Abstract
The horns, ossicones and antlers of ruminants are familiar and diverse examples of cranial appendages. We collectively term ruminant cranial appendages ‘headgear’; this includes four extant forms: antlers (in cervids), horns (in bovids), pronghorns (in pronghorn antelope) and ossicones (in giraffids). Headgear evolution remains an open and intriguing question because phylogenies (molecular and morphological), adult headgear structure and headgear development (where data are available) all suggest different pictures of ruminant evolution. We discuss what is known about the evolution of headgear, including the evidence motivating previous hypotheses of single versus multiple origins, and the implications of recent phylogenetic revisions for these hypotheses. Inclusion of developmental data is critical for progress on the question of headgear evolution, and we synthesize the scattered literature on this front. The areas most in need of attention are early development in general; pronghorn and ossicone development in particular; and histological study of fossil forms of headgear. An integrative study of headgear development and evolution may have ramifications beyond the fields of systematics and evolution. Researchers in organismal biology, as well as those in biomedical fields investigating skin, bone and regenerative medicine, may all benefit from insights produced by this line of research.
Keywords: artiodactyla, Ruminantia, Pecora, evolution, cranial appendage, development
1. Introduction
Cranial appendages are extremely common, conspicuous and diverse among animals. They serve a tremendous diversity of functions, often stemming from mate selection (e.g. [1,2]), but ranging into feeding, sensing [3,4] and even aiding locomotion [5]. Among mammals, cranial appendages are most familiar in artiodactyls, which have been known as the ‘even-toed ungulates’ (e.g. camels, pigs, hippopotami, giraffes, cattle and deer) and are now known to include whales as well [6–10]. The only extant artiodactyls with headgear (a term for artiodactyl cranial appendages first used by Gadow [11]) belong to Ruminantia—a group of artiodactyls that chew cud to support fermentation in their complex, multi-chambered stomachs [12]. Of the six extant families of ruminants, four families possess headgear (cervids, bovids, giraffids and the antilocaprid; figures 1 and 2) [15], each with a different type (antlers, horns, ossicones or pronghorns, respectively).
Figure 1.
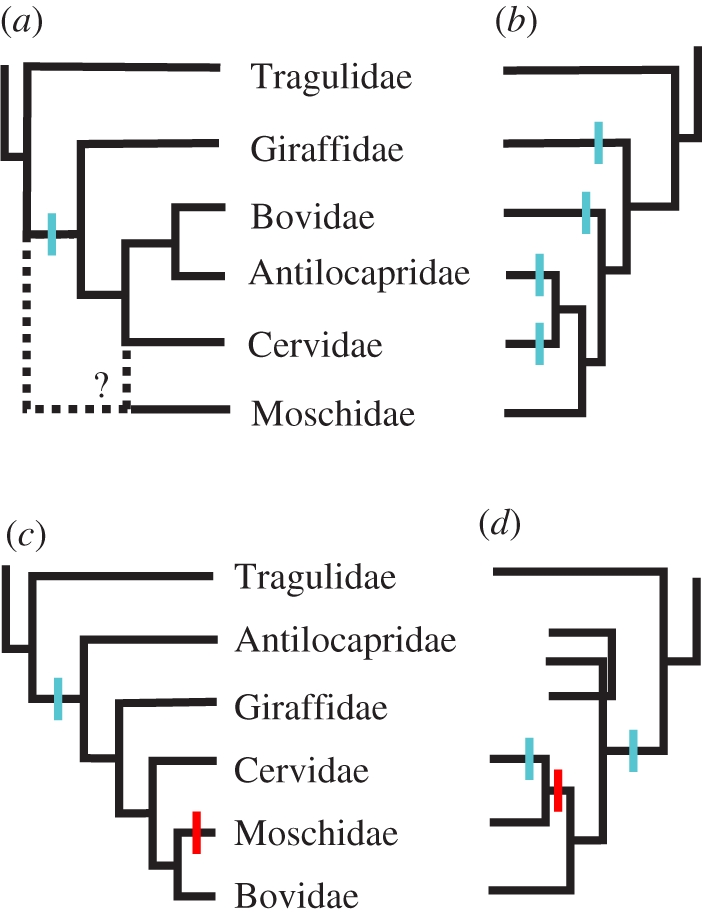
History of phylogenetic hypotheses of ruminant family-level relationships, with superimposed hypotheses of character state transitions for headgear, as referred to in-text; gains are indicated in blue and losses in red. (a) Pilgrim [13], based on morphology, reflecting the putative homology of bovid and antilocaprid headgear and the uncertain position of Moschidae, hypothesized a single origin of headgear with no loss, now rejected. (b) Janis & Scott [14], based on morphology, but down-weighting cranial appendages, hypothesized four independent origins of headgear. (c) The topologies of both Marcot [9] and Spaulding et al. [10] suggest a single origin of headgear with one loss; the former study is based on a supermatrix of 16 different genetic markers; the latter is based on a combined evidence matrix of 12222 informative characters. (d) Hernández Fernández & Vrba [8], a supertree based on molecular and morphological data for extant ruminants, showing one example of an alternative hypothesis of headgear evolution, with two origins and one loss.
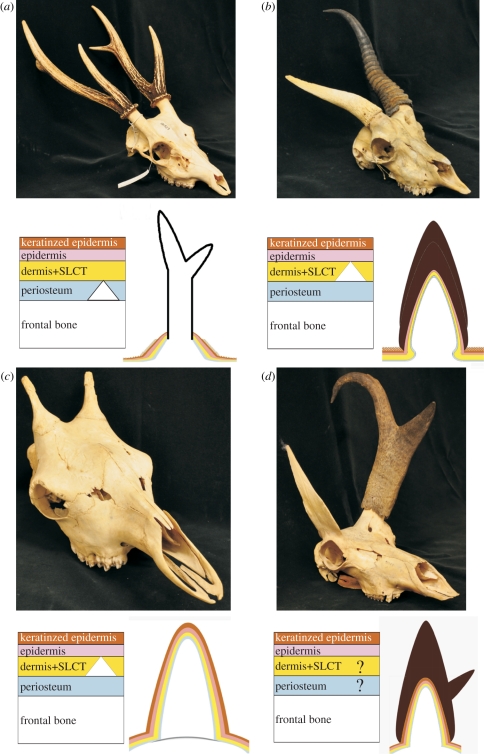
Figure 2.
