Abstract
Purpose of the Review
Infusions of bone marrow derived cells together with “space making” continue to be tested in clinical organ transplant tolerance protocols. These trials are based on the hypothesis that this might produce initial multilineage chimerism. There is some evidence that this in turn induces regulatory cells which control alloimmunity. Although a wealth of knowledge is available from animal models, this review deals with what we know or can speculate about donor bone marrow cells and chimerism in human organ transplantation.
Recent Findings
Calcineurin inhibitors are employed in most of these protocols to blunt the initial immune response. One protocol also has a stepwise regulatory cell generating treatment with sirolimus before total withdrawal. A number of donor chimeric lineages including stem cells, dendritic cells, myeloid precursors and various lymphoid subpopulations cells have been described. Currently, it is recognized that the nature of cells that make up the chimerism could influence graft rejection vs. acceptance. Tolerogenic donor chimeric cells may also generate regulatory subsets thus controlling alloimmunity on two fronts.
Summary
It might be speculated that prolonged and sustained regulation or possible anergy induced by chimerism may eventually lead to clonal deletion, thereby bringing about classical immunologic tolerance.
Keywords: Microchimerism, donor stem cells, immune regulation, graft acceptance, clinical organ transplants
Introduction
Infusions of bone marrow derived cells continue to be tested in clinical protocols intended to induce specific immunologic tolerance of solid organ transplants. This is aside from their more conventional use in conferring engrafted immune and myelopoietic systems into ablated individuals. A wealth of knowledge from experimental animal models has associated chimerism and organ transplant tolerance [1–3]. However, this review deals with what we know or can speculate about donor bone marrow derived cells in human organ transplant recipients, with an emphasis on our own work.
The seminal observations of Billingham, Brent and Medawar [4] in 1953, that H-2 disparate donor bone marrow derived cells infused into fetal or new-born murine recipients could bring about life long specific acquired immunologic tolerance to skin allografts laid the foundation of establishing clinical donor specific tolerance. It was over 20 years later that nonspecific and subsequently donor specific blood transfusions were described to improve human kidney transplant acceptance [5, 6]. The first clinical attempt to use iliac crest donor bone marrow cells (iDBMC) was by Monaco et al. in kidney transplantation [7]. Subsequently, Barber et al. reported initial encouraging results [8], but later observed no significant difference with the control group [9]. However, observations of microchimerism of bone marrow derived cells in several transplant recipients who had stopped immunosuppression (IS) for several years with functioning grafts [10, 11] added impetus to these protocols. In 1994, Fontes et al. [12] reported preliminary clinical results in recipients of several types of organ allografts using vertebral body donor bone marrow cells (vDBMC). Our own clinical studies were performed between 1994 and 2000, in over 350 deceased donor liver (or liver/intestinal), 111 kidney, 25 kidney/pancreas, and 5 kidney/islet transplants accompanied by vDBMC [13–19] as well as 47 living-related-donor (LRD) haploidentical kidney recipients infused with iDBMC [15, 18, 20, 21]. In deceased donor kidney transplant recipients higher graft survival was observed compared to (non-randomized) non-infused controls [17, 22]. Similar observations were also made by others [23–25]. However, in none was immunosuppression withdrawn.
Donor bone marrow cell infusions can bring about a number of immunological effects [21]. These included the infused cells functioning as 1) down-regulators of anti-donor immunity, 2) stimulators that might sensitize, 3) responders that could cause GvHD, and 4) autologous inhibitors of these GvH responses. These theoretical immune effects were studied using non-chimeric marrow from deceased donors in vitro [26–32] suggesting strong inhibitory properties for a number of vDBMC sub-populations that could overcome both responding and stimulatory effects, thereby promoting unresponsiveness [21].
Operational tolerance by Donor Bone Marrow Cell Infusions in Clinical Transplantation
Operational tolerance, i.e. maintenance of the allograft in the absence of immunosuppressive treatment, can be spontaneously achieved in about 20% of liver transplant recipients. The liver contains enormous quantities of passenger leukocytes which generate donor microchimerism in the recipient [33–35]. In contrast, documented occurrences of operational tolerance in kidney transplants are fewer, other than those deliberately induced through donor bone marrow derived cellular infusions involving more potent (ablative or lympho-depleting) induction regimens than in conventional transplants [36–47].
