Abstract
Many biological invasions do not occur as a gradual expansion along a continuous front, but result from the expansion of satellite populations that become established at ‘invasion hubs’. Although theoretical studies indicate that targeting control efforts at invasion hubs can effectively contain the spread of invasions, few studies have demonstrated this in practice. In arid landscapes worldwide, humans have increased the availability of surface water by creating artificial water points (AWPs) such as troughs and dams for livestock. By experimentally excluding invasive cane toads (Bufo marinus) from AWP, we show that AWP provide a resource subsidy for non-arid-adapted toads and serve as dry season refuges and thus invasion hubs for cane toads in arid Australia. Using data on the distribution of permanent water in arid Australia and the dispersal potential of toads, we predict that systematically excluding toads from AWP would reduce the area of arid Australia across which toads are predicted to disperse and colonize under average climatic conditions by 38 per cent from 2 242 000 to 1 385 000 km2. Our study shows how human modification of hydrological regimes can create a network of invasion hubs that facilitates a biological invasion, and confirms that targeted control at invasion hubs can reduce landscape connectivity to contain the spread of an invasive vertebrate.
Keywords: artificial water, biological invasion, Bufo marinus, arid, control strategy, hydrological regime
1. Introduction
Over the last 500 years, human activities have greatly increased the rate at which animal species are translocated around the Earth [1]. Following their introduction to new environments, invasive species often thrive in the absence of population regulation by predators, parasites and diseases with which they have coevolved, and may undergo rapid range expansions. The subsequent disruption to ecological processes caused by the novel interactions of invasive species has been identified as one of the most serious threats to biodiversity at a global scale [2].
Reducing the economic and ecological impacts of invasive species is a key goal of invasive species management, but requires an understanding of factors that influence the population growth, spread and distribution of invaders. One critical step for managers is to identify the pathways through which invasive species spread [1]. Landscape structure, connectivity and the presence of dispersal corridors can all influence the spread of invasive species. Many biological invasions do not occur as gradual expansion along a continuous front, but result from the expansion of satellite populations that become established at ‘invasion hubs’ [3,4]. Invasion hubs can result from random dispersal events, or they may occur in habitat patches preferred by the invader or at locations where individuals are directed during the process of dispersal [1,5]. Once invasion hubs are identified, targeted control efforts at such sites can be an effective way of containing the spread of the invader [6,7]. Although the theoretical significance of biological invasions occurring via invasion hubs has long been recognized [5,7,8], few studies have demonstrated functioning invasion hubs [9,10].
In arid regions, the ability of people to capture and redistribute scarce water has been a key driver of economic growth. However, because water is a limiting resource in arid environments, the modification of hydrological regimes (damming of rivers, depletion of groundwater, and provision of surface water in previously waterless areas) can dramatically alter ecosystems and has facilitated the establishment and spread of non-arid-adapted invasive species [11–15]. Livestock grazing is an important economic activity in arid lands, but is constrained by the scarcity of surface water because horses, cattle, sheep and goats must drink regularly. To increase the livestock carrying capacity of arid rangelands, pastoralists have created artificial water points (AWPs) where water is provided to animals via troughs or dams [11,16].
By providing a reliable water source, AWPs subsidize wildlife with an essential resource for their metabolic homeostasis, growth and reproductive success, and allow ‘water-dependent’ animal species to persist in numbers that would not otherwise be attainable. For example, the provision of AWPs in arid environments has been linked to range expansions and/or population increases of water birds and wild herbivorous mammals that must drink [16,17] and has provided previously unavailable habitat for aquatic organisms [18,19]. Thus, AWPs can influence how arid ecosystems function by facilitating the trophic and competitive interactions of species that need to access water to survive [20,21].
Here, we examine how the redistribution of water in a semi-arid landscape can provide a resource subsidy and subsequently influence the survival and distribution of an invasive species. Our study area was situated in the Victoria River District of the Northern Territory, Australia (electronic supplementary material, figure S1). This semi-arid region has a vast network of bore-holes that supply water to small earthen tanks that in turn supply water to cattle-drinking troughs (electronic supplementary material, figure S2a). The region is currently being invaded by the cane toad (Bufo marinus), a large anuran from tropical America that is toxic to endemic Australian predators [22,23]. Cane toads lack physiological adaptations to aridity and we tested the hypothesis that AWPs function as invasion hubs for toads by providing them with access to water during the extreme aridity of the dry season. We experimentally fenced AWPs to test our predictions that: (i) cane toads require access to AWPs to survive; (ii) exclusion of toads from water in combination with hand collection of toads confined by fences is an effective way of reducing their populations; and (iii) that the movements of toads are focal around water. Finally, we evaluated the usefulness of excluding toads from AWPs by mapping the distribution of permanent waters in the predicted range of toads across the arid regions of Australia and simulating the extent to which the exclusion of toads from AWPs could reduce the availability of dry season refuge sites for toads and the area over which they are likely to disperse and colonize.
2. Methods
(a). Study species
The cane toad B. marinus has spread through more than a million square kilometres of Australia since its introduction to Queensland in 1935 [24]. This spread resulted from both range expansion overland and along watercourses [25] and from inadvertent human-assisted dispersal [26]. Unlike most native Australian desert-dwelling frogs, which possess physiological adaptations (cocoon formation, aestivation, reduced metabolic rate) to survive long dry periods [27,28], cane toads cannot physiologically control evaporative water loss through their skin [29]. Thus, toads are susceptible to dehydration throughout their life cycle, but become increasingly resistant to dehydration as their body size increases owing to a decrease in their surface area to volume ratio [29,30]. To combat dehydration, adult cane toads are active nocturnally and during the daytime they select moist cool microhabitats as shelter sites [29,31]. The arid regions of northern Australia are characterized by distinct wet and dry seasons, with almost all annual rainfall occurring within a brief wet season (December–March). During the dry season, high desiccation rates and limited moisture availability could conceivably restrict cane toads to microhabitats near standing water.
