physical structure and location of the nest [16,21]. The energy invested to maintain egg temperature and re-warm clutches that have cooled during incubation recesses can influence the outcome of current or subsequent breeding attempts [21,22]. A reduced energetic demand of incubation can enable parents to invest more in care of the offspring later in the breeding attempt, in turn increasing fledging success, or have indirect fitness consequences such as an increase in clutch size and decreased incubation periods [21,22]. The energetic demand of incubation largely depends on the rate at which heat energy is lost from the clutch and incubating parent. Therefore, nest insulation is arguably important to the lifetime reproductive success of birds. Yet, it is surprising that there are few investigations of the thermal properties of nests.
Nest insulation is expressed in terms of the conductance of heat through the nesting material, where well-insulated nests have low conductance and vice versa. Surface-specific nest conductance has been investigated in a number of studies that have reported relationships with nest mass [23], thickness [23–25], depth [23], wall porosity [23,24,26], surface area, height above-ground [23] and elevation above sea level [26]. However, surface-specific conductance is an inadequate descriptor of the quality of a nest in terms of the amount of energy that the incubating bird has to expend to maintain the nest temperature. More important to the energetics of the bird is the total conductance of the nest, which is the total quantity of heat that passes through the nest per degree of temperature difference between the inside and outside. Remarkably, total conductance has been measured in only one species, the Eurasian tree sparrow (Passer montanus) [27]. That study measured total conductance of the parent and nest and found that nest-lining within a nest-box decreased conductance from the bird by 23 to 36 per cent, in comparison to an empty nest-box that decreased heat loss by 18 per cent.
Two nests could have the same surface-specific conductance, but have greatly different rates of heat loss under the same conditions, if they were different sizes. Therefore, the only way to assess the thermal qualities of nests of different sizes is to measure total conductance and analyse it allometrically, that is, in relation to size. Allometric relationships are of the form y = aMb, where y is the measured variable of interest and M is body mass [28]. The ‘a’ value defines the elevation (height) of the curve and the exponent ‘b’ describes the way the curve bends. Allometric relationships with a positive exponent describe an increase in the variable of interest as body mass increases, whereas negative exponents describe a decrease. Examination of the scaling exponents provides insights to the factors that affect nest construction. In particular, we pose three alternative hypotheses and test the data against them.
(a). Heat loss scaling
If insulation is a major factor in the evolution of nest design, thermal conductance of the nest would scale with adult bird body mass in a similar way as plumage conductance, that is, with an exponent of 0.48 [29]. Such scaling would support an insulating function.
(b). Isometric scaling
If nest size is proportional to bird size, then the nest should increase isometrically, in which case thermal conductance would scale to the 0.33 power. This exponent emerges because conductance (M0.33) is proportional to the ratio of surface area (M0.67) divided by thickness (M0.33).
(c). Structural scaling
If structural considerations of nest construction are of primary importance, then the nest must be able to support the combined weight of the bird, the young and the nest itself, which are assumed to be proportional to the body mass of the adult bird. In this case, nest thermal conductance would be secondarily related to nest mass.
According to engineering principles, the mass of supporting structures scales to the mass of the supported object with an exponent of 1.33 [28]. The exponent deals with an entirely self-supporting structure, similar to the ideal mass of the skeleton to support an animal's mass [30]. In the case of a nest, the exponent takes into account support of not only the nest itself, but also the clutch and parent. The exponent stems from the fact that the mass of the object must be proportional to the surface area that supports them. To normalize the stress on the cross-sectional surface area of a supporting structure, its area should scale proportionally with mass, that is, with an exponent of 1.0. However, area is two-dimensional and, therefore, the third linear dimension, which scales with mass to the exponent 0.33, must be added to achieve the three-dimensional, self-supporting structure [28].