The first deliberate successful clinical attempt was made at Massachusetts General Hospital. HLA-identical (HLA-Id) LRD-kidney transplants were performed accompanied by DBMC infusions, in patients who had received previous chemotherapy for multiple myeloma, the cause of their end-stage renal disease [36, 37]. Thymic x-irradiation (7 gy) was administered, together with (equine) anti-thymocyte globulin induction therapy (ATGAM®; Upjohn, Kalamazoo, MI), and a short course of cyclosporine, which was then totally withdrawn [36]. These studies were then extended to haploidentical renal transplant recipients [38, 39]. The details are reviewed in this issue of the journal, and hence are not further discussed.
Strober and colleagues [40, 41] initiated a protocol in HLA-identical (HLA-Id) kidney transplant recipients (n=12) by conditioning with ten doses of total lymphoid irradiation (TLI) and five infusions of rabbit anti-thymocyte globulin. This was followed by granulocyte-colony stimulating factor (GCSF) mobilized and purified CD34+ Donor Hematopoetic Stem Cells (DHSC) with low numbers of T cells. Criteria for withdrawal of immunosuppression at ≥ six months were stable chimerism and absence of rejection or graft versus host disease (GVHD). Some patients developed rejection (3/12). In 6 others immunosuppression was withdrawn [41]. Nonetheless, there has been subsequent loss of donor chimerism without deleterious effects.
Trivedi et.al initiated somewhat similar clinical procedures evolving over time [42–44]. They first used GM-CSF mobilized but unpurified DHSC in high dose infusions intraportally, systemically and into the thymus. The majority of recipients were either fully or haplomismatched with the donors. More recently, donor specific blood transfusions to stimulate allospecific immunity have been followed by “deletion” of responding cells with cyclophosphamide, ATG and TLI resulting in 16/69 (23%) patients immunosuppression free or on low dose of steroids at 13–23 months post-operatively. Another modification included the use of rituximab and Bortezomib to eliminate B cells and plasma cells respectively [44].
Currently we are conducting clinical trials using 2 different approaches. In the first, HLA-Id LRD renal transplant recipients are given 4 infusions of CD34+ DHSC, the first purified from iliac crest marrow and the others from GCSF mobilized DHSC in peripheral blood. The infusions extend from day +5 to +270 post-operatively, with alemtuzimab induction on days 0 and +4. Maintenance immunosuppression with tacrolimus is converted to sirolimus by day +80. Mycophenolate, also started at surgery, is discontinued between 12 and 18 months and finally sirolimus withdrawn by 24 months [45]. Chimerism has never reached above 3% and became lower than the detection level of 0.01% in both the peripheral blood and the bone marrow usually after 1 year. Five of 7 recipients are >2 years with immunosuppression withdrawn upto 12 months, thus far with normal renal biopsies and function.
In the second, we have explored combined DHSC and kidney transplantation in HLA mismatched living related and unrelated transplant recipients in collaboration with University of Louisville [46, 47]. This was based on observations that a subpopulation of bone marrow derived cells, the CD8+TcR-αβnegative facilitating cells (FC), significantly increased DHSC engraftment without GVHD in a mouse model [48] as well as in HLA mismatched leukemia [49, 50] and sickle-cell disease patients [51]. A subsequent Phase 1 study of FC-enriched DHSC in renal transplant recipients established the safety of the protocol, although durable chimerism was never achieved [51]. The current study involves nonmyeloablative conditioning pre- and peri-transplant (fludarabine, cyclophosphamide, 200cGy TBI), and infusion of FC-enriched DHSC on Day +1 [46, 47]. Maintenance immunosuppression is with tacrolimus and MMF, with planned total elimination by one year. All initial 8 patients entered into this Phase 2 trial have demonstrated macrochimerism post-transplant, ranging from 6 to 100% at 1 month. Chimerism was lost in 2 subjects due to suboptimal cell dosing and more limited conditioning (less cyclophosphamide was used). However, durable full (100% donor) chimerism has developed in the others, along with evidence of donor-specific hyporesponsiveness. Three patients have been successfully weaned from immunosuppression thus far, one for over 10 months. They are immunocompetent responding to mitogen (PHA) and MHC-disparate third party alloantigens; none have developed donor-specific antibodies using flow crossmatch techniques. Most notably, none have developed GVHD, in spite of high levels of chimerism. These encouraging early results suggest that nonmyeloablative conditioning in conjunction with FC-enriched DHSC preparations can safely achieve durable donor macrochimerism in mismatched kidney transplant recipients, allowing for immunosuppression withdrawal.
