Abstract
Previous work has suggested that larger groups of prey are more conspicuous to predators. However, this ignores that prey populations are finite. As groups get larger they become fewer, hence the encounter rate between predator and prey decreases with prey aggregation. Here, we present a two-dimensional model based on visual angle to unify these encounter and conspicuousness effects of aggregation. With experimental support using three-spined sticklebacks (Gasterosteus aculeatus L.), searching for chironomid larvae, we demonstrate that the increase in visual angle with increasing group size is outweighed by its corresponding decrease as the groups become fewer and thus further away from the searching predator. The net effect is that prey are found with more difficulty when they aggregate, giving an additional anti-predatory benefit to group living rather than a cost.
Keywords: predator–prey, aggregation, encounter, detection, conspicuousness, visual angle
1. Introduction
Explaining why many animal species live in groups in terms of costs and benefits has attracted a vast literature across taxa [1]. One such cost has been believed to be the increased conspicuousness of larger groups of prey to predators [2–4]. For example, Uetz & Hieber [5] demonstrated that approaches by predatory wasps became more frequent with increased colony size of their prey, Metepeira incrassata (an orb-weaving spider). However, increasing aggregation when population size is constant also decreases the number of groups, so that the rate of encounters between predator and prey decreases [6,7]. That is, viewing distance is not fixed, but will increase with increasing aggregation. These two effects of aggregation (encounter and conspicuousness) have so far been treated as separate issues, probably owing to considering encounter and detection as sequentially-occurring, distinct phases of predation [8]. Previous studies have assumed that either encountered prey are always detected [7,9,10] or that groups of different sizes are encountered at the same rate [2,3]. When population size is finite, however, the distance to a prey group and the group's size covary negatively. It remains unknown, therefore, which, if either, of these two effects (distance and group size) have a greater influence on the probability of detecting prey.
This idea was formalized into a simple geometric model by combining two previous published models [6,11]. Following Vine [6], we use the visual angle that a group of prey generates on the retina of the predator as a mechanism of object detection, as visual angle scales with both the distance from an object and with the object's size [12]. The model predictions were then tested by measuring search times of Gasterosteus aculeatus L. (the three-spined stickleback, a small freshwater fish) preying upon chironomid larvae. Treatments were designed to determine the isolated effects of prey group size and group number, and compared this with a situation where population size was constant with both group size and number varying simultaneously. Our goal is not to contribute to the mechanistic understanding of visual detection and/or fish foraging strategies [13,14], but to use an already well-established and widespread property of object detection (visual angle) to question whether larger groups are in fact more conspicuous to predators, given that all previous work has ignored that prey populations are finite.
(a). Model
The ‘encounter’ effect of aggregation consists of reducing the number of prey groups (N), which increases the average distance from predator to prey [7]. Assuming groups are distributed randomly and independently across a two-dimensional environment, the distance from the predator to the kth nearest prey group (dk) is determined by (the derivation of which can be found in Thompson [11]):
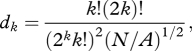 |
1.1 |
where A is the area of the habitat. As group size (G) increases, there is also an increase in the radius r of a circular group (see [6]; the ‘conspicuousness’ effect):
| 1.2 |
where λ is the length of each prey individual. Importantly, this assumes that the density of individuals within a group remains constant as group size changes. These two effects of aggregation can be combined using simple trigonometry (electronic supplementary material, figure S1) to examine the unified effect of group formation on visual angle (Σαk for k = 1–N):
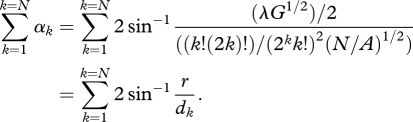 |
1.3 |
Although there is a positive effect of aggregation on visual angle from the increased size of prey groups (equation (1.2); dashed line in figure 1a), the increased distance between predator and prey has a greater, and opposite, effect (equation (1.1); dotted line in figure 1b). The net consequence is that prey become harder to find when they aggregate (solid line in figure 1a,b).
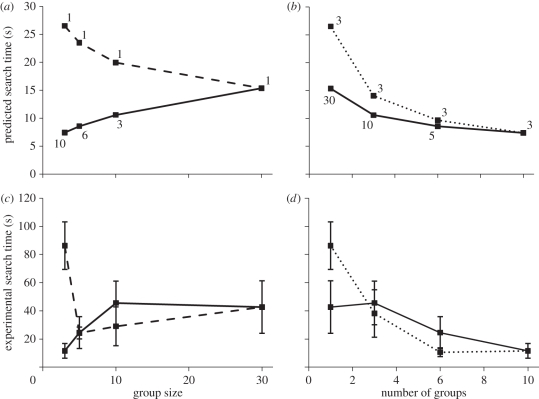
Figure 1.