Ruminant headgear types discussed in this review. Upper photographs illustrate adult osteological forms. Lower left diagrams are simplified representations of the layers of tissue at the loci of headgear ossification during earliest ontogeny, with five tissue types illustrated with different coloured layers. From lowest (most internal) to highest (external), the layers are: white for the frontal bone, blue for the periosteum surrounding the frontal, yellow for the connective tissue (dermis + subcutaneous loose connective tissue (SLCT)), pink for the epidermis and brown for the keratinized epidermal sheath. A white triangle indicates the presumed site of initial ossification of headgear. Lower right diagrams illustrate tissues in adult states. (a) Cervid: Rusa unicolor University of California Museum of Vertebrate Zoology (MVZ) 181527; left diagram showing the extension of the frontal by a modified endochondral ossification; right diagram showing mature form composed primarily of exposed bone (integument and SLCT are shed by this stage). (b) Bovid: Antidorcas marsupialis MVZ 117913; left diagram showing the dermal origin of the horncore-forming os cornu or anlage, which either fuses with the frontal or stimulates outgrowth of bone; right diagram showing adult form composed mostly of bone and keratin, with two nested layers of keratin sheath indicating annual growth. (c) Giraffa camelopardalis MVZ 55148; left diagram showing initial dermal ossification; right diagram showing adult form composed of bone and skin. (d) Antilocapra americana MVZ 98089; left diagram indicating limited knowledge of early development, with suggestion of antler-like extension of frontal; right diagram showing mature form composed of bone, skin and keratin, with two centres of keratinization for each tine of the sheath.
Surprisingly, the origin of such iconic structures as the horns of antelope or the antlers of elk is still unknown. This deficiency can be partly attributed to the comparatively little attention that this group has historically attracted among evolutionary biologists, despite the popular appeal and the economic value of many ruminants. More importantly, the evolution of ruminant headgear is difficult to ascertain for two purely biological reasons: the phylogenetic discordance of adult headgear characters versus other morphological characters, and the sparse but perplexing results from studies of headgear development.
The four extant forms of headgear have developmental and positional commonalities that have inspired hypotheses of homology: they are paired and (usually) symmetric, found on the frontal bones, formed from a bony core covered by integument (skin) and related connective tissues, and their presence is tightly linked to the male sex. By contrast, sharp differences in their development and physiology have led to hypotheses of multiple origins (table 1). Antlers (figure 2a), found only in cervids, are composed of exposed, living bone when mature [16] and are unique among mammalian appendages in their ability to completely and periodically regenerate in adults [17–20]. Horns (figure 2b), found only in bovids, are composed of a scabbard-like keratinous sheath covering a bony horncore, neither of which is ever shed [14,21]. Ossicones (figure 2c), found only in giraffids today ([22], but see [23]), are the simplest form of extant headgear, consisting of bony projections of dermal bones covered by skin and hair [22,24–27]. Finally, pronghorns (figure 2d), found only in Antilocapra americana today, are projections of the frontals that are covered by skin, hair and an annually replaced keratinous sheath [22,28–31].
Table 1.
Summary of ruminant headgear characters currently known for extant groups. (Presence and absence of characters are marked with plus (+) and minus (−) symbols, respectively. Characters with both states found in a group are marked with plus/minus (+/−) symbols. Unknown values are designated by question marks (‘?’). Parentheses indicate a probable state that has not been experimentally confirmed. SLCT, subcutaneous loose connective tissue.)
| cervids | bovids | giraffids | antilocaprids | |
|---|---|---|---|---|
| inducing tissue(s) | ||||
| epidermis | − | ? | ? | ? |
| dermis/SLCT | − | + | (+) | ? |
| periosteum | + | − | ? | ? |
| 1o ossification location | ||||
| frontal bone/periosteum | + | − | − | ? |
| dermis/SLCT | − | + | + | ? |
| mature bony core is | ||||
| live and covered | + | + | + | + |
| live and exposed | + | − | +/− | − |
| dead and exposed | + | − | − | − |
| character of epidermis | ||||
| hair | +/− | − | + | + |
| keratin sheath | − | + | − | + |
| branching/apical growth | ||||
| of bone | + | − | − | − |
| of sheath | − | − | − | + |
| regeneration | ||||
| of bone tissue | + | − | − | − |
| of integument | + | − | − | − |
| of keratin sheath | − | − | − | + |
Is it possible that these different types of ruminant headgear share a single developmental and evolutionary origin, despite differences in adult structure? In the last decade, phylogenetic analyses of the artiodactyls have produced novel hypotheses for the sequence of the evolution of ruminants, both among and within families [6–10]. We suggest that these topologies allow for renewed discussion of the origin of headgear. If ruminant headgear has a single developmental and evolutionary origin, how have the adult structures arisen; independently from headgear-less intermediates that possessed the basic genetic and developmental architecture, though unexpressed? Or did adult structures evolve through a series of transitional forms of headgear?
Understanding the developmental origins of the headgear in each group will be the key to unravelling the problem of ruminant headgear homology. Comparison of the location and morphology of adult structures will often suffice for identifying homologies, as known to generations of biologists [32,33]; however, in some cases, position and morphology can be deceptive. For example, a developmental-positional disconnection was uncovered by direct study of the frame-shift mutation that led from a theropod state of digits I, II and III in the manus to the modern bird state of II, III and IV [34–36]. Homology reflects inherited information, including developmental pathways, not similarity of adult structure [37]. Therefore, it is important to carefully consider the developmental origin of structures before deciding whether they are homologous or analogous.
In addition to answering the age-old question of ‘how the cow got its horns’, the study of ruminant headgear may enable important applications in other fields. In animal husbandry and veterinary medicine, for example, species and breeds with horns must often be dehorned as juveniles to ensure the safety of the animals, their herd-mates and handlers. Current methods are usually time-consuming and painful for the animals [38,39]. Improved techniques for dehorning based on a detailed understanding of horn development would be beneficial. Even more compelling is the potential for biomedical applications derived from insights on the relationship between bone and integument. Horns and antlers are ideal, simplified systems for investigating the way integument can control or modulate bone growth [17]. Antler development, in particular, is seen as a key to understanding a suite of skin–bone interactions, including healing, regeneration and neural control of bone development [40,41]. Beginning with Goss [42], the possibilities for regenerative medicine have become a major line of inquiry [18], raising the possibility that antler regeneration could be co-opted for human medicine, with applications ranging from accelerating skin regeneration for burn victims to delaying the onset of osteoporosis [43,44].
However, it must be emphasized that the idiosyncrasies of antler, horn and other headgear development will only be fully understood in an evolutionary context. It is unsafe to assume that an organ develops in the best way possible; often it was simply the most convenient way to evolve [45], and alternatives in evolutionary and developmental pathways are (or were) possible. Just as development must be considered when studying the evolution of adult organs, evolution must be considered when studying development.