Cane toads are highly toxic, and possess bufogenins which are absent in native Australian frogs [22]. Consequently, most endemic Australian predators lack physiological resistance to bufotoxins [22], and mammalian and reptilian predators can die after attacking or ingesting cane toads [32]. In northern Australia, populations of frog-eating reptiles have declined dramatically in areas invaded by cane toads [33,34]. The cane toad has recently expanded its range into semi-arid regions of the continent, where it poses a serious threat to carnivorous reptiles [34]. Since the 1980s, considerable effort has been expended on developing biological control techniques to reduce toad numbers and hence impacts, however, efforts to date have had little success [35].
(b). Study area
Our study area was in the Victoria River catchment (Camfield Station: 17°02′ S, 131°17′ E) in the Northern Territory (electronic supplementary material, figure S1a) which experiences a mean annual rainfall of approximately 580 mm. Cane toads first invaded the northern part of the study area in 2007–2008 [34] and are expanding their range westward and southwards into increasingly arid regions. The study area experiences a semi-arid, monsoonal climate characterized by a hot humid wet season (December–March) and a hot dry season (April–November). Temperatures are high year round and on average exceed 30°C on 286.4 days each year at Wave Hill (17°27′ S, 130° 50′ E). During the late dry season, study period of September to November 2009, conditions were hot and dry as is typical for this time of year (mean daily maximum temperature = 37.2°C, mean daily relative humidity = 17%; Australian Bureau of Meteorology). The dominant landforms within the study area are undulating plains that support savannah vegetation dominated by Mitchell grass (Astrebla spp.) situated on deep-cracking clay soils.
In most years, no rain falls between April and September, and most stream flow occurs during the wet season (December–March) when monsoonal rains bring more than 80 per cent of the annual precipitation. During the late dry season (September–November), the only sources of natural water are disconnected pools, separated by exposed sand or rock bars in major drainage channels and a small number of permanent natural springs. Discussions with landholders and examination of rainfall records (Australian Bureau of Meteorology) indicated that no rain fell in the study area between 2 March 2009 and the study period in September–November 2009.
Commercial cattle grazing has been conducted throughout the study area since the late nineteenth century. Water is a limiting resource for grazing livestock in this hot semi-arid region. To increase the amount of grazing land available for cattle, pastoralists have established an AWPs at intervals of 5–10 km throughout the landscape.
(c). Fencing experiment
We experimentally fenced AWPs during the late dry season to determine if cane toads are dependent on AWPs for survival in this seasonally arid landscape. The purpose of the fences was twofold. The fences prevented toads that were sheltering in AWPs from leaving and thus facilitated collection of these individuals by hand. The fences also prevented toads that were sheltering away from the AWP from accessing the water. If toads require access to AWPs to survive, we would expect that survival of toads would be greater at sites where toads had access to water. If hand collection and exclusion of toads from water is an effective way of reducing their populations, we would expect that population declines following the implementation of the fences would be greater at fenced AWPs than sites where toads were able to freely access and leave the water. The study was located at nine AWPs (electronic supplementary material, figure S1b). In each case, AWPs were located more than 4 km from the nearest other source of permanent water. There were three treatments: fenced, unfenced controls and procedural controls, with three replicate AWPs per treatment. Treatments were interspersed to account for any spatial heterogeneity.
We constructed fences made of shade-cloth material, wire and metal posts 2–5 m from the water's edge along the flat crest of the AWP (electronic supplementary material, figure S2b). The fences were 600 mm high, to prevent toads from jumping over, and had a soil-covered flange of shade-cloth extending 400 mm outwards along the ground to prevent toads from burrowing beneath the fence. We constructed procedural control fences in the same fashion, but raised the shade-cloth 100 mm above the ground so that toads could access water (electronic supplementary material, figure S2c).
Once fences were established, we removed toads by hand from inside the fence at the fenced treatments and euthanazed them. Each morning we recorded the number of dead toads on the outside of exclusion fences that died while attempting to gain access to the water. Toads were left undisturbed at the unfenced control and procedural control AWPs.
(d). Toad survival
We used radio telemetry to monitor the short-term (72 h) survival of cane toads at fenced, unfenced control and procedural control AWPs. At each AWP, we captured adult cane toads (>90 mm snout urostyle length (SUL) of both sexes 8–10 h after fence installation at night between 20.00 and 22.00 h). All toads were captured within 2 m of water. We then recorded their sex, mass and SUL. We fitted radio-transmitters (Sirtrack, New Zealand, 3.5 g mass) to the toads with a metal chain-link waistband [36]. To ensure that toads were well hydrated prior to release, they were placed in a bucket of water for 20 min. The toads were released approximately 30 min after capture outside of the fences, or 5 m from water in the case of the unfenced controls and procedural control treatments. We released six to seven toads at each of the nine experimental AWPs (total: n = 19 unfenced AWP; n = 20 procedural fence AWP; n = 21 fenced AWP). Each morning following release, we located toads to determine their fate (alive, dead or eaten by a predator). To avoid undue distress to the toads, the animals were not disturbed during tracking. We recorded the mass of toads that died at the fenced dams and the telemetered toads that survived the 72 h tracking period at unfenced and procedural control AWPs.
(e). Toad abundance surveys
We conducted nocturnal strip-surveys using handheld 12 V spotlights with 25 W halogen bulbs at each AWP to measure the abundance of toads along 4 × 150 m strip transects (n = 4 per AWP) radiating away from the water's edge. All AWPs were surveyed 6 and 3 days prior to fence erection and 1, 3, 6, 12, 20 and 70 days after fence installation. To evaluate if fencing had adverse impacts on native fauna, we recorded the number and species of native fauna found dead on the inside and outside of fences.