The predicted (a,b) and experimentally observed (c,d) effects of group size and number on search time. The broken lines (dashed for group size, dotted for number of groups) represent variable prey population sizes; in contrast, population size is held constant with both group size and number covarying together in treatments connected with solid lines. In (c) and (d), mean (±s.e.m.) search times for each treatment are shown (n = 12, except for the 10 groups of three individuals per group treatment where n = 11). For clarity, the number next to each value in (a) and (b) gives the number of groups (a) and group size (b). The model has been parametrized to match the experiment, i.e. A = 0.5281 m2 (area of experimental arena) and λ = 0.00955 m (apparent length of individual prey). In (a) and (b), visual angle has been converted to predicted search times for ease of comparison to the experiment (based on a log–log linear fit between the total visual angle of prey at the start of each trial and the search time, i.e. log10 search time = −0.47 × log10 total visual angle + 1.62).
This trend can be explained by considering the case when only the nearest group can be found. The number of groups can be expressed as N = P/G, where P is the prey population size (another constant), so P/G can be substituted for N. Hence, if only the nearest group is considered (i.e. k = 1), equation (1.3) simplifies to
| 1.4 |
i.e. group size has no effect, as the change in visual angle with distance is perfectly cancelled by the change owing to the group's radius. Thus, when k = 1, visual angle is determined only by the size of the prey, the prey population size and the area of the landscape. If the predator can detect groups other than the nearest, however, these additional groups contribute to the total visual angle. Thus, the difficulty in finding prey should increase with aggregation if predators can detect groups in addition to the nearest (equation (1.3)), and remain constant if only the nearest prey can be detected (equation (1.4)).
2. Methods
Three-spined sticklebacks (mean length ± s.d. = 38.8 ± 4.26 mm) were caught from the estuary of the Great Eau river, UK (53°25′07″ N; 0°11′25″ E). They were kept in a grey fibreglass stock tank (80 × 50 × 50 cm deep) at 16°C on a 13 L : 11 D cycle for at least three months before testing. Fish were not in reproductive condition. Fish were fed defrosted chironomid larvae each day. These larvae were also used as the prey in the experiment. As individuals tend to be more active in groups [15], which can lead to greater conspicuousness to predators [16], dead prey were used to avoid this, and other, potentially confounding effect(s).
The test arena consisted of an opaque white plastic ring (diameter 82 cm) at the centre of a circular pool (diameter 152 cm). The bottom of the pool was a light blue hue, which gave enough contrast to visually track the fish on video, but was dark enough to help habituate the fish compared with a white background. To approximate the two-dimensional model situation above, we used a shallow water depth of 11 cm. A white cloth sheet was placed over the pool and overhead camcorder to minimize disturbance and reflections on the water surface from overhead fluorescent lighting. Transparent Petri dishes with lids (diameter 8.5 cm, height 1.3 cm) were attached to the floor of the arena, arranged in a hexagonal grid. A hexagon of 61 dishes fitted inside the arena, with the dish in one corner (against the arena wall) of the hexagon replaced with a white plastic cylinder (approx. diameter 5 cm, height 6 cm), which would be used to habituate the fish at the start of each trial, acting as a refuge.
Forty sticklebacks were placed in the arena the evening before testing, fed with chironomid larvae haphazardly scattered across the floor of the arena, then habituated overnight. The following day, all fish were removed from the arena into the surrounding pool area. The surrounding pool area had no visual contact with the test arena. Prey were then placed in the Petri dishes and the lids replaced to minimize prey odour cues. The water in the arena was also saturated with stickleback odour, and the small amount of odour released as the prey were placed in the dishes should have been minimal against this odour background. Prey were oriented haphazardly within each dish, and spaced within the dishes so that they were close but not touching one another or overlapping, which kept density within and between groups approximately constant. Dishes were selected randomly to hold the prey groups, on the conditions that none were placed in the outermost ring of dishes (to reduce edge effects), only a single group could be placed in each dish, and that no two groups would be placed in adjacent dishes (to ensure adjacent groups were not perceived as a single, larger group).
