Abstract
Progress in developing animal communication theory is frequently constrained by a poor understanding of sensory systems. For example, while lizards have been the focus of numerous studies in visual signalling, we only have data on the spectral sensitivities of a few species clustered in two major clades (Iguania and Gekkota). Using electroretinography and microspectrophotometry, we studied the visual system of the cordylid lizard Platysaurus broadleyi because it represents an unstudied clade (Scinciformata) with respect to visual systems and because UV signals feature prominently in its social behaviour. The retina possessed four classes of single and one class of double cones. Sensitivity in the ultraviolet region (UV) was approximately three times higher than previously reported for other lizards. We found more colourless oil droplets (associated with UV-sensitive (UVS) and short wavelength-sensitive (SWS) photoreceptors), suggesting that the increased sensitivity was owing to the presence of more UVS photoreceptors. Using the Vorobyev–Osorio colour discrimination model, we demonstrated that an increase in the number of UVS photoreceptors significantly enhances a lizard's ability to discriminate conspecific male throat colours. Visual systems in diurnal lizards appear to be broadly conserved, but data from additional clades are needed to confirm this.
Keywords: vision, pigments, colour, lizard, ultraviolet, photoreceptor
1. Introduction
Over the past several decades, comparative vision scientists have gained a better understanding of the functional and adaptive properties of colour vision systems by studying them in relation to habitat light and the important visual tasks that each species must perform. Studies on fishes and other aquatic organisms, for example, have suggested a strong relationship between habitat light and visual system features [1]. However, among terrestrial animal groups, colour vision systems are broadly conserved and ancestral properties are at least as important as ecology and visual task in visual system evolution [1–5]. Lizards are an important model system for understanding the role of visual ecology, phylogeny and behaviour on the structure of visual systems [6–9]. They are diverse in ecology and habitat and many species rely heavily on vision as their primary sensory modality. Extensive work on the phylogeny of many groups of lizards now makes it possible to relate physiological relationships to evolutionary history. Importantly, recent molecular studies have revised our understanding of higher level relationships among lizard clades [10,11], providing a new framework with which to examine the evolution of lizard visual systems.
Here, we present a study of the visual physiology and retinal anatomy of the Augrabies flat lizard, Platysaurus broadleyi (figure 1a), from South Africa. This is an extremely interesting species to study for two reasons. First, modest variations in the ultraviolet (UV)/violet throat colour of males play a major role in male–male social interactions [12,13], but nothing is known about the species' visual perception in this wavelength range. Second, studies of lizard photoreceptors and retinal physiology are limited to a few major clades. Platysaurus broadleyi belongs to a major group of lizards whose visual system has not been studied, and thus has the potential to offer insights into visual system evolution in lizards as a whole.
Figure 1.
(a) A typical male Platysaurus broadleyi form Augrabies Falls National Park. (b) A male displaying to a rival during the breeding season. Note the expanded throat which is UV-reflective and signals fighting ability.
In male–male interactions, P. broadleyi often use an elaborate display in which their UV/violet throat patch is prominently displayed (figure 1b). The spectral shape of the throat colour is an important predictor of the outcome of male–male fights, and altering male colours strongly influences the course of male–male contests [12,13]. A number of lizard species have been shown to exhibit UV colour patches that may be used as signals (e.g. [9,14–22]) and in a few cases, it has been shown that a high level of UV reflectance influences mate preference [23,24]. However, P. broadleyi is the only species where it has been demonstrated that modest among-individual variations in the shape of the spectral reflectance curve in the UV region have a strong influence on conspecific behaviour [13].
The ability of an animal to make fine-scale discriminations among similar colours is limited by the spectral sensitivity, and relative density of each class of photoreceptor in its retina [25,26]. We were therefore very interested in determining whether the retina of P. broadleyi exhibited any specializations for the detection and discrimination of subtle differences in UV coloration.
Platysaurus broadleyi belongs to the relatively basal Scinciformata clade of lizards [11], for which we currently have no information regarding the physiological or anatomical basis of colour vision. Visual pigment spectra have previously been measured in a number of diurnal species from the Toxicofera–Iguania clade (including the families Iguanidae, Agamidae and Chamaeleonidae) and both nocturnal and diurnal species from the Gekkota clade (families Gekkonidae and Sphaerodactylidae). All Iguania thus far studied show the same basic pattern of retinal photoreceptors. All appear to have tetrachromatic colour vision based on four classes of cone pigments with similar peak absorption wavelengths (λmax) [7,8,27,28]. Among the Gekotta, both nocturnal and diurnal forms have only three classes of photoreceptors (referred to as rods in the nocturnal forms, and cones in the diurnal). The λmax of the two non-UV pigments are shifted to longer wavelengths in the diurnal forms [29–31]. The Scinciformata form a distinct clade which is more closely related to the Gekkota than to the Iguania. These relationships raise some interesting questions about lizard visual systems and phylogeny. Is the Scinciformata clade fundamentally different from either the Iguania or Gekkota clades, more similar to the more closely related Gekkota or more similar to the diurnally active Iguania?
Here, we carried out an analysis of retinal physiology, photoreceptor pigments and oil droplets in the cordylid lizard P. broadleyi. We used electroretinography (ERG) to determine overall spectral sensitivity. For comparison, and as a control, we measured spectral sensitivity in Anolis sagrei using identical methods. We carried out microspectrophotometric (MSP) analysis of the retina in order to identify the pigments and oil droplets found in the different retinal photoreceptors, and estimated the relative abundance of different classes of photoreceptor oil droplets. We used this information to model colour discrimination by P. broadleyi in order to assess the ability to make fine discrimination of UV coloration of conspecifics, and to gain further insight into the role of evolutionary history in determining the visual system structure of diverse lizard species.