Assuming that (i) the nest is a thick-walled hemisphere made of a material with a constant thermal conductivity, (ii) the nest maintains the same shape as it increases in size, and (iii) the nest volume scales with bird mass to the 1.33 power, then the total conductance should scale with bird mass to the 0.25 power. This exponent emerges from geometry because conductance (M0.25) is proportional to geometric mean surface area (M0.77) divided by nest thickness (M0.52). The higher exponent for nest volume (M1.33) increases the external diameter of the nest. This in turn raises the exponents for nest thickness and surface area above what would be expected based on isometric scaling. The derivation of these exponents is provided in the electronic supplementary material.
2. Methods
To establish which factor influences nest conductance, we measured the thermal properties and dimensions of 213 cup-shaped nests across 36 species and parent masses ranging from 8 to 360 g. In brief, nest external and internal diameters (dE and dI, respectively) and heights (hE and hI, respectively) were measured, and thickness (X) and geometric mean surface area (A) calculated. Values of nest mass (MN) were obtained for nests without supporting branches. Then, thermal conductance (G) was measured in still air by relating the rate of heat production by an internal heater (Φ) to the mean temperature difference across the nest (ΔT) according to the equation for Newton's Law of Cooling, G = Φ/ΔT [31].
Statistical analyses on allometric relationships were performed in JMP IN (SAS Institute Inc., v. 4.0.4) using linear regression techniques on log-transformed data. Analysis of covariance (ANCOVA) techniques were used to test if multiple regression slopes differed and to test the model output values against nest data. Residuals of the data met the assumptions required for parametric linear regression tests of normality (Shapiro–Wilk W-test) and equal variance. The significance value was set at 0.05 for all analyses. Data are expressed as mean ± 95% confidence interval (CI).
For details of methods see the electronic supplementary material.
3. Results
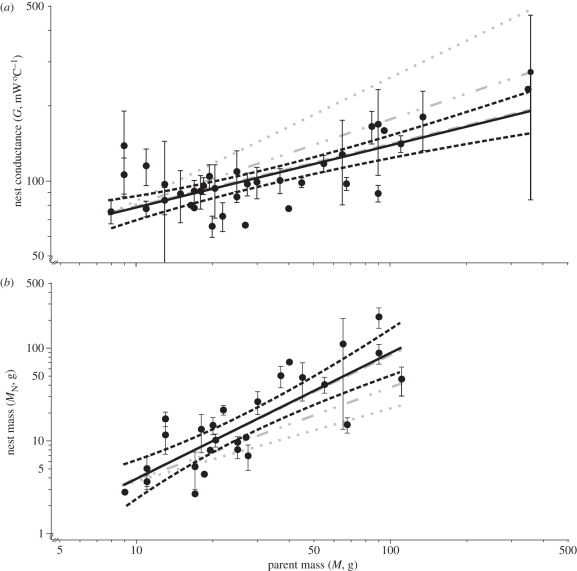
Nest thermal properties are significantly correlated with parent mass (table 1). Values for each species are listed in electronic supplementary material, table S2. Nest conductance scales positively with parent mass (figure 1a). The scaling exponent is 0.25, which is equivalent to the expected exponent for a load-bearing structure, but differs significantly from that of an isometric object and an object designed to prevent heat loss (table 2). The scaling exponent of nest mass is 1.36 (figure 1b), which is also not significantly different from the expected exponent of 1.33, on structural grounds (table 2).
Table 1.
Allometric relationships between parent mass (M, g) and the thermal properties and dimensions of avian cup-shaped nests according to y = aMb. Statistics include the intercept of the regression mean (a) and scaling exponent (slope, b) according to the allometric relationship y = aMb, as well as the correlation coefficient (r2) and F-ratio. The intercept and scaling exponent values represent the mean ± 95% CI for all species. n = 36 and d.f. = 1,34 for all comparisons, except nest mass which has n = 27 and d.f. = 1,25. The replicate for the nest mass measurements is lower as some nests were excluded from analysis owing to the attachment of supporting branches.