(f). The movements and shelter sites of toads
We used telemetry to examine the movement patterns and identify the diurnal shelter sites of 20 toads at an unfenced control AWP after the cessation of the survival study. These toads were individuals that were not tracked in the survival study. The procedure for fitting transmitters was identical to that described in §2c. The location of tracked toads was determined over 12 days both during the day and at night. Toads were not disturbed during tracking. For each shelter site identified, we recorded the habitat type and distance to water (m) using a GPS.
(g). Predicting the broadscale effects of excluding toads from artificial water point
We used information on the potential range of cane toads in Australia [37], their movement potential and the distribution of permanent natural waters and AWPs to quantify the extent to which the exclusion of toads from AWPs could reduce the number of dry season refuge sites for cane toads and the area over which they are likely to disperse and colonize. We restricted our analyses to areas receiving less than 700 mm annual rainfall because it is in these drier areas where permanent waters are likely to function as invasion hubs for toads by providing them with dry season refuges (see §3). A fundamental assumption of our models was that toads cannot survive without access to water during the dry season and thus must disperse from refuge sites that have permanent water (see §3).
We determined the distribution of potential dry season refuges for toads by mapping all permanent water features within the potential range of toads from data published by the Australian Government (AUSLIG mapping data, http://www.ga.gov.au/mapspecs/250k100k/appendix_a.jsp) in a geographical information system (ArcGIS 9.0). The movement potential of toads is likely to vary geographically owing to physiological constraints imposed by climatic variables [37]. To account for this, we modelled the potential dispersal ability of toads using a model in which the distance that toads were able to move each month was a function of toads' estimated body temperature, curtailed by a spatial dataset on the number of rainy days per month ([37]; see Methods for calculating toad dispersal potential in the electronic supplementary material). Rain days were defined as days that received more than 0.2 mm of rainfall. The inter-annual intensity and frequency of rainfall events in Australia is highly variable owing to the influence of coupled oceanic/atmospheric circulation systems, the El Niño Southern Oscillation (ENSO) and the Indian Ocean Dipole (IOD), on the continent's climate [38]. Because of this variability, we simulated the movement potential of toads based on the mean annual number of rain days and also the number of rain days from an unusually wet year (2000) when the dispersal potential of toads was likely to be enhanced. Assuming that each permanent water feature could serve as a dry season refuge for cane toads and that toads could disperse in any direction, we used the buffer wizard of ArcGIS 9.0 to map the physiologically constrained distance that cane toads could potentially disperse from permanent natural water and AWPs (electronic supplementary material, figure S3a,b). We then mapped the connectivity of the landscape for colonizing toads by halving the dispersal distance around each refuge, assuming that toads would only be able to successfully disperse between patches that were spaced at a distance equal to or less than the annual dispersal potential of toads. These predictions assumed that toads experienced the monthly mean temperature, wind speed and cloud cover conditions but high humidity (90%) and hence minimal evaporative cooling. Because rainy nights are often cooler than average and we did not adjust for the dilution of propagules that could be expected to occur with increasing distance from refuges, our predicted distances are likely to be overestimates. We simulated the effect of excluding toads from AWPs by overlaying the predicted dispersal area of toads from permanent natural waters on that from AWP, and then subtracting the area that AWP add to the potential dispersal area of toads from the total area of both layers combined. We conducted these simulations for the average number of rain days per year and the actual number of rain days in a wet year associated with the La Niña phase of ENSO.
(h). Statistical analyses
We compared the survival of radio-tracked toads between each treatment using the product-limit method or Kaplan–Meier estimator (JMP 5.0.1 SAS Institute Inc.). To test the effect of fencing on toad density with time, we used a before-after control-impact (BACI) analysis of variance design with time as a repeated measure. Data were log-transformed (log + 1) to reduce variances and correct a skewed distribution [39]. Parametric test assumptions (normality and homogeneity of variances) were evaluated by checking residual plots. Planned pairwise contrasts (n = 12 contrasts) investigated differences between treatment means in four time periods: 6 and 3 days before fence installation; 1 and 3 days after fence installation; 6 and 12 days after fence installation; and 20 and 70 days after fence installation. Because of the large number of post hoc tests, a sequential Bonferonni adjustment was applied to reduce the significance levels for the pairwise contrasts [39]. Contrasts were deemed statistically significant at p ≤ 0.005. We tested the hypothesis that individual cane toads were more likely to be located in the water both during the day and night using Cochran's Q test [40].
3. Results
(a). The effect of fencing on toad survival
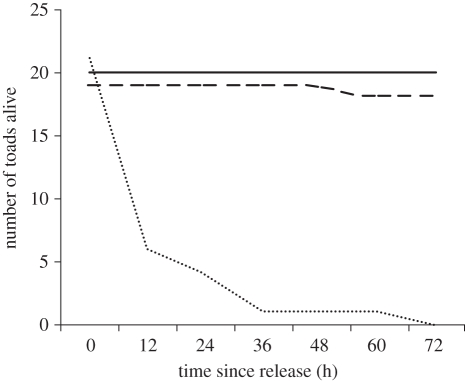
Radio-tagged toads had lower survival at fenced AWPs than at unfenced control or procedural control AWPs (χ2 = 64.5, d.f. = 3, p < 0.0001; figure 1). All 21 radio-tracked toads at fenced AWPs died within 72 h of release (figure 1). Of the 20 toads radio-tracked at unfenced control AWPs, 19 survived the 72 h tracking period, while one toad was killed by a predatory bird 48–60 h after release. All 19 toads monitored at procedural control AWPs survived the 72 h tracking period.
Figure 1.
Mean survival time of radio-tracked toads released at fenced (dotted line), procedural control (solid line) and unfenced (dashed line) control AWP (n = 3 AWP).