2. Material and methods
(a). Study animal and husbandry
Platysaurus broadleyi is an extremely colourful and sexually dimorphic lizard found in the Northern Cape Province of South Africa [32]. Males rely on visual signals at a distance but also use chemical cues when in close quarters [33]. Eight adult males and eight adult females were the subject of this study. The lizards were wild-caught, but were part of a long-term (3–4 years) captive group in Johannesburg before being shipped to the USA. They were housed in large communal cages and exposed to a 12 L : 12 D cycle with a combination of fluorescent, incandescent and UV-emitting mercury vapour lamps that mimicked natural sunlight. They were provided with water ad libitum and vitamin-coated crickets on a daily basis.
(b). Electroretinography
Prior to recording, each lizard was anaesthetized and immobilized with an injection of 0.01 mg g−1 nembutal and 0.03 mg g−1 tubocurarine chloride, and then respired with a small rodent respirator through a tracheal tube. After local application of 2 per cent xylocaine gel (Astra), an indifferent platinum wire electrode was inserted through a small slit in the skin of the neck. The eyelid of one eye was held open with forceps and the cornea was swabbed with a small amount of clear 2 per cent xylocaine gel. The active electrode, mounted on a three-axis micromanipulator, was then brought into contact with the eye. The active electrode consisted of a 0.5 mm diameter steel tube mounted on the end of a sheath holding two 400 µm diameter fused silica optical fibres. Light stimuli and adapting lights were delivered through this pair of fibre optics. Because of the positioning in contact with the cornea, and because light emerging from them diverges quickly, the light from the fibres could not be focused by the animal's lens and diffusely illuminated a broad region of the retina. Light from each of the paired fibres illuminated a nearly identical region. A wire soldered to the active electrode passed the electrical response from the eye to an A-M Systems AC amplifier, where the signal was recorded differentially between the active and inactive electrodes. The signal was filtered (1 Hz high pass, 50 Hz low pass) and passed to an analogue-to-digital conversion system (Powerlab Scope software) and to a Macintosh computer. The output from the eye was signal-averaged 32 times. The amplitude of the ERG b-wave was used to quantify response.
One of the two optical fibres leading to the eye transmitted a constant low intensity-adapting stimulus (ca = 1 µmol m−2 s−1 irradiance measured at the surface of the eye with a Li-Cor quantum irradiance metre) from a tungsten lamp. Chromatic stimuli were delivered through the other fibre optic. Stimuli consisted of 12 ms flashes of monochromatic light, with a 1 s interval between each flash. Light for the chromatic stimulus flashes was created with a 300 W xenon arc lamp focused onto the entrance slit of monochromator, which produced a 20 nm half-intensity bandwidth stimulus. Intensity of the chromatic stimulus was controlled with a continuously variable optical density wedge. The relationship between wedge position and the intensity for each wavelength was determined in advance using an Ocean Optics USB2000 spectroradiometer (calibrated for wavelength with an Ocean Optics HG-1 mercury/argon calibration lamp; for irradiance with a Li-Cor 1800-02 irradiance/radiance standard calibration lamp).
At each test wavelength, four stimulus intensities were employed in sequence from darkest to brightest. The actual range of intensities varied depending on the wavelength, and was adjusted in order to achieve as wide a range of responses as possible. We recorded responses from stimuli ranging from 300 to 700 nm in 10 nm intervals. Prior to the presentation of each set of four intensities at a given test wavelength, the eye was stimulated with a 550 nm stimulus flash at a fixed medium intensity. The responses to the chromatic test stimuli were divided by the response to the 550 nm standard stimulus given just prior to the test stimulus set, which controlled for changes in response sensitivity of the retina over the duration of the experiment.
For each wavelength, we plotted log stimulus intensity versus b-wave amplitude and fitted the data with a straight line (least-squares linear regression). For each individual lizard, we examined the results and chose a criterion response that fell within the range of responses seen for all wavelengths. We then calculated the stimulus intensity for each wavelength that would elicit the criterion response. We plotted wavelength versus the reciprocal of stimulus intensity at criterion. We then normalized the data relative to a maximum sensitivity of 1.0. These data were plotted on a semi-log scale to produce a relative spectral sensitivity curve for each individual.
The same procedure was carried out on three individual A. sagrei in order to confirm that unique results observed for P. broadleyi were not an artefact of differences in methodology from earlier studies. Spectral sensitivity of A. sagrei has been measured earlier using slightly different methods (ERG flicker-photometry [3] and L. J. Fleishman 1997, unpublished data).
(c). Microspectrophotometry and oil droplet counts
Methodological details are described briefly here, and in detail elsewhere [7,30,34]. All preparation was done under dim red or infrared illumination using image converters. Animals were dark-adapted for at least 2 h, after which they were cooled in a refrigerator at 2°C and observed until torporous, after which they were quickly decapitated using a small animal guillotine. Enucleated eyes were hemisected and incubated for 2 h at 5°C in Ca+2/Mg+2-free Puck's medium (Gibco) with added sucrose (final concentration 6%) to make the solution hypertonic. The retina was teased from the pigment epithelium and macerated using razor blade fragments. A drop of the dispersed retina was sandwiched between two cover slips and transferred to the stage of the MSP (see [30] for a description). Absorbance spectra were obtained for all clearly visible outer segments. Whenever possible, the inner segment of the same cell was also scanned to measure the absorbance of the oil droplet or dispersed inner segment pigment. The criteria used for selecting data for inclusion into the analysis pool were the same as those used by Loew [30,34]. Although we attempted to collect MSP data for the inner and outer segments for each cell, in some cases one or the other of these measurements did not meet our selection criteria, and thus sample sizes for oil droplets and pigments differ.
For outer segments (i.e. visual pigments), each acceptable spectrum was normalized by estimating a spectral maximum using the eye and fitting a Gaussian function to the data points 20 nm either side of this wavelength. λmax was then obtained using the method of Mansfield [35] as presented by MacNichol [36] using templates from Lipitz & Cronin [37]. Both A1 and A2 pigment templates were employed to see which produced the best fit. λmax values are accurate to ±1.0 nm and are reported here to the nearest whole integer.
Oil droplet and ellipsoid pigment absorbance spectra were plotted directly in units of optical density. For identification of different oil droplet classes and characterization of variation within class of oil droplet, a value of λmid was determined, which is equal to the wavelength at which the absorbance is half way between the minimum and maximum values measured.