| intercept, a | scaling exponent, b | r2 | F-ratio | |
|---|---|---|---|---|
| internal diameter (dI, cm) | 2.03 ± 1.09 | 0.35 ± 0.02 | 0.96 | 865.23a |
| external diameter (dE, cm) | 2.35 ± 1.18 | 0.42 ± 0.04 | 0.91 | 352.99a |
| internal height (hI, cm) | 1.74 ± 1.24 | 0.26 ± 0.06 | 0.70 | 78.85a |
| external height (hE, cm) | 2.05 ± 1.34 | 0.32 ± 0.08 | 0.66 | 65.12a |
| thickness (X, cm) | 0.30 ± 1.64 | 0.49 ± 0.14 | 0.61 | 53.24a |
| nest mass (MN, g) | 0.17 ± 3.77 | 1.36 ± 0.39 | 0.67 | 51.09a |
| surface area (A, cm2) | 11.65 ± 1.29 | 0.68 ± 0.07 | 0.92 | 394.33a |
| total nest conductance (G, mW °C−1) | 43.98 ± 1.31 | 0.25 ± 0.08 | 0.57 | 44.59a |
| surface-specific nest conductance (GA, W °C−1 m−2) | 37.74 ± 1.42 | −0.44 ± 0.10 | 0.71 | 81.44a |
| thermal conductivity (k, mW °C−1 m−1) | 111.61 ± 1.50 | 0.06 ± 0.11 | 0.03 | 1.09 |
aIndicates that there is a significant effect of parent mass on the variable (p < 0.0001 at α = 0.05) in all cases with exception to thermal conductivity which was not significantly related to mass (p = 0.30).
Figure 1.
Relationship between parent mass (M, g) and (a) total nest conductance (mW °C−1, G = 43.98 × M0.25) and (b) nest mass (g, MN = 0.17 × M1.36) for cup-shaped birds' nests. Each point represents the mean ± 95% CI for a species of bird (n[a] = 36, n[b] = 27). Solid lines represent the regression mean. Black dashed lines represent the 95% confidence bands for the regression mean. The modelled regression for heat loss scaling are represented by a grey dotted line, isometric scaling by a grey dash-dotted line and structural scaling by a grey dashed line.
Table 2.
Model predictions for the allometric relationship between parent mass (M, g) and the thermal properties and dimensions of a hemispherical object according to y = aMb for heat loss, isometric and structural scaling, and tests for significant differences between actual scaling exponents (table 1) and predictions. Nest thermal properties model output values represent the exponent (slope, b) of the regression mean according to the allometric relationship y = aMb. The scaling exponent values represent the mean for all masses. Accompanying statistics include the F-ratio and p-value (the statistical probability of rejecting the null hypothesis (p > F)) for the ANCOVA comparisons between the model predictions and real nest data. n = 117 and d.f. = 1,113 for all comparisons, except nest mass, which has n = 108 and d.f. = 1,104. The replicate for the nest mass measurements is lower as some nests were excluded from analysis owing to the attachment of supporting branches.
| nest parameters | heat loss scaling | isometric scaling | structural scaling |
|---|---|---|---|
| internal diameter (dI, cm) | M0.33, F = 7.41, p = 0.01a | M0.33, F = 7.41, p = 0.01a | M0.33, F = 7.41, p = 0.01a |
| external diameter (dE, cm) | M0.30, F = 65.18, p < 0.001a | M0.33, F = 34.48, p < 0.001a | M0.43, F = 1.37, p = 0.24 |
| internal height (hI, cm) | M0.33, F = 16.69, p < 0.001a | M0.33, F = 16.69, p < 0.001a | M0.33, F = 16.69, p < 0.001a |
| external height (hE, cm) | M0.30, F = 0.63, p = 0.43 | M0.33, F = 0.20, p = 0.66 | M0.43, F = 18.94, p < 0.001a |
| thickness (X, cm) | M0.15, F = 62.58, p < 0.001a | M0.33, F = 13.92, p < 0.001a | M0.52, F = 0.40, p = 0.53 |
| surface area (A, cm2) | M0.64, F = 4.80, p = 0.03a | M0.67, F = 0.57, p = 0.45 | M0.77, F = 14.25, p < 0.001a |
| volume (V, ml) or mass (MN, g) | M0.78, F = 33.94, p < 0.001a | M1, F = 13.41, p < 0.001a | M1.33, F = 0.09, p = 0.76 |
| total nest conductance (G, mW °C−1) | M0.48, F = 99.12, p < 0.001a | M0.33, F = 13.36, p < 0.001a | M0.25, F = 0.01, p = 0.92 |
aIndicates that the scaling exponent for the model output and real nest data are statistically different at the specified alpha value (α = 0.05) following Bonferroni adjustment.