Role of chimerism
The term chimerism was popularized in transplantation biology by Medawar [52] based on the observations of Owen [53] in freemartin calf dizygotic twins describing a mixture of blood cells due to cross-circulation in the common placenta in utero. This type of chimerism, established during the fetal or newborn stages, has been synonymous with a state of lifelong unresponsiveness to donor alloantigens. All the afore-mentioned clinical trials have in common the intent to generate donor chimerism in the adult, some with the additional hypothesis that even transient microchimerism might be sufficient to induce donor specific immune tolerance. Therefore, it is important to analyze the immune reactions that can occur due to chimerism.
Detection of chimerism
Differences between donor and recipient gene polymorphisms or their products have been used to detect chimerism in a variety of fluorescent and molecular methods. These include HLA polymorphism, gender differences (XX-XY chromosome), variable number of tandem repeat sequences (VNTR) and other cytogenetic markers including ABO blood group antigens. Specific methods include polymerase chain reaction (PCR) [54–56], fluorescent in situ sequence hybridization (FISH) [57], flow cytometry (>0.1%) [58] and a combination of PCR and flow cytometry called PCR-Flow. [14, 59]. However, the most widely used and FDA approved method is PCR amplification of short tandem repeats and single-nucleotide polymorphism-specific quantitative real-time PCR (reviewed in [60]*).
Chimerism: friend or foe
There is controversy about the role and the extent of chimerism needed, especially in humans to be associated with drug-free organ transplant acceptance [61, 62]. Microchimerism mediated by blood transfusions, organ transplantation, or pregnancy has even been associated with allo-sensitization and rejection [55, 56, 63, 64] as well as the development of GVHD in liver and small bowel transplant recipients [65, 66]. Conversely, other studies describe microchimerism as either only an epiphenomenon derived from the vascularized organ or helping to induce allograft acceptance. Although we had reported clinical evidence linking increasing microchimerism in the bone marrow compartment with the absence of graft loss [15, 17, 21], it was with ex vivo experiments that we have clarified a role for chimeric cells in amplifying donor-specific unresponsiveness in renal transplant recipients [21, 67, 68]*. Currently, it is recognized that the nature of cells that make up the chimerism could influence rejection vs. graft acceptance.
Distribution and phenotype of chimeric cells
Passenger leukocytes that migrate from the vascularized transplant in non-immunosuppressed rodent recipients were found to first circulate through the blood stream and rapidly disappear [69, 70] possibly into central and secondary lymphoid organs. In immunosuppressed patients given DBMC infusions, chimeric cell numbers were highest in the peripheral circulation during first 3 months and then gradually decreased until they approached minimal detection levels by 1 year post-transplantation [10, 38] with few exceptions [21, 46, 47, 71]. However, cells of donor origin have been detected long-term in bone marrow, skin and lymph nodes of kidney and liver transplant recipients [10, 11, 72, 73]. These belong to a number of lineages including stem cells, dendritic cells, some myeloid precursors and various lymphoid subpopulations, i.e., T, B and NK cells [14, 15, 18, 21, 74, 75]. When isolated using anti-HLA antibodies to the donor mismatched antigens and with magnetic microbeads, a substantial percentage of the recipient derived donor (RdD) chimeric cells were found to be CD3+, TcR-αβ+ and CD28+ T cells with markedly decreased CD40L, CD80 and CD86 receptors [21, 67]. However, a significant proportion of RdD cells remained undetermined (lineage negative). Thus, it appears that the chimerism generated in DBMC infused recipients is of multiple lineages.