Lizard retinal preparations are especially difficult for MSP measurements [7,28] because of the difficulty in isolating individual photoreceptors from the pigment epithelium. For this reason, relatively small numbers of cones were measured, and the counts of cells encountered cannot be used as a reliable indicator of relative abundance of different cell classes. Oil droplets, however, can be observed directly under a microscope from small patches of isolated retina, and their relative numbers can be counted. In all diurnal lizard species studied thus far, ultraviolet-sensitive (UVS) cells and short wavelength-sensitive (SWS) cells are always associated with colourless oil droplets [7,28]. This makes it possible to obtain an estimate of the total numbers of UVS and SWS cells combined, relative to others, by counting colourless and coloured oil droplets. Two small pieces of isolated retina from each of two individual animals were placed in a drop of buffer on a glass slide, covered with a grease-edged coverslip and examined using an Olympus BHT microscope at 40X. The samples came from different retinal regions but we did not attempt to identify the retinal position of each sample. A supercircuits INC PC-33C colour camera was used for video imaging. Images were captured as JPG files and oil droplets were counted from these images. In order to facilitate the identification of different oil droplet classes, a program (Eyepilot) was used to segment images based on colour. For each image we made two counts: one starting from the bottom right and one starting from the bottom left and counts were repeated if they did not match. We did not attempt to systematically sample different regions of the retina and we cannot discount the possibility of variation across the retina in the relative abundance of different cone types. Therefore, these counts can be viewed only as an approximation of the overall abundance of oil droplets across the retina.
(d). Modelling the ability to discriminate among male throat colours
In order to explore how the sensitivity to UV wavelengths in P. broadleyi retina influences their ability to discriminate differences in the throat colours of conspecific males, we implemented a version of the Vorobyev–Osorio model [38] as detailed in Vorobyev et al. [39] and Siddiqi et al. [40]. The model determines the distance in perceptual space between pairs of colours based on the spectral sensitivity of each cone type and its relative abundance in the retina. A distance in perceptual colour space, ΔS, is calculated in units of multiples of just noticeable difference. We assumed that colour perception is based on single cones [41], and the output from each cone type is proportional to the natural log of relative photon capture: determined by multiplying the normalized absorption spectrum by the normalized transmission spectrum of the oil droplet most commonly associated with that cell type. Methodological details and assumptions for our implementation of the model are found in the electronic supplementary material, M1.
We randomly selected pairs of throat colours (spectral reflectance) from a set of 136 males and determined the impact of changes in the relative abundance of the UVS photoreceptor on the value of ΔS for each throat colour pair. We also determined the proportion of throat colour pairs with ΔS ≥ 2.0, as an indication of the proportion of throat colours that could be reliably discriminated by different model retinae. We ran each test under three conditions: (i) UVS photoreceptors equal in abundance to other single cones (typical lizard), (ii) UVS photoreceptors three times as abundant as other photoreceptors (P. broadleyi), and (iii) no UVS photoreceptors.
3. Results
(a). Electroretinography
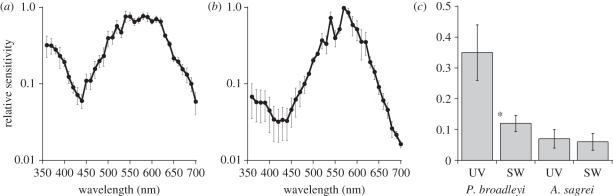
The relative spectral sensitivity curves of the three males and three females were very similar in overall shape, but the small sample size precluded a statistical comparison. However, the range of maximum–minimum values for males and for females overlapped at all but one of the wavelengths measured (450 nm), and at this wavelength the means differed by only 0.04. We therefore combined the data for males and females. The ERG spectral sensitivity curve (figure 2a) consists of an elevated sensitivity at 360–370 nm, drops down to a local minimum at 440 nm, shows a very small peak at 450 nm and then rises smoothly to a broad plateau from 550 to 610 nm. The pattern is similar in broad outlines to that reported for a variety of other lizards [3,29] except that the UV sensitivity is considerably elevated.
Figure 2.
(a) Relative spectral sensitivity of P. broadleyi (n = 6). Average and standard error are shown for six individuals (three males and three females). (b) Relative spectral sensitivity of A. sagrei (n = 3) using identical procedures. Average and standard error are shown for three individuals (2 males, 1 female). (c) Average peak spectral sensitivity for broadleyi and sagrei from a UV region (360–370 nm) and from an SW region (450–460 nm) of the spectrum. Error bars are standard errors. Asterisk indicates a significant difference (pairwise t-test, p < 0.05).
In order to be certain that the high UV sensitivity was not an artefact of recording procedures (e.g. low levels of UV in our adapting stimulus), we measured spectral sensitivity of A. sagrei in our laboratory (figure 2b). This curve was consistent in shape with previous spectral sensitivity curves for A. sagrei (L. J. Fleishman 1997, unpublished data) and most other Anolis lizard curves [3].
Figure 2c shows a direct comparison of peak sensitivity of P. broadleyi and A. sagrei at a region of elevated UV sensitivity (360–370 nm) and at the SWS region (450–460 nm). These regions were chosen because they span the peak sensitivity of the UVS and SWS photoreceptors, respectively (see below). In P. broadleyi, UV sensitivity is significantly greater than SW sensitivity (paired sample t-test, t5 = 3.79, p < 0.05), while in A. sagrei sensitivity in the two regions are not significantly different (t2 = 0.12, p > 0.05).