(a). Hypotheses of homology or analogy
Workers have long acknowledged that the problem of headgear evolution is restricted to the Ruminantia and not related to the evolution of cranial appendages in the other groups of artiodactyls [46]. Ruminantia consists of two main groups, the headgear-less paraphyletic stem-group ‘Tragulina’ and the monophyletic clade Pecora. (Protoceratids, extinct artiodactyls equivocally placed between tylopods and ruminants [47], are thought to have evolved headgear independent of the Pecora.) The extant families of ruminants with four-chambered stomachs are found in Pecora, and four of the five pecoran families have headgear (figures 1 and 2) [12]. Up to the late 1980s, workers explored several ideas about homology in ruminant headgear [13,46,48–51]. While they varied in details, all hypothesized that ruminant headgear had a single history, evolving from ossicone-like tubercles in the common ancestor either of all ruminants or the Pecora, ruminants above tragulids (figure 1a). Most researchers hypothesized that the antler pedicle was homologous to the entire ossicone, horn or pronghorn [26,49,50]. Consequently, the exposed-bone portion of the antler was seen as an evolutionary novelty, growing distally on an ossicone. Most discussions focused on bovid horns and cervid antlers, ignoring ossicones and pronghorns, but hypotheses of homology between the initial ossifications of ossicones and bovid horns go back to Geoffroy Saint-Hilaire in the mid-nineteenth century [21,52]. The placement of Antilocapra was up for debate, with workers either uniting it with bovids because of its keratinous sheath, antelope-like behaviour and dentition [13,29], or uniting it with cervids because of its skeletal characters, annual shedding of the keratinous sheath, and horncore development [14,51]. The equivocal placement of Antilocapra clearly required additional, independent evidence [30].
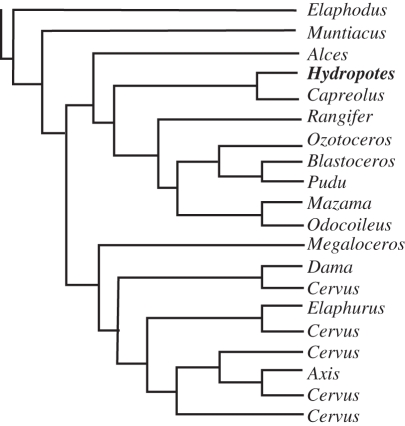
By the late 1980s, enough fossil, anatomical and modern developmental evidence had been gathered to make a strong case for the independent origin of all forms of ruminant headgear [14]. Janis & Scott [14] correctly explained that investigating the origin of a complex structure requires an independently derived cladogram. Consequently, they constructed a phylogeny based on 34 morphological traits of 36 taxa of fossil and living ruminants (figure 1b). Their analysis indicated: (i) headgear-less tragulids were the most basal ruminants, (ii) antilocaprids were nested with cervids, and (iii) the most basal cervid was the antlerless Hydropotes. Because of the distinct developmental modes and the nested positions of headgear-less moschids and Hydropotes, the most parsimonious reconstruction was for an independent origin of headgear in each ruminant family [14,53].
Recent molecular data have changed the topology of Ruminantia yet again (figure 1c,d), placing Antilocapra with giraffids, grouping bovids, cervids and headgear-less moschids, and moving the antlerless Hydropotes well within the cervid radiation (figure 3) [6–10]. The placement of Antilocapra is robust; it is based upon 15 of the 16 genes available to Marcot [9] in his supermatrix analysis, with 100 per cent bootstrap support. The placement of Hydropotes is based on six genes, with 98 per cent bootstrap support [9]. This new topology prompts reconsideration of the hypothesis of a single origin of ruminant headgear. Because the absence of antlers in Hydropotes is now judged to be a derived autapomorphy, only one family of ruminants above tragulids has evidence of a basal headgear-less state: the moschids (sister to the bovids or cervids: figure 1c,d). Fossil moschids have no headgear [14], but the earliest fossil antilocaprids [54–56], giraffids [22,23], bovids [57] and cervids [14,58] all possess headgear. However, this picture is complicated by the fact that these families are diagnosed by their headgear (or lack thereof), which creates circularity problems for investigating their origins [57]. Additionally, the current paucity of early headgear-less forms does not by itself justify rejection of the multiple origins hypothesis.
Figure 3.
Schematic of current genus-level cervid tree, with the antlerless Hydropotes highlighted in bold. Tree from Marcot [9].
The fossil record contains many headgear-less artiodactyls that may lie within the Pecora. Resolving their phylogenetic placement in the context of the rich molecular dataset of extant pecorans is critical to testing hypotheses about the origin of headgear. For example, knowing the positions of the headgear-less Amphitragulus [14] or the enigmatic Hoplitomeryx (which had bovid-like horns and a cervid-like skeleton [59]) would bring us closer to understanding the path of horn and antler evolution. Many headgear-less forms (such as Amphitragulus, Dremotherium and the blastomerycids) were originally placed on the pecoran stem among the moschids, which were considered a basal group at that time (figure 1a) [46], but living moschids are now recognized as nesting high within the tree (figure 1b–d) [6–10,14]. If some or all of these headgear-less fossil forms reside outside Moschidae [14], it would be difficult to argue for a single origin of headgear. Neglecting to include such fossil taxa for study could erroneously suggest that the problem is one of a single headgear loss instead of an issue of multiple origins. Similarly, there has been debate about the homology of extant giraffid ossicones and the headgear of more basal giraffids [60,61]. Resolving the origin of ruminant headgear will require close study of both extant and extinct species, exploration of the internal phylogenies of each family, and thorough investigation of the developmental processes of each type of headgear.
2. Histogenesis of ruminant headgear
Although the term ‘horn’ has been applied to bovid horns, pronghorns and ossicones (e.g. [24,29]) as well as to headgear per se (e.g. [11]), we restrict its use to bovid horns (headgear covered in a keratinous sheath that is not annually replaced). To avoid implying homologies between groups, we use different terms for each type of headgear: antlers (in cervids), horns (in bovids), ossicones (in giraffids) and pronghorns (in Antilocapra). The different types of headgear share important structural, developmental and positional characters, but they also possess distinct developmental and physiological features that suggest convergence in some of these adult characters (table 1). Here, we present brief summaries of the histogenesis of each headgear type; our electronic supplementary material presents a much more detailed review.
Among ruminant headgear, the antler has prompted the most study in part because it is capable of periodically regenerating after complete abscission. The antler is a bony outgrowth from the lateral crest of the frontal bone [20,62]. During growth, the integument and underlying subcutaneous loose connective tissue (SLCT) externally cover the antler [20,41]; interactions between the external and internal tissues are vital to the initiation and modulation of longitudinal growth [63]. Thus, concomitant changes in skin and bone are apparent during antler growth.