A single fish was then netted from the area outside the arena and placed gently into the plastic cylinder. Filming began at 30 frames per second from the camcorder placed 1.8 m above the centre of the arena. When ready, the fish would swim up and leave the cylinder, then search the arena for a period of time before detecting, approaching and attacking prey. The fish was removed after the first approach and attack on prey. Each fish was tested only once. If the trial lasted more than 5 min, it was not included in the analysis (approx. 20% of trials; the stringent 5 min requirement excluded fish that either took a long time to leave the refuge, i.e. those that were risk averse, and/or those fish that took a long time to attack the prey, i.e. were not highly motivated to feed). Censoring at 5 min is supported by the trial times that followed a negative binomial distribution within this time period (i.e. the duration of trials decayed within 5 min).
Before the next trial, chironomids were removed from all dishes and the arena was cleaned with a fine mesh net. This also disturbed the water, homogenizing any possible odour cues from the previous trial. Each chironomid larvae was used only once. Only fish that had been tested that day were replaced at the end of the day with fish from the stock tank (restoring the population to 40 fish). The untested fish were returned to the test arena overnight for further habituation.
Still images were extracted from the videos at three stages: the moment when the fish first emerged from the cylinder, when the fish first detected prey, and when the first attack was made. Search time was recorded as the time taken from leaving the cylinder to the detection of prey. A detection occurred when the fish changed direction immediately followed by acceleration towards a prey group in a straight line, which then always resulted in an attack on that group. Similar behaviour has been observed in other fish, for example, the bluegill sunfish, Lepomis macrochirus, and is an established method to determine the detection of prey and hence to calculate reaction distance [12,17,18]. The coordinates of the fish's snout in each of the three frames and the centre of every dish containing a group were extracted manually using ImageJ [19]. Reaction distance was calculated as the Euclidean distance between the coordinates of the fish's position at the moment of detection and where the attack was made (i.e. the distance between the positions of the fish in the second and third still images captured from each trial).
The visual angle subtended by each prey group at the start of each trial was approximated using group size, the distance from the predator to each group, and average prey length. Twenty chironomids were measured on each of the test days after being used in trials. Mean chironomid length was 13.3 mm (±1.11 s.d.). However, prey were orientated haphazardly, so each prey could be anywhere between 0° and 90° to the fish. Thus, on average, prey will be orientated 45° to the fish, and have an apparent length (λ) of 9.55 mm. This was used to estimate the radius of each group (equation (1.2); [6]), and hence the visual angle of the group could be calculated as in equation (1.3):
| 2.1 |
A single treatment was presented to single fish in a trial, treatments being ordered between trials in a complete random block. Varying group size (a single group of 3, 5, 10 or 30 chironomid larvae) determined a conspicuousness effect and varying the number of groups (one, three, six or 10 groups of three prey) tested for an encounter effect. Finally, population size was held constant, unlike the previous treatments, and both group size and number varied together (single group of 30, three groups of 10, six groups of 5 and 10 groups of three) to test for both the conspicuousness and encounter effects in combination. The total number of treatments was nine (not 12) as there are three pairs of identical treatments. Twelve replicates were carried out for each treatment, although a single video was damaged so that only 107 searches were analysed.
(a). Data analysis
Generalized Linear Models (GLMs) with negative binomial errors were used to analyse search times. Subsets of the data examined the effect on search time of group and population size (n = 83), the number of groups and population size (n = 83), and both group size and the number of groups when population size was constant (n = 47). Note that although some data appeared in more than one of these analyses, no data appeared more than once in any single analysis (avoiding pseudoreplication). Any significant interaction terms included, and so controlled for, all main effects. A linear regression on all data examined the effect of group size and the number on reaction distance (log10 transformed to achieve normality; n = 107).
Separate GLMs on all data (n = 107) were carried out to determine the effects on search time of total visual angle (visual angle of all groups summed), the visual angle of the nearest group and the visual angle of the attacked group at the beginning of the trial. Covariance between these three angles (total, nearest and attacked) excluded using a single model with all three explanatory variables. R v. 2.8.0 was used for all statistical analyses.