(b). Microspectrophotometric and oil droplet measurements
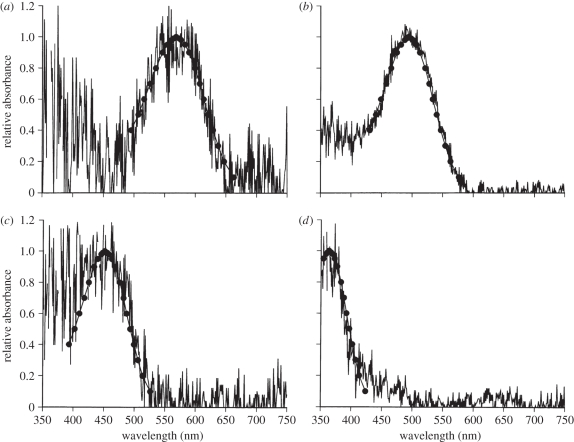
The P. broadleyi retina possesses single cones with oil droplets in their inner segments and double cones that include a principal member with an oil droplet and an accessory member with a dispersed yellow pigment in its inner segment. Four classes of visual pigments were identified, which can be characterized as UVS, SWS, MWS (middle wavelength-sensitive) and LWS (long wavelength-sensitive). A representative example of each pigment is plotted in figure 3. All four were best fit by A1 pigment templates. The pigment λmax values (nm ± SD) were: UVS, 364 ± 1.3; SWS, 451 ± 2.1; MWS, 492 ± 2.9; and LWS single, 570 ± 2.0; LWS double, principal member, 569 ± 1.0; LWS double, accessory member, 572 ± 0.6.
Figure 3.
MSP records from typical examples of the outer segments of four classes of photoreceptors detected in the P. broadleyi retina. Selection criteria and curve fit methods are explained in the text. (a) LWS pigment; (b) MWS pigment; (c) SWS pigment and (d) UVS pigment.
Five distinct classes of oil droplets and one type of dispersed inner segment pigment were found. A representative example of four oil droplets and the dispersed pigment are plotted in figure 4. A second colourless oil droplet that exhibited low density over the entire wavelength range is not shown in the figure. Table 1 summarizes the cell types in which each oil droplet type was found.
Figure 4.
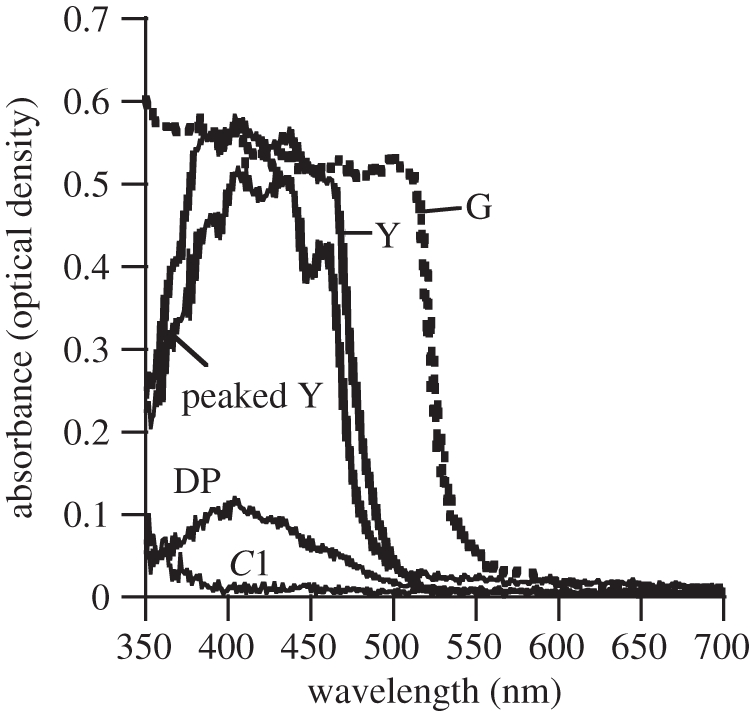
Representative examples of absorption spectra from MSP recordings of four classes of inner segment oil droplets and one example of dispersed inner segment pigment (DP). Each class of oil droplet is described here, followed in parentheses by the number of recordings that met the selection criteria (n), the mean value for λmid (the wavelength at which absorbance is halfway between zero and the maximum value), and the standard deviation of λmid. Names of droplet classes are based on their appearance under light microscopy and/or spectral. Oil droplet classes were: peaked Y (peaked yellow, n = 2, mean λmid = 467, s.d. = 2.1); Y (yellow, n = 8, mean λmid = 476, s.d. = 4.0); G (green, n = 18, mean λmid = 518, s.d. = 3.8); DP (dispersed pigment, n = 2, mean λmid = 462, s.d. = 10.6); C1 (colourless 1, n = 2, mean λmid = 380, s.d. = 0). In addition to the oil droplets and inner segment pigment plotted here, a second colourless oil droplet C2 was found that exhibited low absorption with no measurable increase across the range of wavelengths quantified.
Table 1.
Clearly identified visual pigments found in cones of P. broadleyi with MSP and oil droplet types found in different cell types.
| pigment type (cone type) | number measured that met selection criteria | mean λmax (s.d.) | inner segment oil droplet or dispersed pigment (figures 4 and 5) |
|---|---|---|---|
| UVS (single) | 6 | 364 (1.3) | C2 |
| SWS (single) | 4 | 451 (2.1) | C1 |
| MWS (single) | 5 | 492 (2.9) | G |
| LWS (single) | 11 | 570 (2.0) | Y or G |
| LWS (principal member of double) | 3 | 569 (1.0) | Y |
| LWS (accessory member of double) | 3 | 572 (0.6) | dispersed pigment |
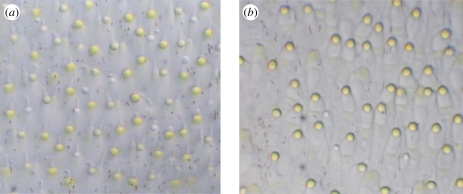
Figure 5 illustrates a light-microscopic section of the P. broadleyi retina when compared with a similar section from an A. sagrei retina. The most noticeable difference is that there appear to be more colourless oil droplets in the P. broadleyi sample. Table 2 summarizes the results of oil droplet counts for P. broadleyi along with percentages of different oil droplet classes from three species of Anolis. The Anolis samples were prepared systematically throughout each retina (excluding the temporal or central foveae) and the percentages were consistent across each retina [42]. The P. broadleyi data are from four non-foveal retinal sections chosen haphazardly and it is possible that other regions of the retina might yield different results. For the samples measured, colourless oil droplets were approximately equal to 20 per cent of all oil droplets sampled. In the three Anolis species, colourless oil droplets represented approximately 10 per cent of the total.