As a primary or first year antler elongates, it passes through three stages of ossification: intramembranous, transitional and endochondral [20,62]. During the stage of intramembranous ossification, the lateral crest of the frontal bone forms by periosteal deposition of spongy bone. The periosteum at this site is unusual because it contains embryonic-like cells (perhaps of neural crest cell origin), and transplantation experiments have demonstrated the ability of this periosteum to form ectopic antlers. More evidence of this intrinsic capacity is provided by induction experiments which show that signalling between skin and bone is not required to form the lateral crest [63]. Continued deposition of spongy bone on top of the lateral crest produces a palpable bump called the pedicle. During the stage of transitional ossification, elongation quickens and produces a short but visible pedicle. The apical periosteum undergoes a partial transformation to a perichondrium, resulting in apical deposition of highly vascularized bony and cartilaginous tissues. Although intensification of pedicle growth typically coincides with male puberty and an increase in testosterone [42,64], additional molecular signalling between the periosteum and integument is required to initiate pedicle formation [63]. Pedicle growth is completed and antlerogenesis begins during the stage of endochondral ossification. The apical periosteum completely transforms into perichondrium and begins to deposit highly vascularized cartilaginous tissue, which ossifies endochondrally, similar to long bones [62]. Likewise, the integument of the antler transforms into a highly glandular form called velvet. Termination of antler growth coincides with a large pulse in circulating androgens and the rutting season [20]. Only the integument covering the antler is shed (the pedicle remains covered), exposing bare bone. The bone tissue towards the periphery probably dies; however, deeper bone tissue remains alive and continues to form compact bone [16]. Antlers are cast as androgen levels decline [65], and a new set of antlers re-grows endochondrally on top of the pedicles when androgen levels are at a minimum [66].
What little is known of early horn development in bovids has been obscured by inconsistent identification and naming of primordial horn structures, compounded by the inclusion of animals with scurs: ‘loose horns’ that have a bony core but no solid connection to the skull. Scurs may arise genetically, as in many breeds, or via pathology, and exhibit a range of severities. In the electronic supplementary material, we establish language to help untangle early horn development.
The three critical structures of the early horn are (in ontogenetic order) the horn anlage (precursor), the os cornu (a detached, palpable bud) and the attached horncore bud. Before birth, the connective tissue components above the future horn site irreversibly differentiate into the anlage [21], which seems to be the primary inducer of horncore development. The next stage may or may not be a distinct os cornu, a palpable nodule in the tissue immediately above the frontal bone. The os cornu is something of a mystery. It is unclear whether it arises from the anlage; it may be made of dermis and/or SLCT [21,67,68] or cartilage [11,69]; and it may ossify independently in the soft tissue (forming a scur) or after attaching to the frontal bone (forming a normal horn) [21]. Cartilage preformation (suggested by Gadow [11] and Atzenkern [69]) would be unexpected in a context of dermal ossification, and has been rejected by most workers [14,67,68]. Several authors reject the existence of the os cornu during normal horn development [21,70–72]. After fusion of the developing anlage/os cornu to the frontal, the bony horncore bud begins to develop. By the time the horncore is observable, it is seamlessly attached to the skull, but its microstructure differs from the frontal [67]. Further growth of the horncore is appositional at both the tip and the surface [14,67,73,74], but with slowing growth, compact bone begins to be deposited in the interior. Unlike antlers, horncores are maintained throughout life and undergo active remodelling [75].
Normal horn sheath growth is dependent on the presence of the anlage/os cornu [21]. The keratinous sheath grows continuously from the skin covering the horncore [14,67]. Temperate species with seasonal fluctuations in sheath growth rates produce a series of distinct nested cones, with the oldest cones at the tip and the youngest against the horncore (figure 2b) [42,76]. The sheath tip is usually much thicker proximodistally than the walls of the sheath [42], suggesting more rapid production of keratin at the tip. The keratinous horn tissue of juveniles is softer and more fibrous [77] and may ‘exfoliate’ before adulthood [31,67,74,77–79]. Distinctive horn shapes are hypothesized to arise by modulating zones of keratin production at the base [17,80]. Overall, the male phenotype is associated with increased expression of horns (earlier and faster growth, greater size and symmetry, etc.) [81]. Castration experiments show that testosterone is important for development of normal male horns [82]. The relationships among hormones, other signalling factors, and horn growth is complex and changes through ontogeny [76,83–89].
Because of the rarity of giraffids, very little is known about the early development of ossicones (figure 2c). Only a few histological studies have been performed on the ossicones of giraffes (Giraffa camelopardalis) [22,24,26,27], and none has investigated the ossicones of okapis (Okapia johnstoni). The ossicone begins as a separate bony core above the frontoparietal suture in giraffes and above the frontals in okapis [22]. The ossicone was originally thought to condense as fibrocartilage within the skin above the bone [26,27]; however, Ganey et al. [24] showed that it is primarily non-cartilaginous. Although initial ossification occurs dermally, the process is intramembranous, as is typical for skull roof bones [24]. Within a week of birth, the ossicones begin to ossify [27], remaining detached until sexual maturity [22] and primarily growing at the intersection with the skull [24]. In immature individuals, ossicones approximate the condition of scurs in bovids [25]. At sexual maturity, the ossicone fuses to the skull; growth ceases at the skull–ossicone interface [22,27], but giraffes continue to deposit lamellar (i.e. dense, layered) bone at the surface of their ossicones [22,27], similar to the primary growth pattern of bovid horncores. While the ossicones of adult male giraffes often have calloused tips [22], in adult okapis, the skin retracts from the tips, leaving the live bone exposed [27]. There is no evidence that the skin of the ossicones is different from that covering the remainder of the skull.
The extinct palaeomerycids possessed headgear generally interpreted as ossicones [14,23], but it is possible that they are more consistent with the headgear of early ‘merycodontine’ antilocaprids; no histological work has investigated this problem. Geraads [60] restricts ‘ossicones’ to crown group giraffids on the basis of development, but Solounias & Moelleken [61] present evidence that suggests this developmental pathway is plesiomorphic in giraffids.
Even less is known of early development of the pronghorn in Antilocapra. Only one study has investigated whether the pronghorn core of Antilocapra begins as a separate ossification or is a direct outgrowth from the frontal [51]. On the basis of an examination of 28 newborn pronghorn cores, Solounias [51] reports no delayed fusion of the pronghorn core, suggesting an even earlier fusion than bovid horncores or a cervid-like apophyseal development. The pronghorn sheath of male Antilocapra grows annually in response to cycles of male hormones [29]. Most females have smaller, irregularly shed, button-like horns, but approximately 30 per cent lack headgear [28]. Unlike bovids, each pronghorn has two centres of keratinization: a distal site for the main spike and an anterior site for the prong. After these two projections are nearly full size, the remainder of the shaft cornifies and elongates, creating a single structure surrounding the pronghorn core [29,31]. The basal, paraphyletic ‘merycodontine’ antilocaprids [54–56] had unshed antler-like headgear of exposed bone [90,91], suggestive of the unshed antlers of the earliest cervids and the bony tips of Okapia ossicones.