(b). Mass loss of telemetered toads
Over the course of radio-tracking, telemetered toads at fenced AWPs lost more body mass (mean ± s.e. = 46% ± 2.9) than toads at unfenced control (3.2% ± 1.1 body mass loss) or procedural control AWP (0.8% ± 1.7 body mass gain) (F = 151.0, d.f. = 2, 51, p < 0.0001).
(c). The number of toads removed and the effect of fencing on toad abundance
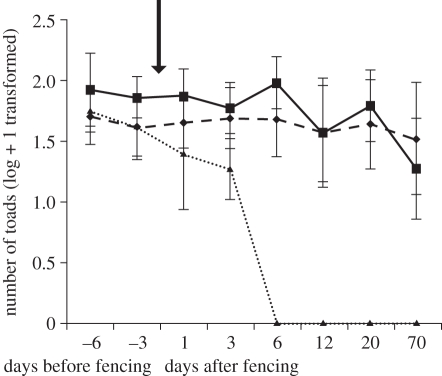
A total of 2016 toads were removed from the three fenced AWPs (table 1). The relative frequency of toads removed from inside and outside of the fences differed between AWPs (table 1; χ2 = 840.6, d.f. = 2, p < 0.001). There was no difference in toad abundance prior to fence installation (figure 2; electronic supplementary material, tables S1 and S2). The abundance of toads at fenced AWPs declined with time since fence installation (figure 2) but varied little at unfenced control or procedural control AWPs (figure 2). No toads were observed on transects conducted at fenced AWPs between 6 and 70 days after fence installation. In contrast, toads were observed on all surveys conducted at unfenced control and procedural control AWPs. Over the course of the study, 16 dead anurans (Litoria inermis) and one lizard (Ctenotus sp.) were found along the outside perimeter at fenced AWPs.
Table 1.
The number of toads removed from the inside and outside of fences at the three fenced AWPs. In : out is the ratio of toads removed from the inside of the fence relative to those removed from the outside of the fence.
| AWP | inside | outside | total | in : out |
|---|---|---|---|---|
| 1 | 62 | 819 | 881 | 0.08 |
| 2 | 716 | 259 | 975 | 2.76 |
| 3 | 61 | 99 | 160 | 0.61 |
Figure 2.
The number of toads observed during nocturnal surveys (log + 1 transformed) before and after exclusion fence installation at fenced (dotted line with filled triangles), procedural control (continuous line with filled squares) and unfenced (dashed line with filled diamonds) control AWP (n = 3 in each case). The arrow indicates the time of fence installation. Values are mean ± s.e.
(d). The movements and shelter sites of toads
Toads were not restricted to the unfenced control AWPs but their movements were focal around it. We obtained 128 fixes of 19 individual toads over the 12 day tracking period. Toads were located in the water or within 0.5 m of the water for 65 per cent of fixes (59% of diurnal and 41% of nocturnal fixes), but were no more likely to be located in the water than away from the water during the day (Cochran's Q test, χ2 = 4.7, d.f. = 4, p = 0.32) or at night (Cochran's Q test, χ2 = 0.17, d.f. = 2, p = 0.92). All tracked toads were located in the water on at least one occasion during the tracking period. Toads were also located in diurnal shelter sites up to 410 m from the AWP, such as soil cracks (29% of all fixes) and logs (6% of all fixes), frequently with other toads.
(e). Predicting the broadscale effects of excluding toads from artificial water points
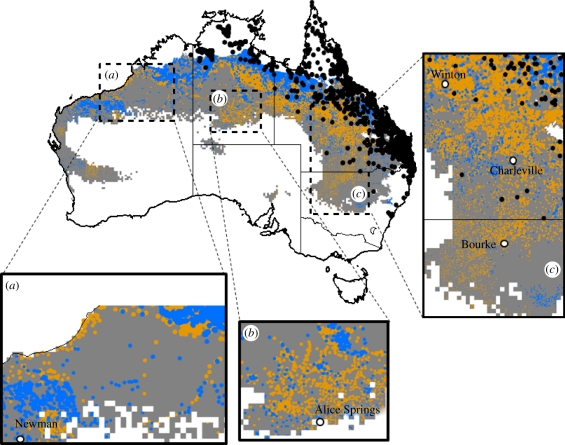
Under average climatic conditions, simulated exclusion of toads from AWPs reduced the area of arid landscape that toads are able to colonize by 38 per cent from 2 242 000 to 1 385 000 km2 (figure 3). Our model suggested that toad exclusion would be more effective during dry years. Under unusually wet conditions, the area available for dispersal and colonization by toads increased to 2 598 000 km2, and simulated exclusion from AWP would reduce the area available for dispersal by toads by 23 per cent to 1 993 000 km2 (electronic supplementary material, figure S4).
Figure 3.
The area available (blue and orange) for dispersal and colonization by toads surrounding potential dry season refuges (sources of permanent water) within the predicted range of cane toads (grey) that receives less than 700 mm of annual rainfall. The orange area indicates the area that our simulation exercise identified could be made unavailable for toads by excluding them from AWPs. The model assumes that each permanent water source could serve as a dry season refuge for toads and that they disperse from such refuges during periods of rain. The annual dispersal potential around water sources has been weighted to reflect the physiological constraints imposed on the movement potential of toads by climatic variables (after [37]). The connectivity of the landscape for toads has been modelled by halving the annual dispersal potential around each refuge, assuming that toads would only be able to successfully disperse between water sources, and thus colonize dry season refuges that were spaced at a distance equal to or less than the annual dispersal potential of toads. The black dots indicate known locality records for toads in 2010. Blue colour, indicates natural water; orange colour, indicates artificial water; grey colour, indicates potential range and black dots, indicate current range.
Mapping indicated three key regions where dry season refuges are naturally sparse and the presence of AWPs provides potential corridors for cane toad dispersal or substantially increase the connectivity of the landscape for toads (figure 3a–c). Our modelling indicates that exclusion of toads at just 40–50 key AWPs could halt invasion from the Kimberly region to the Pilbara region (figure 3a).