Figure 5.
Light microscopy images of a small patch of retina from (a) P. broadleyi and (b) A. sagrei for comparison. Individual photoreceptors and oil droplets are visible in both images. Colourless oil droplets, associated with UVS and SWS photoreceptors, are more common in the P. broadleyi retina.
Table 2.
Percentage of oil droplet classes in retinal samples for P. broadleyi and Anolis species for comparison (from [43]).
| species | % yellow | % green | % colourless |
|---|---|---|---|
| P. broadleyi (n = 8) | 43 | 36 | 22 |
| range for P. broadleyi | 39–46 | 34–37 | 20–24 |
| Anolis carolinensis | 52 | 38 | 10 |
| Anolis cristatellus | 10 | 80 | 10 |
| A. sagrei | 82 | 8 | 10 |
(c). Modelling throat-colour discrimination
Figure 6 illustrates the relative quantum catch of the four single cones (with their most common oil droplet) in P. broadleyi, and illustrates three typical throat colour spectra roughly covering the range of observed variation. It is apparent that most of the differences in throat colour occur over the region of the spectrum where the UVS and SWS cone sensitivities overlap, and the differences over the region covered by the MWS and LWS cones are much smaller.
Figure 6.
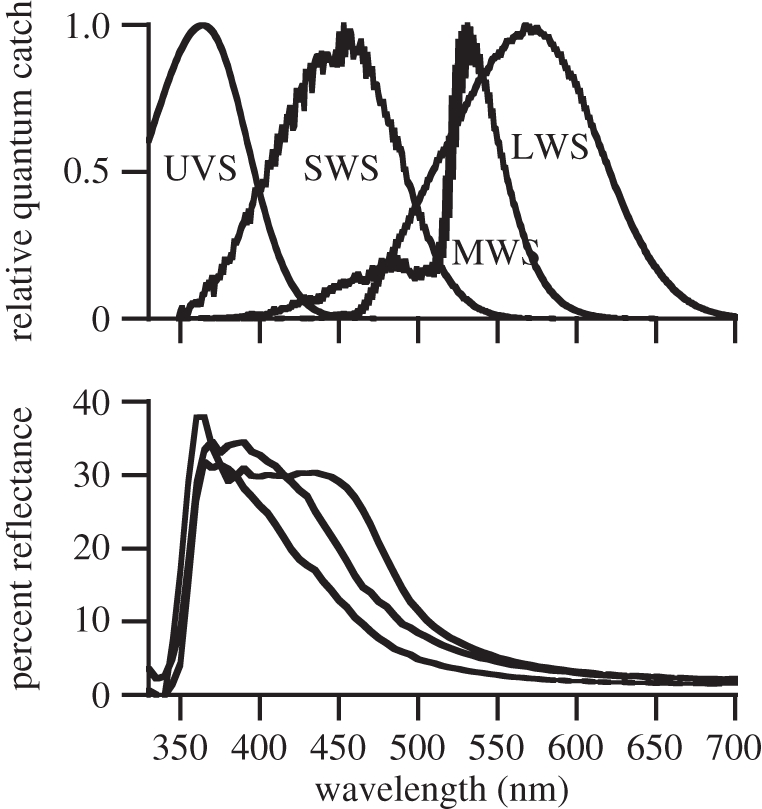
The upper graph shows the estimated relative quantal catch for each of the four single cone types including the filtering effects of its typical oil droplet. Absorbance spectra were calculated using Lamb's pigment template [43] and the average λmax for each cone class. Following Hart & Vorobyev [44], these were multiplied by the transmission spectrum, normalized from 0 to 1, of the most commonly associated oil droplet (see electronic supplementary material, M1). The lower graph shows spectral reflectance graphs of the throats of three different male P. broadleyi spanning the range of observed throat colours.
We were unable to accurately estimate the relative abundance of MWS and LWS single cones. However, as differences in the throat spectra mainly occur over the sensitivity range of the UVS and SWS photoreceptors, their relative abundance and spectral position largely determine the ability of P. broadleyi to discriminate these colours. For this reason, and because the major difference between visual sensitivity in broadleyi and other lizards is in the UV, in our modelling we explored the effects of altering relative UVS cone abundance. For v. 1 of our model (UVS : SWS : MWS : LWS = 1 : 1 : 1 : 1), the mean ΔS was 3.0 (s.e. = 0.19). For v. 2, with elevated UVS abundance (3 : 1 : 1 : 1), mean ΔS = 3.7 (s.e. = 0.24). For v. 3, with no UVS cone present (0 : 1 : 1 : 1), mean ΔS was 1.87 (s.e. = 0.14). The means were significantly different overall (ANOVA, F2,297 = 22.4, p < 0.0001), and each of the three means differed significantly (orthogonal contrasts: p < 0.001 for all three comparisons). The proportion of pairs with ΔS ≥ 2.0 were 0.63 (1 : 1 : 1 : 1), 0.71 (3 : 1 : 1 : 1) and 0.13 (0 : 1 : 1 : 1). Thus, the presence of a UVS cone, and the relatively high number of UVS cones (compared with the SWS cone for example) found in the P. broadleyi retina significantly increased the ability to discriminate among male throat colours.