3. Results
The response of search times to aggregation was sensitive to whether the prey population size was fixed or allowed to vary inadvertently with group size or the number of groups. As predicted by the model, the effect of group size on search time depended on whether the number of prey individuals was constant or variable (figure 1c; GLM: group size × population size interaction: likelihood-ratio test (LRT)1,79 = 4.61, p < 0.05), as was the effect of the number of groups (figure 1d; number of groups × population size interaction: LRT1,79 = 4.69, p < 0.05). Hence, the decision to allow population size to change with the variable of interest (group size or number) or to keep it constant has an effect on the apparent relationship between search time and group size or number.
As is evident in figure 1, the effect of aggregation was greatly reduced when population size was held constant and both group size and number varied concurrently. However, there remained a net effect of aggregation in delaying finding prey. When population size was held constant, search time significantly decreased with an increasing number of groups. In fact, as predicted by the model, the number of groups had a greater effect than group size when both variables were included in the analysis (GLM: number of groups: LRT1,44 = 5.04, p < 0.05; group size: LRT1,44 = 0.62, p = 0.43). This unified effect was not as dramatic as an encounter effect in isolation, as the larger groups were more conspicuous. Reaction distance to a group, defined as the distance at which the fish made an accelerated, straight-line approach that resulted immediately in an attack, increased with group size (linear model: group size: F1,104 = 9.20, p < 0.005) but not the number of groups (F1,104 = 2.57, p = 0.11). Nonlinear least-squares regression was then used to compare linear and logarithmic fits between reaction distance and group size, with no constant included in the model (forcing the function through 0,0 as a predator cannot react at any distance to non-existent prey). The logarithmic (figure 2; reaction distance = 69.5 × ln(group size + 1)) provided a better visual fit to the data than the linear (figure 2; reaction distance = 10.3 × group size), and is supported by a lower Akaike Information Criterion value for the logarithmic (1225.4 versus 1306.0).
Figure 2.
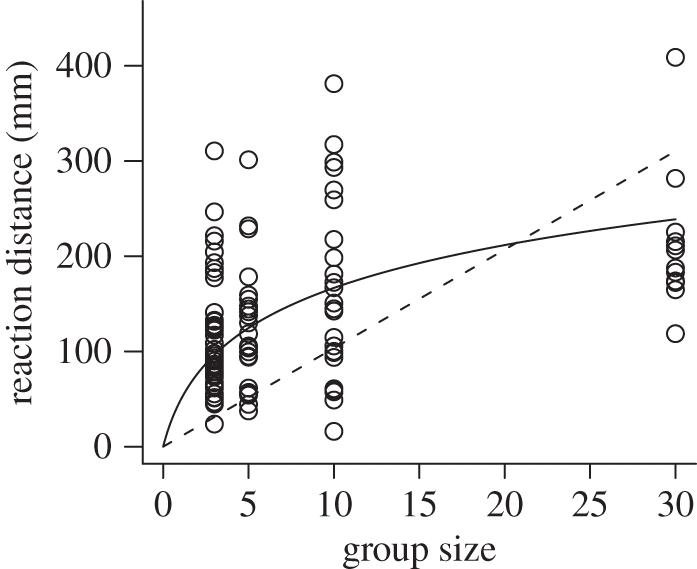
The effect of prey group size on the reaction distance of three-spined sticklebacks. Reaction distance is the distance at which the fish turns towards the prey and makes an accelerated, straight line approach. Linear (dashed) and logarithmic (solid) fits to the data are shown. Sample sizes for group sizes 3, 5, 10 and 30 are 47, 24, 24 and 12, respectively.
Of the 59 trials where more than one group of prey was present, in only 28 trials was the nearest group to the fish at the start of the trial, i.e. the group with the greatest visual angle, attacked. Accordingly, search times significantly decreased with total visual angle at the start of the trial (GLM: LRT1,105 = 6.73, p < 0.01), but were unrelated to the visual angle of the nearest group (LRT1,105 = 0.45, p = 0.50) or the visual angle of the attacked group (LRT1,105 = 0.47, p = 0.49) at the start of the trial.
4. Discussion
The results suggest that rather than being a cost associated with group living, group formation can actually decrease the likelihood that prey are located by predators. More numerous but smaller groups create a greater total visual angle than the same population of prey arranged in fewer but larger groups. This benefit to living in groups operates in a similar manner to other anti-predatory benefits of grouping, such as the confusion effect and group vigilance and defence, in that it decreases the absolute per capita risk of prey and/or increases the cost of foraging for the predator [1]. These other mechanisms operate after the prey and/or predator are detected [8], compared with the mechanism presented here that reduces the chance of prey being found and an interaction occurring at all.