Nest dimensions are also significantly correlated with parent mass (table 1). Values for each species are listed in electronic supplementary material, table S3. Nest thickness (M0.49; electronic supplementary material, figure S3b) and external diameter (M0.42; electronic supplementary material, figure S3c) have a scaling exponent higher than that of isometric or heat loss scaling, but not significantly different from that of a structural object (table 2). Internal diameter (M0.35; electronic supplementary material, figure S3c) has a scaling exponent higher than would be expected based on isometric, structural and heat loss scaling. On the other hand, the scaling exponent for internal nest height (M0.26; electronic supplementary material, figure S3d) is statistically lower than that for isometric, structural and heat loss scaling. The scaling exponent for external nest height (M0.32; electronic supplementary material, figure S3d) is statistically similar to isometric and heat loss scaling, but differs significantly from scaling of a structural object. Furthermore, the scaling exponent for nest surface area (M0.68; electronic supplementary material, figure S3a) is statistically indistinguishable from that of an isometric object; however, it differs significantly from that of a structural object as well as an object designed to prevent heat loss.
There is a distinct pattern for nest diameter to increase at a greater rate in comparison to nest height as bird mass increases. This is true for both internal (F1,68 = 9.35, p = 0.003, n = 72) and external (F1,38 = 4.23, p = 0.04, n = 72) dimensions. Therefore, large birds build nests that are shallower than the nests of small birds.
Surface-specific conductance scales negatively with parent mass (electronic supplementary material, figure S4a). Large birds build nests that have a lower conductance per unit area compared with small birds. On the other hand, the conductance value of the materials used in the nest, as determined by the thermal conductivity, is independent of parent mass (electronic supplementary material, figure S4b).
4. Discussion
This study indicates that the requirement for adequate structural support is the primary selective influence on nest construction, not the requirement for insulation. As birds increase in size, nest surface area increases isometrically, however, nests become much thicker than what we would expect based on isometric or heat loss scaling. The thick walls provide structural support for the parent and clutch, with the consequence that the exponent for thermal conductance has to decrease, according to the relationship outlined in electronic supplementary material, equation S20.
Because the measured scaling exponent for thermal conductance is 0.25, but the hypothetical exponent for heat loss scaling is 0.48, it is clear that structurally adequate nests achieve a lower thermal conductance (higher insulation) than expected, as they increase in size. The consequence of this is that thermal conductance would be important only in small birds, if at all. Exactly, how small is not clear, but this study focuses on nests from birds weighing between 8 and 360 g, a range representing the largest proportion of the world's birds [32]. It is significant that there is little tendency for thermal conductance to drop below the regression line in the smallest nests of this study (figure 1a), which suggests that they are not overly insulated. Nevertheless, as nests become larger, they also become shallower, which may represent a relaxation of the role of insulation. This confirms previous suggestions by Ricklefs ([33] cited in [3]) regarding nest shape. Hansell [3] further proposed that the change in shape means that eggs of small birds may be more protected from the elements. This may in turn offset the need for small birds, with high metabolic demands, to be so attentive to eggs, enabling them to forage more often.