4. Discussion
Although P. broadleyi belongs to a previously unstudied major clade (Scinciformata, [11]), more closely related to the Gekkota than to the Iguania, the photoreceptor make-up of its retina more closely resembles that of diurnal lizards from the Iguania than it does either the nocturnal or diurnal Gekkota. This suggests that the general pattern observed here is a widespread ancestral pattern shared by diurnal lizards from multiple major lizard clades. All of these species have a combination of single cones with oil droplets in the inner segment and double cones in which the primary member contains an oil droplet and the accessory member contains a dispersed pigment. Single cones fall into the same four spectral classes: UVS, SWS, MWS and LWS, while double cones are LWS. This pattern is also similar to that described for most bird retinas, although in many species, the UVS cone is replaced with a violet-sensitive cone [4]. This supports the general conclusion that diurnal lizards share an ancestral pattern of tetrachromatic vision and that the Gekkota visual system differs because of its nocturnal ancestry, and probably does not represent a widespread pattern in the non-iguanine lizard clades. Future studies that focus on diurnal lizards from multiple clades outside the Iguania that depend less on vision and more on olfaction will be of great interest.
The ERG relative sensitivity curve arises from the summed retinal response of all photoreceptors types, weighted by their relative densities [3,29,45]. In regions of the spectrum where photoreceptor sensitivities overlap, it is difficult to discern the relative input of the different classes to the summed output. As in Anolis [7], at wavelengths greater than 470 nm, P. broadleyi has three cone classes that add their outputs to produce a summed ERG response. Below 470 nm, for the most part, only SWS and UV cones contribute to the response, and these have non-overlapping peaks. It is thus possible to estimate the relative density of these two cone classes in the retina from the ERG sensitivity curve. The ERG curve was determined in 10 nm intervals, and the UVS cone peaks between 360 and 370 nm, while the SWS cone peaks fell between 450 and 460 nm. We thus looked at peak values over these two wavelength ranges in order to estimate relative numbers of these two cone classes in the retina. As illustrated in figure 2b, this evidence suggests that in P. broadleyi, UVS cones are roughly three times as common as SWS cones, while in A. sagrei, the two cone types are roughly equal in abundance.
If this estimate is correct, it should be reflected in the count of colourless oil droplets. It is impossible to distinguish C1 and C2 oil droplets by visual inspection. In A. sagrei, we expect them to be roughly equal in numbers. If P. broadleyi possesses three times as many UVS cones, and therefore three times the number of C2 oil droplets, with no difference in the number of C1 oil droplets, we would expect colourless oil droplets to be twice as common in the P. broadleyi retina as in A. sagrei. This prediction is borne out (table 2). One important caveat is that the sample from P. broadleyi came from four retinal sections chosen haphazardly, and in some animal species there are variations in relative numbers of photoreceptors over the retina. This, however, is not the case for Anolis, where relative abundances of different oil droplet classes are consistent throughout the retina [42]. While we cannot be certain that our count for P. broadleyi is representative of the entire retina, the agreement between the colourless oil droplet count and the ERG sensitivity data provides strong circumstantial evidence that the elevated sensitivity in the UV region seen in the ERG recordings is owing to a roughly threefold increase in the relative abundance of UVS cones when compared with other lizard species that have been examined.
(a). The implications of having more ultraviolet-sensitive cones
Enhanced UV sensitivity might simply function to make P. broadleyi more sensitive (i.e. more able to detect at the limits of low light levels) to conspecific males or some other stimuli. However, the animals are found in brightly lit habitats, and it seems unlikely that absolute sensitivity to UV is an important issue. We therefore hypothesized that the apparent increase in UVS cone density functions to enhance the ability to detect small variations in male throat colours. In order to explore this possibility, we modelled chromatic discrimination thresholds in P. broadleyi assuming different relative abundances of UVS cones using a variant of the model proposed in Vorobyev & Osorio [39]. We found that a threefold increase in UVS cones significantly increased the ability of the model retina to detect small differences in conspecific throat colour. This occurred because the noise in the UVS photoreceptor channel is reduced by the square root of the relative numbers of detectors contributing to each opponent channel [38,46]. It is clear that the presence of UVS cones in the retina is critical to the discrimination process, as indicated by the low number of throat colour pairs that could be discriminated reliably by a visual system consisting of only three classes of single cones (excluding the UVS cone).
The P. broadleyi retina is thus well adapted for the visual task of discriminating among conspecifics with subtle difference in body coloration in the short wavelength portion of the spectrum. We cannot distinguish between two possibilities that might explain the evolution of this pattern. First, elevated UVS densities may be a widespread feature of the group, which allowed P. broadleyi to evolve a communication system based on short wavelength variation in throat colour. Second, the communication system and visual system may have co-evolved with increased UV sensitivity evolving in concert with the evolution of the UV/violet signal. Additional species within the Cordylidae will have to be studied to distinguish among these possibilities. The strong overall similarity of the Platysaurus visual system with that of the distantly related Iguaninae highlights the critical importance of considering broad, conservative evolutionary patterns in the study of visual systems and visual ecology.
Acknowledgements
Experiments were carried out under Union College IACUC protocol no. 1056 and followed guidelines of Pough [47]. Platysaurus broadleyi were collected and shipped to New York State under permit no. L16354, issued by the Province of Gauteng, South Africa.
We thank Marissa Zarchy, Laura Maloney and Brian Oveson who collected and analysed major portions of the ERG data used in this paper. Sarah Pryke is thanked for statistical assistance. We thank two anonymous reviewers for the suggestions on an earlier version of this manuscript. The research was supported in part by a grant from the Howard Hughes Medical Institute to Union College.