Many ecological models approximate an encounter between two agents as occurring when a critical proximity between them is reached, for example, in the common ‘ideal gas’ approach [7,10]. In most sensory systems, however, detection is better described by a gradual decline as the separating distance increases, i.e. a step function relating distance and conspicuousness is not appropriate. Visual angle as an alternative approach has been used to successfully predict the relative consumption rates of differently sized planktonic prey [20,21] and has been shown to have a neurophysiological basis (e.g. [22,23]). The implication is that disentangling encounter and detection may be impossible in habitats where prey are distributed at a similar scale to the visual constraints of the predator. Search times were in fact best predicted by the visual angle of all the groups summed at the start of the trial (total visual angle) as used in the model. In contrast, the visual angle subtended by the nearest group or the group that was actually attacked had no effect on search time. This is consistent with the fish having multiple groups within their visual field, although further experimental work is needed to explicitly test this assumption of the model. Interestingly, this may depend on the colour contrast of the prey against their background owing to neuronal mechanisms, as has recently been shown to be the case in bees [24].
That the experimental results show similar trends to that predicted by the model supports our contention that we present a minimal, but adequate, model. This was in spite of excluding a number of biologically relevant factors to minimize the number of parameters. Our intention in this study was to present a very general model that can be elaborated upon in further studies and made specific to particular systems. Even without considering predator and prey movement or the specifics of the fish's visual system, there is a reasonably good fit between model and experiment. The model is thus more biologically realistic than assuming that encounters occur within a set distance from the prey [7], but lacks the specificity (and accuracy) of other studies [24,25]. We expect that including additional parameters will improve the fit between model and experiment and partially account for the relatively large variability of search times within each treatment. For example, we ignored foveal vision and the blind-spot of the fish and assumed instead a 360° field-of-vision. Ecological variables, such as turbidity in water and atmospheric attenuation in air, are also expected to affect the relationship between prey distribution and search times.
Some discrepancy existed between the model predictions and experimental results. In agreement with previous experimental work [2,3], the effect of group size was most pronounced at small group sizes with the effect saturating at larger groups (this also applies more generally, i.e. to object size [25]). The model, in contrast, predicted a more gradual response. Interestingly, within the range of group sizes studied, the relationship between reaction distance and group size was approximately linear. This suggests that the discrepancy between experiments and model arose during the search of the fish before prey were detected. One possibility is that odour from the bloodworm once they were placed in the dishes gave a cue to the fish for the presence of food, changing the search behaviour of the fish. In contrast to the visual detection response, odour cue detection would not result in an abrupt change of direction and direct approach to the prey, and hence could have occurred before the prey had been visually detected. The use of odourless dummies in future experiments could solve this issue [26]. Changes in the search strategy of the fish as the search progressed could also account for the difference between model and experiment. The model implicitly assumes that the movement of the fish is constant during the search. Ruxton [27] demonstrated that a less-than-proportional relationship between prey density and encounter rate could be explained by foragers increasing speed while searching [28]. However, this specific mechanism would be expected to affect the number of groups to a greater extent than group size, whereas the discrepancy was observed particularly in the effect of group size. Changes in search strategy depending on the size (i.e. conspicuousness) and distribution of detected prey have been shown to be important in both vertebrates [29] and invertebrates [25], although this factor was not considered in the current study by analysing only the first prey detected.
Although aggregation decreases the rate at which predators find prey, risk will be greater in larger groups if the whole group is consumed: a dilution effect still needs to operate for group living to be adaptive [4]. In addition, other effects of group size and density on predation, such as the confusion effect and group vigilance, might have different optimal packing densities and/or group sizes [1]. These factors were intentionally minimized in the current study by using dead prey items to isolate the effects of aggregation on finding prey. However, a study examining the complete interaction between predator and prey is overdue.
The influential theory of attack abatement [4] predicts that individuals should be safer in larger groups whenever attack rate is less than proportional to group size. Previous theory [30,31] and experiments [2,3] have demonstrated this saturating effect of group size is the case, a result we confirm in this study. An implication of attack rates being less than proportional to group size is that when population size is constant, encounter rates should decline faster than the increase in conspicuousness of the groups. Using the visual angle subtended by the group on the retina of the predator as a mechanism of visual detection, we provide the first theoretical and experimental evidence for this prediction. This mechanism of avoiding risk via group living does not require information transfer or coordination between individuals, which is needed for group vigilance and group defence mechanisms [1], nor even the movement of individuals to induce a confusion effect [32]. For these reasons, the reduced rate of finding prey through aggregation may be very general and widespread across taxa. In fact, it may apply more generally to any situation where visual search is conducted for targets distributed in an open environment, such as pollinators searching for inflorescences [24,25].