3. Major questions
Although current knowledge does not let us definitively answer the question ‘how did ruminant headgear evolve?’, we can offer several viable hypotheses and reject some old ideas. First, it is still possible that all types of headgear have independent origins (as with Janis & Scott [14]: figure 1b). In this case, the developmental origins of the types might be independent, or they might share deep genetic and developmental homologies. The latter case would bear many similarities to the other major hypothesis: a single origin of headgear as an adult structure (figure 1c). In either case, we would expect shared pathways underpinning the bovid horncore anlage/os cornu and the early ossicone and pronghorn core structures. The complete antler or just the pedicle might be homologous with the anlage/os cornu, but antler–horn homology would require that the cervid lineage shifted from an integumentary to a periosteal centre of ossification in the transition to modern antler development. We can clearly reject early ideas of headgear homology that grouped the musk deer (Moschidae) with the basal tragulids on the basis of their ‘primitive’ hornlessness (figure 1a) [46]. Any complete model of headgear evolution has to account for the absence of headgear in moschids, nested high within the Pecora. In addition, we can reject the hypothesis of Solounias [51] that supposed two origins of headgear, one for pronghorns and antlers and another for ossicones and horns; the current molecular topologies will not allow this evolutionary scenario (figure 1c,d). In the end, the historical path of headgear evolution was probably messy, with homology among some lineages and independent origins of morphology and/or developmental pathways in others. For example, we could see a scenario in which the headgear of giraffids, antilocaprids and bovids are homologous structures, but that ancestral type of headgear was lost along the lineage to cervids + moschids and antlers were independently derived (figure 1d). We cannot reach a clear conclusion until a series of new investigations of histogenesis are completed.
As we related in §2, knowledge of early development of headgear is imbalanced among the pecoran families, decreasing in knowledge from antlers, known very well from experimental manipulation [18], to pronghorns, known from only one study of neonate skulls [51]. For proper comparisons of antler, horn, ossicone and pronghorn developments, it will be necessary to know, from equivalent investigations, the sequences of development and the regulatory processes governing the adult forms. Transplantation and other manipulative studies of primordial headgear tissues, as pioneered in bovids (e.g. [21]) and cervids (e.g. [63,66,92–94]), must be extended to other clades of pecorans. Additionally, tissues from foetal animals require immunohistochemical study to determine which genes, molecules and tissues contribute to headgear development at each stage of early development. Both these lines of investigation will require access to breeding populations, which is easier for cervids, bovids and antilocaprids than for giraffids. Until these studies can be completed, no one will know which tissues induce the development of pronghorns or ossicones, nor can anyone completely test hypotheses of homology among headgear tissues and developmental processes.
We have synthesized the state of knowledge of headgear development in our histogenesis review (here and expanded in the electronic supplementary material), but we can complement that review with a list of the most important questions to be addressed by transplantation and histological studies.
— For antilocaprids: how do pronghorn cores originate and develop?
— For giraffids: fundamentally, how do ossicones originate?
— For bovids: how do the anlage and the os cornu of horns differ, and what role, if any, does each have in normal horn development? How is fusion with, or outgrowth of, the frontal bone achieved? How do normal and scurred horns differ in their development?
— For cervids: how and when does the periosteum of the frontal bone become antlerogenic and capable of transforming into different tissues? Does it have a predetermined developmental pathway, perhaps originating from neural crest cells [92]? Addressing the formation of this pathway will require a shift in focus from postnatal to embryological development.
Heterotypic interactions (between different types of tissues) play an important role in tissue differentiation in all types of headgear, but most of our current knowledge comes from studies of antlers. To test a hypothesis of deep developmental homology, it will be necessary to link our knowledge of heterotypic interactions in antlers to interactions in other forms of headgear. First, it will be necessary to understand what signalling mechanisms cause stem cells in growing antlers to differentiate into pre-osteoblasts at one time and pre-chondroblasts at another, and then to test for those signalling mechanisms in the development of other types of headgear. It will be important also to answer: which heterotypic interactions are essential to induce growth, and which simply modulate growth? What signals the transformation of normal skin into rapidly growing velvet or keratinized horn sheath?
Complete understanding of headgear evolution will only emerge when we can reconstruct headgear development in extinct species using osteological correlates of the processes observed in extant animals. One of the goals of ruminant biologists (both neontologists and palaeontologists) must be to document the details of bone microstructure that would allow for identification of the developmental pathways used by early bovids, cervids, giraffids and antilocaprids. With this knowledge, it should be possible to trace the evolution of headgear development within each family, generating hypotheses of ancestral character states. For example, given unambiguous results from modern developmental studies, one could examine fossils of Hoplitomeryx-grade animals to distinguish between the hypotheses that early cervids modified a plesiomorphic ossicone-like developmental path, with a separate centre of ossification, or that they independently derived headgear, with no macro-scale homology. Also, this sort of study would be the only way to determine the developmental process and potential homology of the headgear of the extinct Palaeomerycidae. Reconstructing the plesiomorphic developmental paths should provide further insight into the phylogeny of Ruminantia and the evolution of their most distinctive features, allowing more fossil data to be included on equal footing with molecular and developmental data from extant forms.
Acknowledgements
We would like to thank W. Clemens for first suggesting that someone ought to think about the broader implications of the new ruminant tree. Three anonymous reviewers made significant contributions, improving an earlier version of this work. We also thank C. Conroy of the MVZ for access to specimens shown in figure 2 as well as K. Padian, S. Hopkins and her students at U.O. for their constructive criticism.