References
- 1.Osorio D., Vorobyev M. 2005. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 10.1098/rspb.2005.3156 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chittka L. 1996. Does bee color vision predate the evolution of flower color? Naturwissenschaften 83, 136–138 10.1007/BF01142181 (doi:10.1007/BF01142181) [DOI] [Google Scholar]
- 3.Fleishman L. J., Bowman M., Saunders D., Miller W. E., Rury M. J., Loew E. R. 1997. The visual ecology of Puerto Rican anoline lizards: habitat light and spectral sensitivity. J. Comp. Physiol. A. 181, 446–460 10.1007/s003590050128 (doi:10.1007/s003590050128) [DOI] [Google Scholar]
- 4.Hart N. S., Hunt D. M. 2007. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 169, S7–S26 10.1086/510141 (doi:10.1086/510141) [DOI] [PubMed] [Google Scholar]
- 5.Hunt D. M., Carvalho L. S., Cowing J. A., Davies W. L. 2009. Evolution and spectral tuning of visual pigments in birds and mammals. Phil. Trans. R. Soc. B 364, 2941–2955 10.1098/rstb.2009.0044 (doi:10.1098/rstb.2009.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleishman L. J., Persons M. 2001. The influence of stimulus and background colour on signal visibility in the lizard Anolis cristatellus. J. Exp. Biol. 204, 1559–1575 [DOI] [PubMed] [Google Scholar]
- 7.Loew E. R., Fleishman L. J., Foster R. G., Provencio I. 2002. Visual pigments and oil droplets in diurnal lizards: a comparative study of Caribbean anoles. J. Exp. Biol. 205, 927–938 [DOI] [PubMed] [Google Scholar]
- 8.Macedonia J. M., Lappin A. K., Loew E. R., McGuire J. A., Hamilton P. S., Plasman M., Brandt Y., Lemos-Espinal J. A., Kemp D. J. 2009. Conspicuousness of Dickerson's collared lizard (Crotaphytus dickersonae) through the eyes of conspecifics and predators. Biol. J. Linn. Soc. 97, 749–765 10.1111/j.1095-8312.2009.01217.x (doi:10.1111/j.1095-8312.2009.01217.x) [DOI] [Google Scholar]
- 9.Stuart-Fox D., Moussalli A., Whiting M. J. 2007. Natural selection on social signals: signal efficacy and the evolution of chameleon display coloration. Am. Nat. 170, 916–930 10.1086/522835 (doi:10.1086/522835) [DOI] [PubMed] [Google Scholar]
- 10.Townsend T. M., Larson A., Louis E., Macey J. R. 2004. Molecular phylogenetics of Squamata: the position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Syst. Biol. 53, 735–757 10.1080/10635150490522340 (doi:10.1080/10635150490522340) [DOI] [PubMed] [Google Scholar]
- 11.Vidal N., Hedges S. B. 2009. The molecular evolutionary tree of lizards, snakes, and amphisbaenians. C. R. Biol. 332, 129–139 10.1016/j.crvi.2008.07.010 (doi:10.1016/j.crvi.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 12.Stapley J., Whiting M. J. 2006. Ultraviolet signals fighting ability in a lizard. Biol. Lett. 2, 169–172 10.1098/rsbl.2005.0419 (doi:10.1098/rsbl.2005.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting M. J., Stuart-Fox D. M., O'Connor D., Firth D., Bennett N. C., Blomberg S. P. 2006. Ultraviolet signals ultra-aggression in a lizard. Anim. Behav. 72, 353–363 10.1016/j.anbehav.2005.10.018 (doi:10.1016/j.anbehav.2005.10.018) [DOI] [Google Scholar]
- 14.Fleishman L. J., Loew E. R., Leal M. 1993. Ultraviolet vision in lizards. Nature 365, 397. 10.1038/365397a0 (doi:10.1038/365397a0) [DOI] [Google Scholar]
- 15.Font E., Pérez I., DeLanuza G., Sampredo C. 2009. Ultraviolet reflectance and cryptic sexual dichromatism in the ocellated lizard, Lacerta (Timon) lepida (Squamat:Lacertidae). Biol. J. Linn. Soc. 97, 766–780 10.1111/j.1095-8312.2009.01251.x (doi:10.1111/j.1095-8312.2009.01251.x) [DOI] [Google Scholar]
- 16.Gehring P.-S., Witte K. 2007. Ultraviolet reflectance in Malagasy chameleons of the genus Furcifer (Squamata: Chamaeleonidae). Salamandra 43, 43–48 [Google Scholar]
- 17.Lappin A. K., Brandt Y., Husak J. F., Macedonia J. M., Kemp D. J. 2006. Gaping displays reveal and amplify a mechanically based index of weapon performance. Am. Nat. 168, 100–113 10.1086/505161 (doi:10.1086/505161) [DOI] [PubMed] [Google Scholar]
- 18.Leal M., Fleishman L. J. 2002. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc. R. Soc. Lond. B 269, 351–359 10.1098/rpsb.2001.1904 (doi:10.1098/rpsb.2001.1904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macedonia J. M. 2001. Habitat light, colour variation and ultraviolet reflectance in the Grand Cayman anole, Anolis conspersus. Biol. J. Linn. Soc. 73, 299–320 10.1111/j.1095-8312.2001.tb01365.x (doi:10.1111/j.1095-8312.2001.tb01365.x) [DOI] [Google Scholar]
- 20.Martín J., López P. 2009. Multiple colour signals may reveal multiple messages in male Schreiber's green lizards, Lacerta schreiberi. Behav. Ecol. Sociobiol. 63, 1743–1755 10.1007/s00265-009-0794-6 (doi:10.1007/s00265-009-0794-6) [DOI] [Google Scholar]
- 21.Stoehr A., McGraw K. J. 2001. Ultraviolet reflectance of color patches in male Sceloporus undulates and Anolis carolinensis. J. Herpetol. 35, 168–171 10.2307/1566045 (doi:10.2307/1566045) [DOI] [Google Scholar]
- 22.Thorpe R. S., Murielle R. 2001. Evidence that ultraviolet markings are associated with patterns of molecular gene flow. Proc. Natl Acad. Sci. USA 98, 3929–3934 10.1073/pnas.071576798 (doi:10.1073/pnas.071576798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajer K., Molnár O., Török J., Herczeg G. 2010. Female European green lizards (Lacerta viridis) prefer males with high ultraviolet throat reflectance. Behav. Ecol. Sociobiol. 64, 2007–2014 10.1007/s00265-010-1012-2 (doi:10.1007/s00265-010-1012-2) [DOI] [Google Scholar]