4. Discussion
(a). The effects of excluding toads from artificial water point
Cane toads at AWPs required access to standing water for survival during the late dry season and their movements were focal around water. Correspondingly, our failure to detect toads between 6 and 70 days after fence installation suggests that we eradicated toads from fenced AWPs and their immediate vicinity. Collectively, these results provide evidence that sites with permanent water serve as dry season refuges for toads and thus act as invasion hubs. Because toads must have access to water during the late dry season, the likely process of invasion in our study area has been gradual range expansion through dispersal from dry season refuges in the wet season following rainfall events. According to this patchy population model (sensu [41]), water becomes a limiting resource for toads during the dry season and their distribution contracts to the immediate vicinity of sites with permanent water from which individuals can disperse during the next wet season. Presumably, toads that have not located permanent sources of water by the start of the dry season die from dehydration.
Potentially, toad absence at fenced AWPs could have occurred if toads that were excluded from the water had selected suitable microhabitats (e.g. deep soil cracks) which prevented dehydration and allowed them to survive the observation period without being detected in our surveys [31]. However, we contend that this scenario was unlikely to occur for several reasons. First, toads excluded from AWPs lost on average 46 per cent of their body mass compared to negligible changes in body mass among toads from unfenced AWPs. Presumably, this loss of body mass observed in toads from fenced AWPs was due to dehydration. A previous study has shown that toads are likely to experience fatal dehydration if they lose more than 40 per cent of their body mass [23]. Second, all but one of the toads excluded from AWPs died within 12 h of release and the only individual that survived more than 12 h died within 72 h. In contrast, only one individual at a non-fenced AWP died, due to predation by a bird. Third, although radio-tracked toads at an unfenced dam moved and sheltered in deep cracks away from the AWP, all of the individuals were observed in the water during the tracking period. Together, these results suggest that in the late dry season, cane toads were restricted to the immediate vicinity of sites where standing water was available to avoid dehydration. A similar pattern of water dependency has been documented for adult cane toads during the dry season in the more mesic environments of the wet-dry tropics of Queensland, Australia [29,42].
The massive reduction in toad density following fence installation demonstrates that fencing combined with hand collection can reduce toad numbers around AWPs during the late dry season. The number of dead toads on the outside of fences also decreased with time since fence installation, suggesting that toad densities were reduced as a result of mortality. Moreover, it is likely that any toads that attempted to move to alternative water sources located several kilometres away under the hot and dry climatic conditions prevalent at the time of the study would have died from dehydration.
We recorded negligible mortality of small native anurans at fenced AWPs. In addition, birds, large pythons and large mammals such as dingoes and kangaroos were able to move over the fences unimpeded (authors, September–October 2009, personal observations). Thus, the results show that fencing effectively reduced toad numbers, yet had minimal negative impact on native fauna. ‘Wildlife gates’ constructed of a mesh size able to be traversed by small native anurans, but not by adult toads, could be incorporated into fences to ameliorate any negative impacts. Metamorph or juvenile toads were not observed at the AWPs, so it appears that the inclusion of wildlife gates would not reduce the effectiveness of the fences as barriers to toads in the late dry season.
(b). Artificial waters as invasion hubs for toads
The spatial configuration of landscapes can be an important influence on the dispersal of invasive species [4,43]. For example, the presence of suitable habitat corridors can focus the movements and dispersal of invaders into some habitats, but not others [1,36]. Likewise, the presence of isolated patches of particularly favourable habitat for invaders, or barriers that prevent successful dispersal can result in invasive species having spatially structured populations [44]. The establishment and subsequent expansion of small satellite populations may allow invaders to colonize new areas faster than through gradual expansion of a larger contiguous population [7]. Both theoretical and field studies indicate that concentrating control efforts on satellite populations, rather than on large focal populations, can be an effective strategy to reduce the rate of spread of invasive plant species [6,7,45,46]. Our study shows that modification of hydrological regimes by humans can create a network of invasion hubs and that targeted control at invasion hubs can be an effective way to contain the spread of an invasive vertebrate.
The presence of AWPs has substantially increased the availability of standing water and spatial distribution of water in the rangelands of Australia and other arid regions of the Earth [11,18,47]. In Australia, the proliferation of AWPs over the last 150 years has generated a landscape where few places are now more than 10 km from water across the approximately 70 per cent of the continent that is used for livestock grazing [16]. Prior to European settlement, surface water was comparatively rare in this low rainfall region and normally occurred only in the channels of major rivers and isolated springs, and was only widespread for brief periods following large rainfall events [47].
By providing a resource subsidy for toads in the dry season, AWPs increase the number of refuges available for adult cane toads, and the connectivity of arid landscapes for toads and potentially other non-arid-adapted wildlife (figure 3; [16]). AWPs probably serve as ‘stepping stones’ that have facilitated the invasion of cane toads into naturally waterless landscapes where without AWPs toads would be unable to reach or persist owing to scarcity of water. By increasing the number of dry season refuges, AWPs also elevate the regional toad population and may be expected to exacerbate the ecological impacts of toads (e.g. [33]) by increasing their encounter rates with terrestrial predators.
Our experiment shows that excluding adult toads from water coupled with the hand collection of toads confined within the fences can be an effective method for controlling satellite populations at isolated AWPs. Fencing is not the only technique available to exclude toads from AWP. An equivalent approach would be to replace earthen tanks with above-ground plastic tanks that do not allow toads access to water. Despite the success of our exclusion fences, the considerable dispersal potential of toads and the presence of natural waterholes situated on major catchment drainage lines poses a major problem for toad-control programmes. During extended periods of wet weather, toads are likely to re-invade ‘treated’ dry season refuges from untreated sites and, if they have access to water, re-establish refuge populations. The dispersal abilities of cane toads are evidenced by studies from the wet-dry tropics of Australia, which show that toads move as far as 1 km in a single night and 50 km in a calendar year [25]. No data are available on the annual movements of individual toads in semi-arid Australia. In our semi-arid Victoria River study area, cane toads have colonized AWPs located up to 9.5 km from the nearest source of permanent water (M.L. personal observation). Natural waterholes situated on major drainage lines also function as dry season refuges for toads. Many of these sites are not amenable to fencing because of the length of the waterholes and complexity of their vegetation, and are far too large for hand collection of toads to be feasible. Thus, where they occur, natural waterholes in arid areas will probably be an ever-present source of toads which can then invade surrounding landscapes.