References
- 1.Jarman P. J. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48, 215–267 10.1163/156853974X00345 (doi:10.1163/156853974X00345) [DOI] [Google Scholar]
- 2.Reeves R. R., Tracey S. 1980. Monodon monoceros Mamm. Spec. 127, 1–7 10.2307/3503952 (doi:10.2307/3503952) [DOI] [Google Scholar]
- 3.Schneider D. 1964. Insect antennae. Annu. Rev. Entomol. 9, 103–122 10.1146/annurev.en.09.010164.000535 (doi:10.1146/annurev.en.09.010164.000535) [DOI] [Google Scholar]
- 4.Krieger J., Breer H. 1999. Olfactory reception in invertebrates. Science 286, 720–723 10.1126/science.286.5440.720 (doi:10.1126/science.286.5440.720) [DOI] [PubMed] [Google Scholar]
- 5.Fay F. H. 1985. Odobenus rosmarus. Mamm. Spec. 238, 1–7 10.2307/3503810 (doi:10.2307/3503810) [DOI] [Google Scholar]
- 6.Hassanin A., Douzery E. J. P. 2003. Molecular and morphological phylogenies of Ruminantia and the alternative position of the moschidae. Syst. Biol. 52, 206–228 10.1080/10635150390192726 (doi:10.1080/10635150390192726) [DOI] [PubMed] [Google Scholar]
- 7.Geisler J. H., Theodor J. M., Uhen M. D., Foss S. E. 2007. Phylogenetic relationships of cetaceans to terrestrial artiodactyls. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 19–31 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 8.Hernández Fernández M., Vrba E. S. 2005. A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants. Biol. Rev. 80, 269–302 10.1017/S1464793104006670 (doi:10.1017/S1464793104006670) [DOI] [PubMed] [Google Scholar]
- 9.Marcot J. D. 2007. Molecular phylogeny of terrestrial artiodactyls. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 4–18 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 10.Spaulding M., O'Leary M. A., Gatesy J. 2009. Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution. PLoS ONE 4, 1–14 10.1371/journal.pone.0005361 (doi:10.1371/journal.pone.0005361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadow H. 1902. The evolution of horns and antlers. Proc. Zool. Soc. Lond. 1, 206–222 [Google Scholar]
- 12.Nowak R. M. 1999. Walker's mammals of the world, 6 edn. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 13.Pilgrim G. E. 1946. The evolution of the buffaloes, oxen, sheep and goats. Zool. J. Linn. Soc. 41, 272–286 [Google Scholar]
- 14.Janis C. M., Scott K. M. 1987. The interrelationships of higher ruminant families with special emphasis on the members of the Cervoidea. Am. Mus. Novitates 2893, 1–85 [Google Scholar]
- 15.Nowak M. A., Michor F., Iwasa Y. 2003. The linear process of somatic evolution. Proc. Natl Acad. Sci. USA 100, 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockstedt-Rasmussen H., Sørensen P. L., Ewald H., Melsen F. 1987. The rhythmic relation between antler and bone porosity in Danish deer. Bone 8, 19–22 10.1016/8756-3282(87)90127-X (doi:10.1016/8756-3282(87)90127-X) [DOI] [PubMed] [Google Scholar]
- 17.Hall B. K. 2005. Bones and cartilage: developmental skeletal biology. San Diego, CA: Academic Press [Google Scholar]
- 18.Kierdorf U., Kierdorf H. 2010. Deer antlers—a model of mammalian appendage regeneration: an extensive review. Gerontology, 57, 53–65 [DOI] [PubMed] [Google Scholar]
- 19.Li C., Suttie J. M., Clark D. E. 2005. Histological examination of antler regeneration in red deer (Cervus elaphus). Anatomical Rec. A 282A, 163–174 10.1002/ar.a.20148 (doi:10.1002/ar.a.20148) [DOI] [PubMed] [Google Scholar]
- 20.Price J. S., Allen S., Faucheux C., Althnaian T., Mount J. G. 2005. Deer antlers: a zoological curiosity or the key to understanding organ regeneration in mammals? J. Anatomy 207, 603–618 10.1111/j.1469-7580.2005.00478.x (doi:10.1111/j.1469-7580.2005.00478.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dove W. F. 1935. The physiology of horn growth: a study of the morphogenesis, the interaction of tissues, and the evolutionary processes of a Mendelian recessive character by means of transplantation of tissues. J. Exp. Zool. 69, 347–405 10.1002/jez.1400690302 (doi:10.1002/jez.1400690302) [DOI] [Google Scholar]
- 22.Churcher C. S. 1990. Cranial appendages of giraffoidea. In Horns, pronghorns, and antlers: evolution, morphology, physiology, and social significance (eds Bubenik G. A., Bubenik A. B.), pp. 180–194 New York, NY: Springer; [DOI] [PubMed] [Google Scholar]
- 23.Solounias N. 2007. Family giraffidae. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 257–277 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 24.Ganey T., Ogden J., Olsen J. 1990. Development of the giraffe horn and its blood supply. Anatomical Rec. 227, 497–507 10.1002/ar.1092270413 (doi:10.1002/ar.1092270413) [DOI] [PubMed] [Google Scholar]
- 25.Kosasih R. 1959. Observations on the structure of ‘loose horns’ in a zebu cow. Commun. Veterinariae 3, 2–19 [Google Scholar]
- 26.Lankester E. R. 1907. On the origin of the horns of the giraffe in foetal life on the area of the parietal bones. Proc. Zool. Soc. Lond. 4, 100–115 [Google Scholar]
- 27.Spinage C. A. 1968. Horns and other bony structures of the skull of the giraffe, and their functional significance. East African Wildl. J. 6, 53–61 [Google Scholar]
- 28.O'Gara B. 1969. Horn casting by female pronghorns. J. Mammal. 50, 2–5 [Google Scholar]
- 29.O'Gara B. W. 1990. The pronghorn (Antilocapra americana). In Horns, pronghorns, and antlers: evolution, morphology, physiology, and social significance (eds Bubenik G. A., Bubenik A. B.), pp. 231–264 New York, NY: Springer [Google Scholar]
- 30.O'Gara B. W., Janis C. M. 2004. Scientific classification. In Pronghorn: ecology and management (eds O'Gara B. W., Yoakum J. D.), pp. 3–25 Boulder, CO: University Press of Colorado [Google Scholar]
- 31.O'Gara B. W., Matson G. 1975. Growth and casting of horns by pronghorns and exfoliation of horns by bovids. J. Mammal. 56, 4–46 [PubMed] [Google Scholar]
- 32.Owen R. 1843. Lectures on the comparative anatomy and physiology of the invertebrate animals. London, UK: Longman Brown Green and Longmans [Google Scholar]
- 33.Wake D. B. 2003. Homology and homoplasy. In Keywords and concepts in evolutionary developmental biology (eds Hall B. K., Olson W. M.), pp. 191–200 New Delhi, India: Discovery Publishing House [Google Scholar]
- 34.Vargas A. O., Fallon J. F. 2005. Birds have dinosaur wings: the molecular evidence. J. Exp. Zool. Part B Mol. Dev. Evol. 304B, 86–90 10.1002/jez.b.21023 (doi:10.1002/jez.b.21023) [DOI] [PubMed] [Google Scholar]