- 24.LeBas N. R., Marshall N. J. 2000. The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus. Proc. R. Soc. Lond. B 267, 445–452 10.1098/rspb.2000.1020 (doi:10.1098/rspb.2000.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osorio D., Smith A. C., Vorobyev M., Buchanan-Smith H. M. 2004. Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 164, 696–708 10.1086/425332 (doi:10.1086/425332) [DOI] [PubMed] [Google Scholar]
- 26.Vorobyev M. 2003. Coloured oil droplets enhance colour discrimination. Proc. R. Soc. Lond. B 270, 1255–1261 10.1098/rspb.2003.2381 (doi:10.1098/rspb.2003.2381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbor H. R., Archer M. A., Hart N. S., Thomas N., Dunlop S. A., Beazley L. D., Shand J. 2002. Retinal characteristics of the ornate dragon lizard, Ctenophorus ornatus. J. Comp. Neurol. 450, 334–344 10.1002/cne.10308 (doi:10.1002/cne.10308) [DOI] [PubMed] [Google Scholar]
- 28.Bowmaker J. K., Loew E. R., Ott M. 2005. The cone photoreceptors and visual pigments of chameleons. J. Comp. Physiol. A 191, 925–932 10.1007/s00359-005-0014-4 (doi:10.1007/s00359-005-0014-4) [DOI] [PubMed] [Google Scholar]
- 29.Ellingson J. M., Fleishman L. J., Loew E. R. 1995. Visual pigments and spectral sensitivity of the diurnal gecko Gonatodes albogularis. J. Comp. Physiol. 177, 559–567 10.1007/BF00207185 (doi:10.1007/BF00207185) [DOI] [PubMed] [Google Scholar]
- 30.Loew E. R. 1994. A third, ultraviolet-sensitive, visual pigment in the Tokay gecko, Gekko gecko. Vision Res. 34, 1427–1432 10.1016/0042-6989(94)90143-0 (doi:10.1016/0042-6989(94)90143-0) [DOI] [PubMed] [Google Scholar]
- 31.Loew E. R., Govardovskii V. I., Röhlich R., Szél Á. 1996. Microspectrophotometric and immunocytochemical identification of ultraviolet photoreceptors in geckos. Vis. Neurosci. 13, 247–256 10.1017/S0952523800007483 (doi:10.1017/S0952523800007483) [DOI] [PubMed] [Google Scholar]
- 32.Branch W. R., Whiting M. J. 1997. A new Platysaurus (Squamata: Cordylidae) from the Northern Cape Province, South Africa. Afr. J. Herpetol. 46, 124–136 10.1080/21564574.1997.9649987 (doi:10.1080/21564574.1997.9649987) [DOI] [Google Scholar]
- 33.Whiting M. J., Webb J. K., Keogh J. S. 2009. Flat lizard female mimics use sexual deception in visual but not chemical signals. Proc. R. Soc. B 276, 1585–1591 10.1098/rspb.2008.1822 (doi:10.1098/rspb.2008.1822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loew E. R. 1995. Determinants of visual pigment spectral location and photoreceptor cell spectral sensitivity. In The outer retina (eds Djamgoz M. B. A., Archer S. N., Vallerga S.), pp. 57–78 London, UK: Chapman and Hall [Google Scholar]
- 35.Mansfield R. J. W. 1985. Primate photopigments and cone mechanisms. In The visual system (eds Fein A., Levine J. S.). New York, NY: Alan R. Liss [Google Scholar]
- 36.MacNichol E. F., Jr 1986. A unifying presentation of photopigment spectra. Vision Res. 26, 1543–1556 10.1016/0042-6989(86)90174-4 (doi:10.1016/0042-6989(86)90174-4) [DOI] [PubMed] [Google Scholar]
- 37.Lipitz L. E., Cronin T. W. 1988. Application of an invariant spectral form to the visual pigments of crustaceans: implications regarding the binding of the chromophore. Vision Res. 24, 597–604 10.1016/0042-6989(84)90114-7 (doi:10.1016/0042-6989(84)90114-7) [DOI] [PubMed] [Google Scholar]
- 38.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vorobyev M., Brandt R., Petisch D., Laughlin S. B., Menzel R. 2001. Colour thresholds and receptor noise: behaviour and physiology compared. Vision Res. 41, 639–653 10.1016/S0042-6989(00)00288-1 (doi:10.1016/S0042-6989(00)00288-1) [DOI] [PubMed] [Google Scholar]
- 40.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison dart frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485 10.1242/jeb.01047 (doi:10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 41.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill I. C. 1998. Colour thresholds and receptor noise: behaviour and physiology compared. Vision Res. 41, 639–653 10.1016/S0042-6989(00)00288-1 (doi:10.1016/S0042-6989(00)00288-1) [DOI] [PubMed] [Google Scholar]
- 42.Campbell D. 2006. Using oil droplets to map the distribution of cones in the anoline retina. PhD thesis, Cornell University, Ithaca, New York [Google Scholar]
- 43.Lamb T. D. 1995. Photoreceptor spectral sensitivities: common shape in the long wavelength region. Vision Res. 35, 3083–3091 10.1016/0042-6989(95)00114-F (doi:10.1016/0042-6989(95)00114-F) [DOI] [PubMed] [Google Scholar]
- 44.Hart N. S., Vorobyev M. 2005. Modeling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J. Comp. Physiol. A 191, 381–392 10.1007/s00359-004-0595-3 (doi:10.1007/s00359-004-0595-3) [DOI] [PubMed] [Google Scholar]
- 45.Jacobs G. H., Neitz M., Deegan J. F., Neitz J. 1996. Trichromatic colour vision in New World monkeys. Nature 382, 156–158 10.1038/382156a0 (doi:10.1038/382156a0) [DOI] [PubMed] [Google Scholar]
- 46.Lind O., Kelber A. 2009. Avian colour vision: effects of variation in receptor sensitivity and noise data on model predictions as compared to behavioural results. Vision Res. 49, 1939–1947 10.1016/j.visres.2009.05.003 (doi:10.1016/j.visres.2009.05.003) [DOI] [PubMed] [Google Scholar]
- 47.Pough F. H. 1991. Recommendations for the care amphibians and reptiles in academic institutions. ILAR J. Suppl. 33, 1–21 [Google Scholar]