Nevertheless, if toad exclusion devices (e.g. fences, plastic tanks) were strategically established at adjacent AWPs so that the distance between suitable habitat patches was greater than the ‘wet-season’ dispersal potential of toads, it may be possible to suppress toad populations and prevent their overland spread across vast areas of arid Australia (figure 3). Such a strategy is likely to be particularly effective in regions where natural waterholes are scarce. Our modelling exercise shows that there are several key areas in Australia where excluding toads from AWPs could prevent their overland spread (figure 3a–c). These areas occur in arid and semi-arid rangelands where natural waters are few and the proliferation of AWPs has increased the connectivity of the landscape for toads.
Rainfall variability will be a key issue affecting the ability to manage toads using water exclusion. Toads' dispersal abilities are enhanced during periods of ‘wet’ climatic conditions that can be expected during the La Niña phase of ENSO and negative phase of the IOD [38]. During these periods, the capacity to contain the spread of toads using water exclusion would be reduced in comparison with ‘average’ climatic years, but is likely to remain effective over large areas (electronic supplementary material, figure S4). Moreover, if toads did disperse into areas distant from permanent natural water during unusually wet periods, populations isolated during the inevitable drying out of the landscape would be vulnerable to both dehydration and physical control.
Most research on methods to control cane toads has focused on identifying and developing biological control agents; all have been unsuccessful to date [35]. A growing body of research now indicates that toad populations and impacts can in some areas be managed using physical control and by manipulating the behaviour of predators that attack toads ([48,49]; this study). While we do not propose water exclusion as a ‘silver bullet’ for toad control, our study shows that exclusion of toads from AWPs can effectively reduce toad numbers and could prevent their overland spread in arid regions.
5. Conclusion
Understanding the spatial dynamics of invasions can provide key insights into the development of strategic approaches to control invasive species. In arid regions of Australia, human modification of hydrological regimes has created a network of invasion hubs in to which invasive cane toads require access in order to survive through dry seasons. Excluding cane toads from AWPs can effectively reduce their local populations, and if conducted strategically at a large spatial scale, has the potential to prevent toads from using AWPs as ‘stepping stones’ into arid Australia.
Acknowledgements
Ethics approval was provided by the University of Sydney Animal Ethics Committee approval L04/9-2009/3/5132.
We thank Mick Tasker (Australian Agricultural Company), Graeme Sawyer (Frogwatch) and Kim Hands (Stop the Toad Foundation) for logistical and/or financial support. Heloise Gibb commented on a draft. Additional financial support was provided by the University of Sydney (M.L.).
References
- 1.Hulme P. E. 2009. Trade transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18 10.1111/j.1365-2664.2008.01600.x (doi:10.1111/j.1365-2664.2008.01600.x) [DOI] [Google Scholar]
- 2.Clavero M., Garcia-Berthou E. 2005. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110. 10.1016/j.tree.2005.01.003 (doi:10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- 3.Suarez A. V., Holway D. A., Case T. J. 2001. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc. Natl Acad. Sci. USA 98, 1095–1100 10.1073/pnas.98.3.1095 (doi:10.1073/pnas.98.3.1095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.With K. A. 2002. The landscape ecology of invasive spread. Conserv. Biol. 16, 1192–1203 10.1046/j.1523-1739.2002.01064.x (doi:10.1046/j.1523-1739.2002.01064.x) [DOI] [Google Scholar]
- 5.Muirhead J. R., MacIsaac H. J. 2005. Development of inland lakes as hubs in an invasion network. J. Appl. Ecol. 42, 80–90 10.1111/j.1365-2664.2004.00988.x (doi:10.1111/j.1365-2664.2004.00988.x) [DOI] [Google Scholar]
- 6.Cook G. D., Setterfield S. A., Maddison J. P. 1996. Shrub invasion of a tropical wetland: implications for weed management. Ecol. Appl. 6, 531–537 10.2307/2269389 (doi:10.2307/2269389) [DOI] [Google Scholar]
- 7.Moody M. E., Mack R. N. 1988. Controlling the spread of plant invasions: the importance of nascent foci. J. Appl. Ecol. 25, 1009–1021 10.2307/2403762 (doi:10.2307/2403762) [DOI] [Google Scholar]
- 8.Havel J., Lee C., Vander Zanden M. J. 2005. Do reservoirs facilitate invasion into landscapes. Bioscience 55, 518–525 10.1641/0006-3568(2005)055[0518:DRFIIL]2.0.CO;2 (doi:10.1641/0006-3568(2005)055[0518:DRFIIL]2.0.CO;2) [DOI] [Google Scholar]