Acknowledgements
C.C.I. was funded by a University of Leeds Research Scholarship. F.B. acknowledges the Spanish Ministry of Science and Innovation (Ramón y Cajal Programme REF. 09-449-06-01). We thank Arthur Lembo for guiding us to equation (1.1) and Amy S. I. Wade, Iain D. Couzin, Caroline E. Farrior, Lars Chittka and two anonymous reviewers for comments on the article.
References
- 1.Krause J., Ruxton G. D. 2002. Living in groups. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Riipi M., Alatalo R. V., Lindstrom L., Mappes J. 2001. Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514 10.1038/35097061 (doi:10.1038/35097061) [DOI] [PubMed] [Google Scholar]
- 3.Ioannou C. C., Krause J. 2008. Searching for prey: the effects of group size and number. Anim. Behav. 75, 1383–1388 10.1016/j.anbehav.2007.09.012 (doi:10.1016/j.anbehav.2007.09.012) [DOI] [Google Scholar]
- 4.Turner G. F., Pitcher T. J. 1986. Attack abatement: a model for group protection by combined avoidance and dilution. Am. Nat. 128, 228–240 10.1086/284556 (doi:10.1086/284556) [DOI] [Google Scholar]
- 5.Uetz G. W., Hieber C. S. 1994. Group size and predation risk in colonial web-building spiders: analysis of attack abatement mechanisms. Behav. Ecol. 5, 326–333 10.1093/beheco/5.3.326 (doi:10.1093/beheco/5.3.326) [DOI] [Google Scholar]
- 6.Vine I. 1971. Risk of visual detection and pursuit by a predator and the selective advantage of flocking behaviour. J. Theor. Biol. 30, 405–422 10.1016/0022-5193(71)90061-0 (doi:10.1016/0022-5193(71)90061-0) [DOI] [PubMed] [Google Scholar]
- 7.Travis J. M. J., Palmer S. C. F. 2005. Spatial processes can determine the relationship between prey encounter rate and prey density. Biol. Lett. 1, 136–138 10.1098/rsbl.2004.0293 (doi:10.1098/rsbl.2004.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lima S. L., Dill L. M. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 9.Holling C. S. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 10.4039/Ent91385-7 (doi:10.4039/Ent91385-7) [DOI] [Google Scholar]
- 10.Hutchinson J. M. C., Waser P. M. 2007. Use, misuse and extensions of ‘ideal gas’ models of animal encounter. Biol. Rev. 82, 335–359 10.1111/j.1469-185X.2007.00014.x (doi:10.1111/j.1469-185X.2007.00014.x) [DOI] [PubMed] [Google Scholar]
- 11.Thompson H. R. 1956. Distribution of distance to nth neighbour in a population of randomly distributed individuals. Ecology 37, 391–394 10.2307/1933159 (doi:10.2307/1933159) [DOI] [Google Scholar]
- 12.Strickler J. R., Udvadia A. J., Marino J., Radabaugh N., Ziarek J., Nihongi A. 2005. Visibility as a factor in the copepod-planktivorous fish relationship. Sci. Mar. 69, 111–124 [Google Scholar]
- 13.Wright D. I., O'Brien W. J. 1984. The development and field test of a tactical model of the planktivorous feeding of white crappie (Pomoxia annularis). Ecol. Monogr. 54, 65–98 10.2307/1942456 (doi:10.2307/1942456) [DOI] [Google Scholar]
- 14.Aksnes D. L., Giske J. 1993. A theoretical model of aquatic visual feeding. Ecol. Model. 67, 233–250 10.1016/0304-3800(93)90007-F (doi:10.1016/0304-3800(93)90007-F) [DOI] [Google Scholar]
- 15.Grand T. C., Dill L. M. 1999. The effect of group size on the foraging behaviour of juvenile coho salmon: reduction of predation risk or increased competition? Anim. Behav. 58, 443–451 10.1006/anbe.1999.1174 (doi:10.1006/anbe.1999.1174) [DOI] [PubMed] [Google Scholar]