Regulatory functions of chimeric cell of donor origin
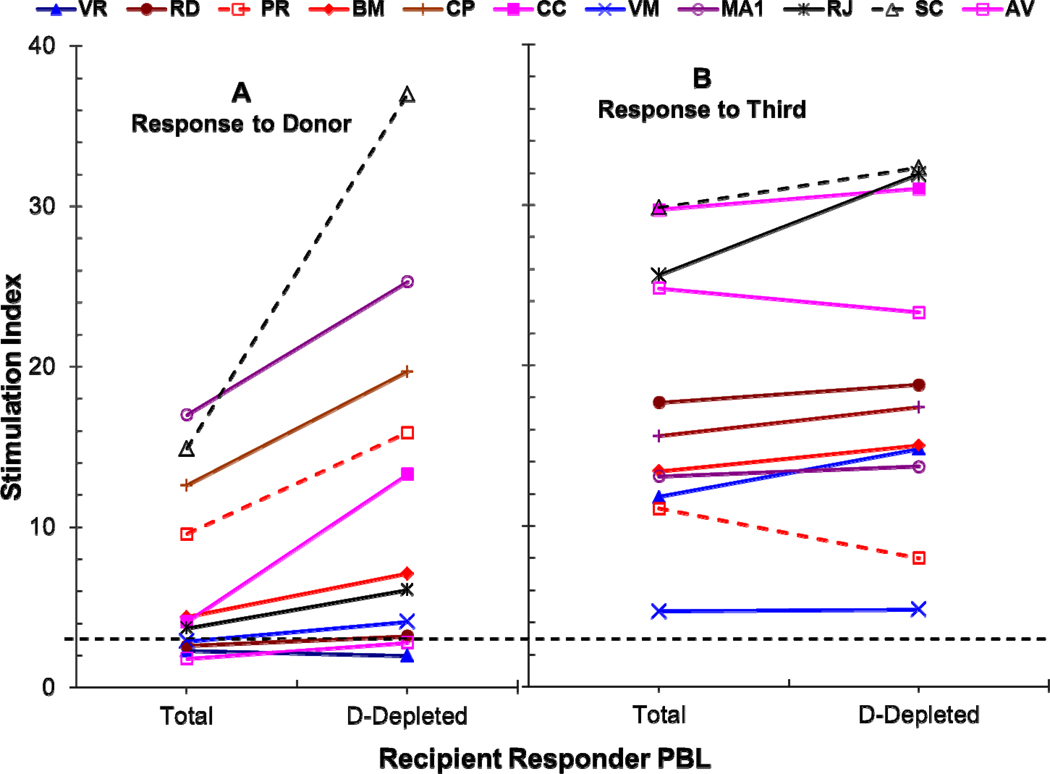
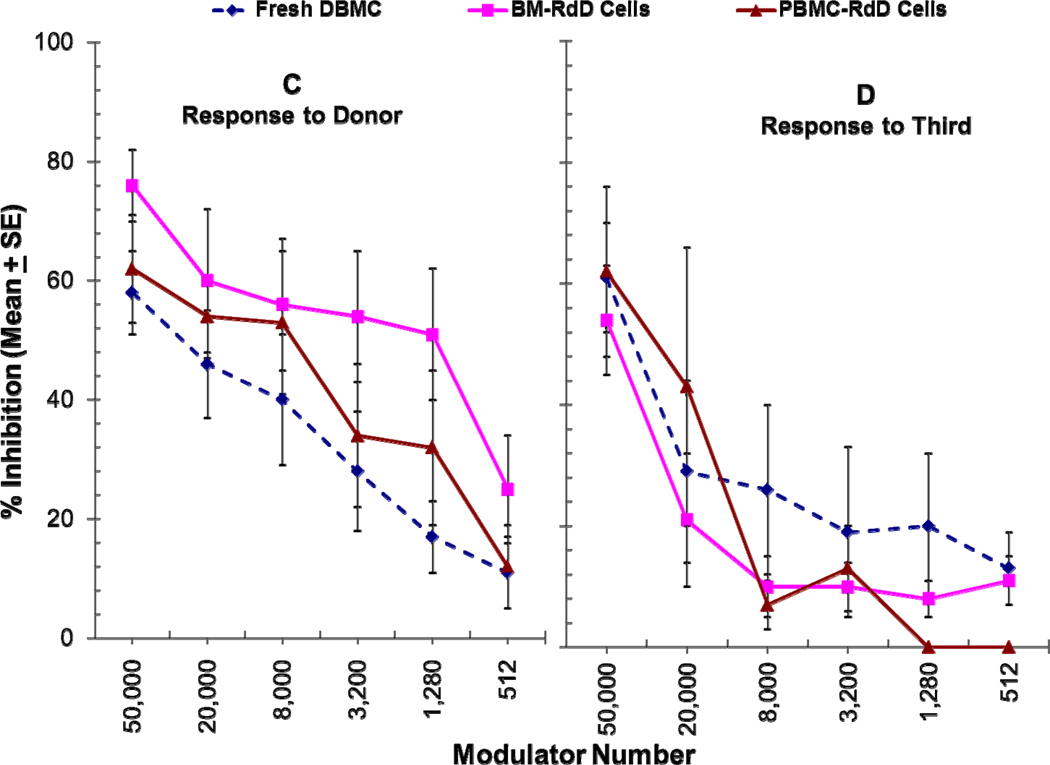
Very few studies directly demonstrated regulatory functions of chimeric cells of donor or even recipient origin post-transplant. In 1995, Burlingham et al. [76] observed that removal of the donor chimeric cells failed to reverse CTL unresponsiveness in a “chimeric” patient functionally tolerant to a maternal kidney allograft without immunosuppression. However, although restimulation of primary cultures with donor cells plus exogenous IL-2 completely reversed unresponsiveness, addition of fresh patient PBMC subsets to tertiary MLR cultures, inhibited the generation of anti-donor CTL. In our own studies, in addition to phenotypically characterizing RdD cells from the iDBMC LRD-renal transplant recipients (see above), we have tested them in functional ex vivo assays. In the recipients with residual anti-donor responses, depletion of cells of donor phenotype allo-specifically increased donor-specific mixed lymphocyte reactions (Figure 1A&B) and addition of these cells back into the culture inhibited them more potently than freshly isolated non-chimeric iDBMC from the non-immunosuppressed LRD volunteers (Figure 1C) [67, 68]*. This inhibition was quasi-antigen-specific, in that at higher doses RdD cells inhibited non-specifically but as the doses decreased non-specific inhibition disappeared while donor-specific inhibition still occurred (Figure 1C vs D) [68]*. Analogously, Demirkiran et.al. observed that up to 5% of CD4+CD25+CTLA4+ T cells in liver transplant recipient blood were derived from the donor liver within the first weeks, and that when purified using monoclonal antibodies specific to the donor, these cells inhibited recipient’s anti-donor MLRs [77].
Figure 1. Role of chimeric cells of donor phenotype present in DBMC recipients at 1 year post-transplantation on MLR regulation.