- 9.Johnson L. E., Ricciardi A., Carlton J. T. 2001. Overland dispersal of aquatic invasive species: a risk assessment of transient recreational boating. Ecol. Appl. 11, 1789–1799 10.1890/1051-0761(2001)011[1789:ODOAIS]2.0.CO;2 (doi:10.1890/1051-0761(2001)011[1789:ODOAIS]2.0.CO;2) [DOI] [Google Scholar]
- 10.Simberloff D., Boecklen W. 1991. Patterns of extinction in introduced Hawaiian avifauna: a re-examination of the role of competition. Am. Nat. 138, 300–327 10.1086/285219 (doi:10.1086/285219) [DOI] [Google Scholar]
- 11.Perkins J. S., Thomas D. S. G. 1993. Spreading deserts or spatially confined environmental impacts? Land degradation and cattle ranching in the Kalahari Desert of Botswana. Land. Degrad. Rehabil. 4, 179–194 10.1002/ldr.3400040307 (doi:10.1002/ldr.3400040307) [DOI] [Google Scholar]
- 12.Stromberg J. C., Tiller R., Richter B. 1996. Effects of groundwater decline on riparian vegetation of semiarid regions: the San Pedro, Arizona. Ecol. Appl. 6, 113–131 10.2307/2269558 (doi:10.2307/2269558) [DOI] [Google Scholar]
- 13.Stromberg J. C., Lite S. J., Marler R., Paradzick C., Shafroth P. B., Shorrock D., White J. M., White M. S. 2007. Altered stream-flow regimes and invasive plant species: the Tamarix case. Glob. Ecol. Biogeogr. 16, 381–393 10.1111/j.1466-8238.2007.00297.x (doi:10.1111/j.1466-8238.2007.00297.x) [DOI] [Google Scholar]
- 14.Tolley K. A., Davies S. J., Chown S. L. 2008. Deconstructing a controversial local range expansion: conservation biogeography of the painted reed frog (Hyperolius marmoratus) in South Africa. Divers. Distrib. 14, 400–411 10.1111/j.1472-4642.2007.00428.x (doi:10.1111/j.1472-4642.2007.00428.x) [DOI] [Google Scholar]
- 15.Merritt D. M., Poff N. L. R. 2010. Shifting dominance of riparian Populus and Tamarix along gradients of flow alteration in western North American rivers. Ecol. Appl. 20, 135–152 10.1890/08-2251.1 (doi:10.1890/08-2251.1) [DOI] [PubMed] [Google Scholar]
- 16.James C. D., Landsberg J., Morton S. R. 1999. Provision of watering points in the Australian arid zone: a review of effects on biota. J. Arid Environ. 41, 87–121 10.1006/jare.1998.0467 (doi:10.1006/jare.1998.0467) [DOI] [Google Scholar]
- 17.Dawson T. J., McTavish K. J., Munn A. J., Holloway J. 2006. Water use and the thermoregulatory behaviour of kangaroos in arid regions: insights into the colonisation of arid rangelands in Australia by the Eastern Grey Kangaroo (Macropus giganteus). J. Comp. Physiol. B. 176, 45–53 10.1007/s00360-005-0030-2 (doi:10.1007/s00360-005-0030-2) [DOI] [PubMed] [Google Scholar]
- 18.Burkett D. W., Thompson B. C. 1994. Wildlife associated with human-altered water sources in semiarid vegetation communities. Conserv. Biol. 8, 682–690 10.1046/j.1523-1739.1994.08030682.x (doi:10.1046/j.1523-1739.1994.08030682.x) [DOI] [Google Scholar]
- 19.Knutson M. G., Richardson W. B., Reineke D. M., Gray B. R., Parmalee J. R., Weisk S. E. 2004. Agricultural ponds support amphibian populations. Ecol. Appl. 14, 669–684 10.1890/02-5305 (doi:10.1890/02-5305) [DOI] [Google Scholar]
- 20.Chamaille-Jammés S., Valeix M., Herve F. 2007. Managing heterogeneity in elephant distribution: interactions between elephant population density and surface water availability. J. Appl. Ecol. 44, 625–633 10.1111/j.1365-2664.2007.01300.x (doi:10.1111/j.1365-2664.2007.01300.x) [DOI] [Google Scholar]
- 21.Smit I. P. J., Grant G. C., Devereux B. J. 2007. Do artificial waterholes influence the way herbivores use the landscape? Herbivore distribution patterns around rivers and artificial water sources in a large African savanna park. Biol. Conserv. 136, 85–99 10.1016/j.biocon.2006.11.009 (doi:10.1016/j.biocon.2006.11.009) [DOI] [Google Scholar]
- 22.Smith J. G., Phillips B. L. 2006. Toxic tucker: the potential impact of cane toads on Australian reptiles. Pacific Conserv. Biol. 12, 40–49 [Google Scholar]
- 23.Zug G. R., Zug P. B. 1979. The marine toad, Bufo marinus: natural history resume of native populations. Smithson. Contrib. Zool. 284, 1–58 [Google Scholar]
- 24.Urban M. C., Phillips B. L., Skelly D. K., Shine R. 2008. A toad more traveled: the heterogenous invasion dynamics of cane toads in Australia. Am. Nat. 171, 134–148 10.1086/527494 (doi:10.1086/527494) [DOI] [PubMed] [Google Scholar]
- 25.Phillips B., Brown G. P., Greenlees M., Webb J. K., Shine R. 2007. Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral. Ecol. 32, 169–176 10.1111/j.1442-9993.2007.01664.x (doi:10.1111/j.1442-9993.2007.01664.x) [DOI] [Google Scholar]
- 26.White A. W., Shine R. S. 2009. The extra-limital spread of an invasive species via ‘stowaway’ dispersal: toad to nowhere? Anim. Conserv. 12, 38–45 10.1111/j.1469-1795.2008.00218.x (doi:10.1111/j.1469-1795.2008.00218.x) [DOI] [Google Scholar]
- 27.Withers P. C. 1993. Metabolic depression during aestivation in the Australian frogs, Neobatrachus and Cyclorana. Austr. J. Zool. 41, 467–473 10.1071/ZO9930467 (doi:10.1071/ZO9930467) [DOI] [Google Scholar]