There are over 9000 species of birds worldwide, ranging in size from the bee hummingbird (Calypte helenae, 2 g) that constructs nests approximately two centimetres in diameter to the ostrich (Struthio camelus, 100 kg) that uses a 1 m wide scrape in the ground in which to lay its eggs [32]. Cup-shaped nests are but a fraction of the nest types used by birds. Nests can involve complicated construction such as the 5 m wide incubation mounds of the mallee-fowl (Leipoa ocellata), the excavated cavities of the red-headed woodpecker (Melanerpes erythrocephalus) and underground burrows of the storm petrel (Hydrobates pelagicus) [34–36]. Other species use pre-existing tree hollows (e.g. glossy-black cockatoo, Calyptorhynchus lathami halmaturinus), cliff edges (e.g. bank swallow, Riparia riparia) or scrapes on bare ground (e.g. piping plovers, Charadrius melodus) as their nesting substrate [37–39]. For these species, structural support for the eggs is provided by a solid surface and the parent is less able to modify the insulation value of the nest. Furthermore, a few species do not use nests at all—such as the emperor penguin (Aptenodytes forsteri) that rests a single egg on their feet, which is then enfolded with their brood flap [40]. In this case, support of the egg is paramount and incubation is constant—negating the need for an insulative nest altogether. When considering the above findings in the context of nests, in general, it is not surprising that nest conductance is not driven by heat loss from the incubating parent, as bird species are able to employ different incubation strategies and use nests of various types to maintain an appropriate egg temperature for embryo development.
While this study shows that the requirement for structural support is the main driver on nest dimensions and, therefore, the thermal properties of nests across multiple species, it is possible that ambient temperature may still have some influence at a species-specific level. For example, the surface-specific conductance of nests of the Hawaiian honeycreeper (Hemignathus virens virens) at high elevations is lower than those at low elevations [26]. Nest insulation is clearly important for the nests of some birds, as a distinct layer of well-insulating materials can be found in the nests of many species [16]. The eider duck (Somateria mollissima) is a well-known example—females cover the eggs with down feathers prior to incubation recesses to reduce the extent of egg cooling, however, the nests of this species are constructed on the ground where structural support of the eggs is inherent [41].
This study was conducted on nests from within Australia and, therefore, it would be of interest to repeat the study using fresh samples and over greater geographical and climatic ranges. While further sampling seems unlikely to influence significantly the exponent of the allometric relationship with conductance, it may shed light on the full extent of variation in nest design by including species that nest in warmer and colder climates than those included in this analysis.
An understanding of the influence of climate, wind and rain on nest thermal properties may help to clarify some of the within-species variation observed in this study. Greater knowledge regarding the influence of clutch size on nest dimensions and, in turn, the thermal properties of the nest may also prove useful.
Acknowledgements
We thank the South Australian Museum (in particular, Philippa Horton) and Queensland Museum (mainly, Heather Janetzki) for use of their nest collections and Richard Norrish (The University of Adelaide) for designing and constructing the controlled temperature power monitor circuit. We thank the University of Queensland (principally, Craig White) for allowing the use of their laboratory facilities. We also thank the reviewers for their useful comments and improving the clarity of the text.
References