(A & B): Recipient PMBC were used as responders in MLR either before (Total) or after the depletion of donor cells (D-Depleted) using monoclonal antibodies to mismatched HLA-Class I and Miltenyi magnetic microbeads. Statistically significant differences were obtained in the MLR responses to the donor between Total versus D-depleted recipient responders (p<0.01).
(C & D): 1×105 PMBC from renal transplant recipients depleted of donor chimeric cells were stimulated with 1×105 irradiated PMBC from the living related donors (C) or third Party (D) in presence of the indicated number of donor modulator cells and standard 3H-thymidine incorporation assays were performed on day 7. Data are shown as percentage inhibition ± SE (n=6). Statistically significant differences were obtained in the inhibition of anti-donor MLR between fresh DBMC versus RdD cells from the bone marrow (BM-RdD; p< 0.001) and fresh DBMC versus RdD cells from the peripheral blood (PBL-RdD; p<0.01).
[Previously published in Human Immunology: 2010; 71(6): 566–576]
“Regulation Recruitment”: of cells that develop in the recipient
Microchimerism may have its greatest and long-lasting effect by inducing a regulatory profile within the recipient. Initially in parallel to the immunoregulatory studies with donor chimeric RdD cells described above [67, 68]*, we also tested purified recipient-derived recipient (RdR) “chimeric” cells from the peripheral blood and bone marrow of iDBMC infused LRD-kidney transplant recipients for donor specific regulatory functions [18]. When used as modulators, the RdR cells also inhibited the recipient anti-donor MLR (and CML) responses. In a number studies, depletion of CD25+ cells from recipient responding PBMC increased their donor specific MLR or CTL responses [41, 68]*; but there were exceptions [37, 78]. Conversely, addition of purified CD4+CD25+ cells from the post-transplant PBMC inhibited recipient’s responses in a dose dependent manner [68, 79–81]*.