- 35.Vargas A. O., Kohlsdorf T., Fallon J. F., VandenBrooks J., Wagner G. P. 2008. The evolution of HoxD-11 expression in the bird wing: insights from alligator mississippiensis. PLOS ONE 3, e3325. 10.1371/Journal.Pone.0003325 (doi:10.1371/Journal.Pone.0003325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X., et al. 2009. A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature 459, 940–944 10.1038/nature08124 (doi:10.1038/nature08124) [DOI] [PubMed] [Google Scholar]
- 37.Van Valen L. M. 1982. Homology and causes. J. Morphol. 173, 3–12 [DOI] [PubMed] [Google Scholar]
- 38.Stafford K. J., Mellor D. J. 2005. Dehorning and disbudding distress and its alleviation in calves. Vet. J. 169, 337–349 10.1016/j.tvjl.2004.02.005 (doi:10.1016/j.tvjl.2004.02.005) [DOI] [PubMed] [Google Scholar]
- 39.Alvarez L., Nava R. A., Ramírez A., Ramírez E., Gutiérrez J. 2009. Physiological and behavioural alterations in disbudded goat kids with and without local anaesthesia. Appl. Anim. Behav. Sci. 117, 190–196 10.1016/j.applanim.2009.01.001 (doi:10.1016/j.applanim.2009.01.001) [DOI] [Google Scholar]
- 40.Bubenik G. A. 1990. The antler as a model in biomedical research. In Horns, pronghorns, and antlers: evolution, morphology, physiology, and social significance (eds Bubenik G. A., Bubenik A. B.), pp. 474–487 New York, NY: Springer; [DOI] [PubMed] [Google Scholar]
- 41.Li C., Suttie J. M. 2000. Histological studies of pedicle skin formation and its transformation to antler velvet in red deer (Cervus elaphus). Anatomical Rec. Part A 260, 1–71 [DOI] [PubMed] [Google Scholar]
- 42.Goss R. J. 1983. Deer antlers: regeneration, function, and evolution. New York, NY: Academic Press [Google Scholar]
- 43.Mundy G. R., Gutierrez G., Gallwitz W., Feng J., Chen D., Garrett R., Harris S. 2001. Antler-derived bone growth factors and their potential for use in osteoporosis. In Antler science and product technology (eds Sim J. S., Sunwoo H. H., Hudson R. J., Jeon B. T.), pp. 171–187 Edmonton, Canada: Antler Science and Production Technology Research Centre [Google Scholar]
- 44.Li C. 2010. Exploration of the mechanism underlying neogenesis and regeneration of postnatal mammalian skin—deer antler velvet. Int. J. Med. Biol. Front. 16, 1–19 [Google Scholar]
- 45.Gould S. J., Lewontin R. C. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 1161–1198 [DOI] [PubMed] [Google Scholar]
- 46.Webb S. D., Taylor B. E. 1980. The phylogeny of hornless ruminants and a description of the cranium of archaeomeryx. Bull. Am. Mus. Nat. Hist. 167, 3–57 [Google Scholar]
- 47.Prothero D. R., Ludtke J. A. 2007. Family Protoceratidae. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 169–176 Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- 48.Coope G. R. 1968. The evolutionary origin of antlers. Deer 1, 215–217 [Google Scholar]
- 49.Pilgrim G. E. 1941. The relationship of certain variant fossil types of ‘horns’ to those of the living Pecora. Annu. Mag. Nat. Hist. 7, 172–184 [Google Scholar]
- 50.Bubenik A. B. 1990. Epigenetical, morphological, physiological, and behavioral aspects of evolution of horns, pronghorns, and antlers. In Horns, pronghorns, and antlers: evolution, morphology, physiology, and social significance (eds Bubenik G. A., Bubenik A. B.), pp. 3–113 New York, NY: Springer [Google Scholar]
- 51.Solounias N. 1988. Evidence from horn morphology on the phylogenetic relationships of the pronghorn (Antilocapra americana). J. Mammal. 69, 1–3 [Google Scholar]
- 52.Saint-Hilaire M. G. 1837. Sur le nouveau genre Sivatherium, trouvé fossile au bas du versant méridional de l'Himalaya, dans la vallée du Markanda; animal gigantesque de l'ancien honde, que je propose de rapporter au genre Camelopardalis. C. R. hebd Séances Acad. Sci. Semetre 1, 53 [Google Scholar]
- 53.Nieto M., et al. 2004. Origin of ruminant cranial appendages: biochronological and biogeographical aspects. J. Morphol. 260, 316 [Google Scholar]
- 54.Davis E. B. 2007. Family antilocapridae. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 227–240 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 55.Janis C. M., Manning E. 1998. Antilocapridae. In Evolution of tertiary mammals of North America volume 1: terrestrial carnivores, ungulates, and ungulatelike mammals (eds Janis C. M., Scott K. M., Jacobs L. L.), pp. 491–507 Cambridge, UK: Cambridge University Press [Google Scholar]
- 56.O'Gara B. W., Janis C. M. 2004. The fossil record. In Pronghorn: ecology and management (eds O'Gara B. W., Yoakum J. D.), pp. 27–39 Boulder, CO: University Press of Colorado [Google Scholar]
- 57.Bibi F., Bukhsianidze M., Gentry A. W., Geraads D., Kostopoulos D. S., Vrba E. S. 2009. The fossil record and evolution of bovidae: state of the field. Palaeontol. Electron. 12, 10A [Google Scholar]
- 58.Groves C. P. 2007. Family cervidae. In The evolution of artiodactyls (eds Prothero D. R., Foss S. E.), pp. 249–256 Baltimore, CO: Johns Hopkins University Press [Google Scholar]
- 59.Leinders J. 1983. Hoplitomerycidae fam. nov. (Ruminantia, Mammalia) from Neogene fissure fillings in Gargano (Italy). Scr. Geol. 70, 1–68 [Google Scholar]
- 60.Geraads D. 1991. Derived features of giraffid ossicones. J. Mammal. 72, 1–4 [Google Scholar]
- 61.Solounias N., Moelleken S. M. C. 1991. Evidence for the presence of ossicones in Giraffokeryx punjabiensis (Giraffidae, Mammalia). J. Mammal. 72, 1–7 [Google Scholar]
- 62.Li C., Suttie J. M. 1994. Light microscopic studies of pedicle and early first antler development in red deer (Cervus elaphus). Anatomical Rec. 239, 198–215 10.1002/ar.1092390211 (doi:10.1002/ar.1092390211) [DOI] [PubMed] [Google Scholar]
- 63.Li C., Yang F., Xing X., Gao X., Deng X., Mackintosh C., Suttie J. M. 2008. Role of heterotypic tissue interactions in deer pedicle and first antler formation: revealed via a membrane insertion approach. J. Exp. Zool. Part B Mol. Dev. Evol. 310B, 267–277 10.1002/jez.b.21210 (doi:10.1002/jez.b.21210) [DOI] [PubMed] [Google Scholar]
- 64.Bubenik G. A., Brown R. D., Schams D. 1991. Antler cycle and endocrine parameters in male axis deer (Axis axis): seasonal levels of LH, FSH, testosterone, and prolactin and results of GnRH and ACTH challenge tests. Comp. Biochem. Physiol. Part A Physiol. 99, 4–50 [DOI] [PubMed] [Google Scholar]