- 1.Ar A., Sidis Y. 2002. Nest microclimate during incubation. In Avian incubation (ed. Deeming D. C.), pp. 143–160 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Collias N. E., Collias E. C. 1984. Nest building and bird behaviour. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Hansell M. 2000. Bird nests and construction behaviour. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Bartholomew G. A., White F. N., Howell T. R. 1976. The thermal significance of the nest of the sociable weaver, Philetairus socius: summer observations. Ibis 118, 402–410 10.1111/j.1474-919X.1976.tb02027.x (doi:10.1111/j.1474-919X.1976.tb02027.x) [DOI] [Google Scholar]
- 5.Caccamise D. F., Weathers W. W. 1977. Winter nest microclimate of monk parakeets. Wilson Bull. 89, 346–349 [Google Scholar]
- 6.Hadley N. F. 1969. Microenvironmental factors influencing the nesting sites of some subalpine fringillid birds in Colorado. Arct. Alp. Res. 1, 121–126 10.2307/1550018 (doi:10.2307/1550018) [DOI] [Google Scholar]
- 7.Howell T. R., Dawson W. R. 1954. Nest temperatures and attentiveness in the Anna hummingbird. Condor 56, 93–97 10.2307/1364665 (doi:10.2307/1364665) [DOI] [Google Scholar]
- 8.Orr Y. 1970. Temperature measurements at the nest of the desert lark (Ammomanes deserti deserti). Condor 72, 476–478 10.2307/1366401 (doi:10.2307/1366401) [DOI] [Google Scholar]
- 9.Ricklefs R. E., Hainsworth F. R. 1969. Temperature regulation in nestling cactus wrens: the nest environment. Condor 71, 32–37 10.2307/1366045 (doi:10.2307/1366045) [DOI] [Google Scholar]
- 10.Webb D. R. 1987. Thermal tolerance of avian embryos: a review. Condor 89, 874–898 10.2307/1368537 (doi:10.2307/1368537) [DOI] [Google Scholar]
- 11.White F. N., Bartholomew G. A., Howell T. R. 1975. The thermal significance of the nest of the sociable weaver, Philetairus socius: winter observations. Ibis 117, 171–179 10.1111/j.1474-919X.1975.tb04205.x (doi:10.1111/j.1474-919X.1975.tb04205.x) [DOI] [Google Scholar]
- 12.Deeming D. C., Ferguson M. W. J. 1991. Physiological effects of incubation temperature on embryonic development in reptiles and birds. In Egg incubation: its effects on embryonic development in birds and reptiles (eds Deeming D. C., Ferguson M. W. J.), pp. 147–171 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Gloutney M. L., West N., Clark R. G. 1996. Metabolic costs of incubation and clutch size determination in the red junglefowl, Gallus gallus spadiceus. Comp. Biochem. Phys. A 114, 265–270 10.1016/0300-9629(96)00006-0 (doi:10.1016/0300-9629(96)00006-0) [DOI] [Google Scholar]
- 14.Haftorn S., Reinertsen R. E. 1985. The effect of temperature and clutch size on the energetic cost of incubation in a free-living blue tit (Parus caeruleus). Auk 102, 470–478 [Google Scholar]
- 15.Töien O., Aulie A., Steen J. B. 1986. Thermoregulatory responses to egg cooling in incubating bantam hens. J. Comp. Physiol. B 156, 303–307 10.1007/BF01101091 (doi:10.1007/BF01101091) [DOI] [Google Scholar]
- 16.Hilton G. M., Hansell M. H., Ruxton G. D., Reid J. M., Monaghan P. 2004. Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 121, 777–787 10.1642/0004-8038(2004)121[0777:UANTTI]2.0.CO;2 (doi:10.1642/0004-8038(2004)121[0777:UANTTI]2.0.CO;2) [DOI] [Google Scholar]
- 17.Walsberg G. E. 1978. The energetic consequences of incubation for two passerine species. Auk 95, 644–655 [Google Scholar]
- 18.Walsberg G. E., King J. R. 1978. The heat budget of incubating mountain white-crowned sparrows (Zonotrichia leucophrys oriantha) in Oregon. Physiol. Zool. 51, 92–103 [Google Scholar]
- 19.Ricklefs R. E. 1983. Avian postnatal development. In Avian biology (eds Farner D. S., King J. R., Parkes K. C.), pp. 1–83 New York, NY: Academic Press [Google Scholar]
- 20.Williams J. B. 1996. Energetics of avian incubation. In Avian energetics and nutritional ecology (ed. Carey C.), pp. 375–415 New York, NY: Chapman and Hall [Google Scholar]