- 16.Krause J., Godin J. G. J. 1995. Predator preferences for attacking particular prey group sizes: consequences for predator hunting success and prey predation risk. Anim. Behav. 50, 465–473 10.1006/anbe.1995.0260 (doi:10.1006/anbe.1995.0260) [DOI] [Google Scholar]
- 17.Confer J. L., Blades P. I. 1975. Omnivorous zooplankton and planktivorous fish. Limnol. Oceanogr. 20, 571–579 10.4319/lo.1975.20.4.0571 (doi:10.4319/lo.1975.20.4.0571) [DOI] [Google Scholar]
- 18.Kerfoot W. 1982. A question of taste: crypsis and warning coloration in freshwater zooplankton communities. Ecology 63, 538–554 10.2307/1938969 (doi:10.2307/1938969) [DOI] [Google Scholar]
- 19.Rasband W. S. 2004. Image J, v. 1.34s Bethesda, MD: National Institutes of Health [Google Scholar]
- 20.Wetterer J. K. 1989. Mechanisms of prey choice by planktivorous fish: perceptual constraints and rules of thumb. Anim. Behav. 37, 955–967 10.1016/0003-3472(89)90140-1 (doi:10.1016/0003-3472(89)90140-1) [DOI] [Google Scholar]
- 21.O'Brien W. J., Slade N. A., Vinyard G. L. 1976. Apparent size as the determinant of prey selection by bluegill sunfish (Lepomis macrochirus). Ecology 57, 1304–1310 10.2307/1935055 (doi:10.2307/1935055) [DOI] [Google Scholar]
- 22.Murray S. O., Boyaci H., Kersten D. 2006. The representation of perceived angular size in human primary visual cortex. Nat. Neurosci. 9, 429–434 10.1038/nn1641 (doi:10.1038/nn1641) [DOI] [PubMed] [Google Scholar]
- 23.Roth G. 1987. Visual behavior in salamanders. New York, NY: Springer [Google Scholar]
- 24.Wertlen A., Niggebrugge C., Vorobyev M., Hempel de Ibarra N. 2008. Detection of patches of coloured discs by bees. J. Exp. Biol. 211, 2101–2104 10.1242/jeb.014571 (doi:10.1242/jeb.014571) [DOI] [PubMed] [Google Scholar]
- 25.Spaethe J., Tautz J., Chittka L. 2001. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl Acad. Sci. USA 98, 3898–3903 10.1073/pnas.071053098 (doi:10.1073/pnas.071053098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith C., Barber I., Wootton R. J., Chittka L. 2004. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. Lond. B 271, 949–955 10.1098/rspb.2004.2690 (doi:10.1098/rspb.2004.2690) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruxton G. D. 2005. Increasing search rate over time may cause a slower than expected increase in prey encounter rate with increasing prey density. Biol. Lett. 1, 133–135 10.1098/rsbl.2004.0292 (doi:10.1098/rsbl.2004.0292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannou C. C., Ruxton G. D., Krause J. 2008. Search rate, attack probability, and the relationship between prey density and prey encounter rate. Behav. Ecol. 19, 842–846 10.1093/beheco/arn038 (doi:10.1093/beheco/arn038) [DOI] [Google Scholar]
- 29.Thomas G. 1974. The influences of encountering a food object on subsequent searching behaviour in Gasterosteus aculeatus L. Anim. Behav. 22, 941–952 10.1016/0003-3472(74)90017-7 (doi:10.1016/0003-3472(74)90017-7) [DOI] [Google Scholar]
- 30.Vine I. 1973. Detection of prey flocks by predators. J. Theor. Biol. 40, 207–210 10.1016/0022-5193(73)90127-6 (doi:10.1016/0022-5193(73)90127-6) [DOI] [PubMed] [Google Scholar]
- 31.Treisman M. 1975. Predation and the evolution of gregariousness. I. Models for concealment and evasion. Anim. Behav. 23, 779–800 10.1016/0003-3472(75)90106-2 (doi:10.1016/0003-3472(75)90106-2) [DOI] [Google Scholar]
- 32.Ruxton G. D., Jackson A. L., Tosh C. R. 2007. Confusion of predators does not rely on specialist coordinated behavior. Behav. Ecol. 18, 590–596 10.1093/beheco/arm009 (doi:10.1093/beheco/arm009) [DOI] [Google Scholar]