- 65.Goss R. J. 1968. Inhibition of growth and shedding of antlers by sex hormones. Nature 220, 83–85 10.1038/220083a0 (doi:10.1038/220083a0) [DOI] [PubMed] [Google Scholar]
- 66.Lincoln G. A., Tyler N. J. C. 1994. Role of gonadal hormones in the regulation of the seasonal antler cycle in female reindeer, Rangifer tarandus. J. Reprod. Fertil. 101, 129–138 10.1530/jrf.0.1010129 (doi:10.1530/jrf.0.1010129) [DOI] [PubMed] [Google Scholar]
- 67.Dürst J. U. 1902. Versuch einer Entwicklungsgeschichte der Hörner der Cavicornier nach Untersuchungen am Hausrinde. Frauenfeld, Switzerland: Verlag von J. Huber [Google Scholar]
- 68.Brandt K. 1928. Die Entwicklung des Hornes beim Rinde bis zum Beginn der Pneumatisation des Hornzapfens. Morphol. Jahrb. 60, 428–468 [Google Scholar]
- 69.Atzenkern J. 1923. Zur entwicklung der os cornu der cavicornier. Anatomischer Anzeiger 57, 125–130 [Google Scholar]
- 70.Bubenik A. B. 1983. Taxonomy of Pecora in relation to morphophysiology of their cranial appendices. In Antler development in cervidae (ed. Brown R. D.), pp. 163–185 Kingsville, TX: Caesar Kleberg Wildlife Research Institute [Google Scholar]
- 71.Kyselý R. 2010. Breed character or pathology? Cattle with loose horns from the Eneolithic site of Hostivice-Litovice (Czech Republic). J. Archaeol. Sci. 37, 1241–1246 [Google Scholar]
- 72.Sandifort G. 1829. Over de Vorming en Ontwickkeling der Horens van zogende dieren in het algmeen en van die der Hertenbeesten in het bijzonder. Nieuwe Verh. i Kl. Kiningl Ned. Inst Wet. 2, 67–106 [Google Scholar]
- 73.Dürst J. U. 1902. Sur le developpement des cornes chez les cavicorne. Bull. Mus. d'Hist. Nat. Paris, 3, 197–203 [Google Scholar]
- 74.Dürst J. U. 1926. Das Horn der Cavicornia. Seine Entstchungsursache, seine Entwicklung, Gestaltung auf den Schadel der horntragenden Wiederkauer. Denkschriften Schweiz. Nat. Ges. Zurich 68, 1–180 [Google Scholar]
- 75.Albarella U. 1995. Depressions on sheep horncores. J. Archaeol. Sci. 22, 699–704 10.1016/S0305-4403(95)80155-3 (doi:10.1016/S0305-4403(95)80155-3) [DOI] [Google Scholar]
- 76.Santiago-Moreno J., Gomez-Brunet A., Toledano-Diaz A., Gonzalez-Bulnes A., Picazo R. A., Lopez-Sebastian A. 2005. Influence of age on the relationship between annual changes in horn growth rate and prolactin secretion in the European mouflon (Ovis gmelini musimon). Anim. Reprod. Sci. 85, 251–261 10.S0378432004001186[pii]10.1016/j.anireprosci.2004.04.042 (doi:10.S0378432004001186[pii]10.1016/j.anireprosci.2004.04.042) [DOI] [PubMed] [Google Scholar]
- 77.George A. N. 1956. The post-natal development of the horn tubules and fibres (intertubular horn) in the horns of sheep. Br. Vet. J. 112, 1–4 [Google Scholar]
- 78.Fambach O. 1909. Geweih und Gehorn. Ein kritisches Referat. Z. Nat. 81, 225–264 [Google Scholar]
- 79.Khan I. A., Biddulph C. H., D'Abreu E. A. 1937. Horn growth in black buck and nilgai. J. Bombay Nat. Hist. Soc. 39, 1–2 [Google Scholar]
- 80.Kingdon J. 1982. East African mammals: an atlas of evolution in Africa. IIIC Bovids. Chicago, IL: The University of Chicago Press [Google Scholar]
- 81.Kiltie R. A. 1985. Evolution and function of horns and hornlike organs in female ungulates. Biol. J. Linn. Soc. 24, 299–320 10.1111/j.1095-8312.1985.tb00377.x (doi:10.1111/j.1095-8312.1985.tb00377.x) [DOI] [Google Scholar]
- 82.Sykes N., Symmons R. 2007. Sexing cattle horn-cores: problems and progress. Int. J. Osteoarchaeol. 17, 514–523 10.1002/oa.891 (doi:10.1002/oa.891) [DOI] [Google Scholar]
- 83.Lincoln G. A. 1998. Reproductive seasonality and maturation throughout the complete life-cycle in the mouflon ram (Ovis musimon). Anim. Reprod. Sci. 53, 87–105 10.1016/S0378-4320(98)00129-8 (doi:10.1016/S0378-4320(98)00129-8) [DOI] [PubMed] [Google Scholar]
- 84.Lincoln G. A., Clarke I. J. 1995. Evidence that melatonin acts in the pituitary gland through a dopamine-independent mechanism to mediate effects of daylength on the secretion of prolactin in the ram. J. Neuroendocrinol. 7, 8–43 [DOI] [PubMed] [Google Scholar]
- 85.Lincoln G. A., Tyler N. J. C. 1999. Role of oestradiol in the regulation of the seasonal antler cycle in female reindeer, Rangifer tarandus. Reproduction 115, 1–74 [DOI] [PubMed] [Google Scholar]
- 86.Reiter R. J. 1991. Pineal gland interface between the photoperiodic environment and the endocrine system. Trends Endocrinol. Metab. 2, 1–9 10.1016/1043-2760(91)90055-R (doi:10.1016/1043-2760(91)90055-R) [DOI] [PubMed] [Google Scholar]
- 87.Santiago-Moreno J., Toledano-Diaz A., Gomez-Brunet A., Lopez-Sebastian A. 2006. Effect of constant-release implants of melatonin on horn growth in mouflon ram lambs Ovis gmelini musimon. Folia Zool. 55, 1–8 [Google Scholar]
- 88.Szeligowski E. 1985. Blocking of horn development in cattle by a disturbance in local embryonic induction. Medycyna Weterynaryjna 41, 3–4 [Google Scholar]
- 89.Toledano-Diaz A., Santiago-Moreno J., Gomez-Brunet A., Pulido-Pastor A., Lopez-Sebastian A. 2007. Horn growth related to testosterone secretion in two wild Mediterranean ruminant species: the Spanish ibex (Capra pyrenaica hispanica) and European mouflon (Ovis orientalis musimon). Anim. Reprod. Sci. 102, 300–307 10.S0378-4320(06)00504-5[pii]10.1016/j.anireprosci.2006.10.021 (doi:10.S0378-4320(06)00504-5[pii]10.1016/j.anireprosci.2006.10.021) [DOI] [PubMed] [Google Scholar]
- 90.Matthew W. D. 1924. Third contribution to the Snake Creek Fauna. Bull. Am. Mus. Nat. Hist. 50, 59–210 [Google Scholar]
- 91.Furlong E. L. 1927. The occurrence and phylogenetic status of Merycodus from the Mohave Desert Tertiary. Univ. Calif. Publ. Bull. Dept. Geol. Sci. 17, 145–186 [Google Scholar]
- 92.Goss R. J., Powel R. S. 1985. Induction of deer antlers by transplanted periosteum. I. Graft size and shape. J. Exp. Zool. 235, 359–373 10.1002/jez.1402350307 (doi:10.1002/jez.1402350307) [DOI] [PubMed] [Google Scholar]
- 93.Goss R. J. 1987. Induction of deer antlers by transplanted periosteum. II. Regional competence for velvet transformation in ectopic skin. J. Exp. Zool. 244, 1–11 [Google Scholar]
- 94.Goss R. J. 1991. Induction of deer antlers by transplanted periosteum. III. Orientation. J. Exp. Zool. 259, 2–51 [DOI] [PubMed] [Google Scholar]