- 28.Withers P. C. 1998. Evaporative water loss and the role of cocoon formation in Australian frogs. Austr. J. Zool. 46, 405–418 10.1071/ZO98013 (doi:10.1071/ZO98013) [DOI] [Google Scholar]
- 29.Seebacher F., Alford R. A. 2002. Shelter microhabitats determine body temperature and dehydration rates of a terrestrial amphibian (Bufo marinus). J. Herpetol. 36, 69–75 [Google Scholar]
- 30.Child T., Phillips B. L., Shine R. 2008. Abiotic and biotic influences on the dispersal behavior of metamorph cane toads (Bufo marinus) in tropical Australia. J. Exp. Zool. 309A, 215–224 10.1002/jez.450 (doi:10.1002/jez.450) [DOI] [PubMed] [Google Scholar]
- 31.Cohen M. P., Alford R. A. 1996. Factors affecting diurnal shelter use by the cane toad, Bufo marinus. Herpetologica 52, 172–181 [Google Scholar]
- 32.Covacevich J., Archer M. 1975. The distribution of the cane toad, Bufo marinus, in Australia and its effects on indigenous vertebrates. Mem. Qld. Mus. 17, 305–310 [Google Scholar]
- 33.Doody J. S., Green B., Rhind D., Castellano C. M., Sims R., Robinson T. 2009. Population-level declines in Australian predators caused by an invasive species. Anim. Conserv. 12, 46–53 10.1111/j.1469-1795.2008.00219.x (doi:10.1111/j.1469-1795.2008.00219.x) [DOI] [Google Scholar]
- 34.Letnic M., Webb J. K., Shine R. 2008. Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol. Conserv. 141, 1773–1782 10.1016/j.biocon.2008.04.031 (doi:10.1016/j.biocon.2008.04.031) [DOI] [Google Scholar]
- 35.Shanmuganathan T., et al. 2010. Biological control of the cane toad in Australia: a review. Anim. Conserv. 13(Suppl. 1), 16–23 10.1111/j.1469-1795.2009.00319.x (doi:10.1111/j.1469-1795.2009.00319.x) [DOI] [Google Scholar]
- 36.Brown G. P., Phillips B. L., Webb J. K., Shine R. 2006. Toad on the road: use of roads as dispersal corridors by cane toads (Bufo marinus) at an invasion front in tropical Australia. Biol. Conserv. 133, 88–94 10.1016/j.biocon.2006.05.020 (doi:10.1016/j.biocon.2006.05.020) [DOI] [Google Scholar]
- 37.Kearney M., Phillips B. L., Tracy C. R., Christian K. A., Betts G., Porter W. P. 2008. Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434 10.1111/j.0906-7590.2008.05457.x (doi:10.1111/j.0906-7590.2008.05457.x) [DOI] [Google Scholar]
- 38.Meyers G., McIntosh P., Pigot L., Pook M. 2007. The years of El Niño, La Niña, and interactions with the tropical Indian Ocean. J. Clim. 20, 2872–2880 10.1175/JCLI4152.1 (doi:10.1175/JCLI4152.1) [DOI] [Google Scholar]
- 39.Quinn G. P., Keough M. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 40.Sokal R., Rolf F. 1981. Biometry: the principles and practice of statistics in biological research, 2nd edn. San Fancisco, CA: W.H. Freeman [Google Scholar]
- 41.Harrison S. 1991. Local extinction in a metapopulation context: an empirical evaluation. Biol. J. Linn. Soc. 42, 73–88 10.1111/j.1095-8312.1991.tb00552.x (doi:10.1111/j.1095-8312.1991.tb00552.x) [DOI] [Google Scholar]
- 42.Schwarzkopf L., Alford R. A. 1996. Desiccation and shelter-site use in a tropical amphibian: comparing toads with physical models. Funct. Ecol. 10, 193–200 10.2307/2389843 (doi:10.2307/2389843) [DOI] [Google Scholar]
- 43.Higgins S., Richardson D. 1996. A review of models of alien plant spread. Ecol. Model. 87, 249–265 10.1016/0304-3800(95)00022-4 (doi:10.1016/0304-3800(95)00022-4) [DOI] [Google Scholar]
- 44.Hulme P. E. 2006. Beyond control: wider implications for the management of biological invasions. J. Appl. Ecol. 43, 835–847 10.1111/j.1365-2664.2006.01227.x (doi:10.1111/j.1365-2664.2006.01227.x) [DOI] [Google Scholar]
- 45.Grevstad F. S. 2005. Simulating control strategies for spatially structured weed invasion: Spartina alterniflora (Loisel) in pacific coastal estuaries. Biol. Invas. 7, 665–677 10.1007/s10530-004-5855-1 (doi:10.1007/s10530-004-5855-1) [DOI] [Google Scholar]
- 46.Taylor C., Hastings A. 2004. Finding optimal control strategies for invasive species: a density structured model for Spartina alterniflora. J. Appl. Ecol. 41, 1049–1057 10.1111/j.0021-8901.2004.00979.x (doi:10.1111/j.0021-8901.2004.00979.x) [DOI] [Google Scholar]
- 47.Fensham R. J., Fairfax R. J. 2008. Water-remoteness for grazing relief in Australian arid-lands. Biol. Conserv. 141, 1447–1460 10.1016/j.biocon.2008.03.016 (doi:10.1016/j.biocon.2008.03.016) [DOI] [Google Scholar]
- 48.O'Donnell S., Webb J. K., Shine R. S. 2010. Conditioned taste aversion enhances the survival of an endangered predator imperilled by a toxic invader. J. Appl. Ecol. 47, 558–565 10.1111/j.1365-2664.2010.01802.x (doi:10.1111/j.1365-2664.2010.01802.x) [DOI] [Google Scholar]
- 49.Ward-Fear G., Brown G. P., Shine R. S. 2010. Using a native predator (the meat ant Iridomyrmex reburrus) to reduce the abundance of an invasive species (the cane toad, Bufo marinus) in tropical Australia. J. Appl. Ecol. 47, 273–280 10.1111/j.1365-2664.2010.01773.x (doi:10.1111/j.1365-2664.2010.01773.x) [DOI] [Google Scholar]