- 21.Reid J. M., Monaghan P., Ruxton G. D. 2000. Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proc. R. Soc. Lond. B 267, 37–41 10.1098/rspb.2000.0963 (doi:10.1098/rspb.2000.0963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinbergen J. M., Williams J. B. 2002. Energetics of incubation. In Avian incubation (ed. Deeming D. C.), pp. 299–313 Oxford, UK: Oxford University Press [Google Scholar]
- 23.Kern M. 1984. Racial differences in nests of white-crowned sparrows. Condor 86, 455–466 10.2307/1366826 (doi:10.2307/1366826) [DOI] [Google Scholar]
- 24.Skowron C., Kern M. 1980. The insulation in nests of selected North-American songbirds. Auk 97, 816–824 [Google Scholar]
- 25.Whittow F. N., Berger A. J. 1977. Heat loss from the nest of Hawaiian honeycreeper, ‘Amakihi’. Wilson Bull. 89, 480–483 [Google Scholar]
- 26.Kern M. D., Van Riper C. 1984. Altitudinal variations in nests of the Hawaiian honeycreeper Hemignathus virens virens. Condor 86, 443–454 10.2307/1366825 (doi:10.2307/1366825) [DOI] [Google Scholar]
- 27.Pinowski J., Haman A., Jerzak L., Pinowska B., Barkowska M., Grodzki A., Haman K. 2006. The thermal properties of some nests of the Eurasian tree sparrow Passer montanus. J. Therm. Biol. 31, 573–581 10.1016/j.jtherbio.2006.05.007 (doi:10.1016/j.jtherbio.2006.05.007) [DOI] [Google Scholar]
- 28.Schmidt-Nielsen K. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Schleucher E., Withers P. C. 2001. Re-evaluation of the allometry of wet thermal conductance for birds. Comp. Biochem. Phys. A 129, 821–827 10.1016/S1095-6433(01)00356-7 (doi:10.1016/S1095-6433(01)00356-7) [DOI] [PubMed] [Google Scholar]
- 30.Prange H. D., Anderson J. F., Rahn H. 1979. Scaling of skeletal mass to body mass in birds and mammals. Am. Nat. 113, 103–122 10.1086/283367 (doi:10.1086/283367) [DOI] [Google Scholar]
- 31.Tracy C. R. 1972. Newton's law: its application for expressing heat losses from homeotherms. BioScience 22, 656–659 10.2307/1296267 (doi:10.2307/1296267) [DOI] [Google Scholar]
- 32.Blackburn T. M., Gaston K. J. 1994. The distribution of body sizes of the world's bird species. Oikos 70, 127–130 10.2307/3545707 (doi:10.2307/3545707) [DOI] [Google Scholar]
- 33.Ricklefs R. E. 1974. Energetics of reproduction in birds. In Avian energetics (ed. Paynter R. A.), pp. 152–297 Cambridge, MA: Nuttall Ornithological Club [Google Scholar]
- 34.Bolton M. 1996. Energy expenditure, body-weight and foraging performance of storm petrels Hydrobates pelagicus breeding in artificial nesting chambers. Ibis 138, 405–409 10.1111/j.1474-919X.1996.tb08058.x (doi:10.1111/j.1474-919X.1996.tb08058.x) [DOI] [Google Scholar]
- 35.Conner R. N. 1975. Orientation of entrances to woodpecker nest cavities. Auk 92, 371–374 [Google Scholar]
- 36.Frith H. J. 1962. The mallee-fowl: the bird that builds an incubator. Sydney, Australia: Angus and Robertson [Google Scholar]
- 37.Garnett S. T., Pedler L. P., Crowley G. M. 1999. The breeding biology of the glossy black-cockatoo Calyptorhynchus lathami on Kangaroo Island, South Australia. Emu 99, 262–279 10.1071/MU99032 (doi:10.1071/MU99032) [DOI] [Google Scholar]
- 38.Mayer P. M., Smith L. M., Ford R. G., Watterson D. C., McCutchen M. D., Ryan M. R. 2009. Nest construction by a ground-nesting bird represents a potential trade-off between egg crypticity and thermoregulation. Oecologia 159, 893–901 10.1007/s00442-008-1266-9 (doi:10.1007/s00442-008-1266-9) [DOI] [PubMed] [Google Scholar]
- 39.Petersen A. J. 1955. The breeding cycle in the bank swallow. Wilson Bull. 67, 235–286 [Google Scholar]
- 40.Handrich Y. 1989. Incubation water loss in king penguin egg. I. change in egg and brood pouch parameters. Physiol. Zool. 62, 96–118 [Google Scholar]
- 41.Erikstad K. E., Tveraa T. 1995. Does the cost of incubation set limits to clutch size in common eiders Somateria mollissima? Oecologia 103, 270–274 10.1007/BF00328614 (doi:10.1007/BF00328614) [DOI] [PubMed] [Google Scholar]