Recently, we have approached this as an ancillary study to our HLA-identical renal transplant DHSC infusion trial. The percentages of CD4+CD127−CD25highFOXP3+ cells in the PBMC of all patients increased by 10-fold from the pre-operative values during the first 6 months and remained >4-fold even after 24 months. When these post-op recipient PBMC containing these high percentages of putative Tregs were added as third component modulators, they inhibited the donor-specific proliferation of cryopreserved pre-op recipient CFSE-labeled PBMC responders. Noteworthy is the post-op PBMC modulators enhanced the newly generated CD4+CD127−CD25highFOXP3+ cells in the CFSE labeled proliferating responders [45, 82]. We described this generation of additional Tregs ex vivo as “regulation recruitment” [82, 83]. These inhibition and recruitment effects identified donor-specific Tregs operating in HLA-id renal transplant recipients undergoing thus far successful immunosuppression withdrawal.
Conclusions and Synthesis
Infusions of bone marrow derived cells together with “space making” continue to be tested in clinical protocols to induce specific immunologic tolerance in solid organ transplants [36–47]. These trials are based, among other possible mechanisms, on an hypothesis that this might produce initial multilineage chimerism which in turn induces regulatory cells controlling alloimmunity [21]. Conventional immunosuppression with calcineurin inhibitors is employed to blunt the initial immune response amplified by inflammation [36–41, 45–47]. In addition stepwise regulatory cell generating immunosuppression with sirolimus may be beneficial before total withdrawal [45].
A variety of regulatory cell subsets have been described in transplantation and a number of studies including our own have demonstrated regulatory roles played by chimeric cells of both donor and recipient origin [21, 67, 68]*. We introduce the terminology “regulation recruitment” to describe the latter phenomenon. It might be speculated that regulation by donor chimeric cells may also involve the induction of anergy [76] possibly by incomplete antigen presentation in the absence of costimulatory molecules [84], tolerogenic allopeptides [85] or by the transduction of an as yet undefined negative signal, perhaps even involving B cells [86, 87] with a memory and inhibitory phenotype [88]. Prolonged and sustained regulation or anergy may eventually lead to the clonal deletion, thereby bringing about classical immunologic tolerance [89].
These clinical studies have generated more questions than answers. Is “operational tolerance” a ticking time bomb, i.e., a balancing act that can easily be tipped over by an immune stimulus as “mundane” as a viral infection or is it long-lasting? More definition of mechanisms is needed. Are anergy or deletion eventually involved? Does the thymus and central tolerance play a role? Answers are essential before these protocols can be routine in clinical transplantation.
KEY POINTS.
-
○
Infusions of bone marrow derived cells together with “space making” continue to be tested in clinical organ transplant protocols with one hypothesis being that this might produce initial multilineage chimerism which in turn induces regulatory cells controlling alloimmunity.
-
○
Conventional immunosuppression with calcineurin inhibitors is employed to blunt the initial immune response.
-
○
Stepwise treatment with sirolimus may augment regulatory subsets before total withdrawal.
-
○
Chimeric cells of various subsets of both donor and recipient origin have been shown to play regulatory roles
-
○
It might be speculated that prolonged and sustained regulation or possible anergy induced by chimerism may eventually lead to clonal deletion, thereby bringing about classical immunologic tolerance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH Grant 2R01DK25243-25A2 and VA Merit review funding 5723.07 (J.M.)
REFERENCES
- 1.Slavin S, Fuks Z, Strober S, Kaplan H, Howard RJ, Sutherland DE. Transplantation tolerance across major histocompatibility barriers after total lymphoid irradiation. Transplantation. 1979 Nov;28(5):359–361. doi: 10.1097/00007890-197911000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Monaco AP, Wood ML. Studies on heterologous antilymphocyte serum in mice. VII. Optimal cellular antigen for induction of immunologic tolerance with antilymphocyte serum. Transplant Proc. 1970 Dec;2(4):489–496. [PubMed] [Google Scholar]
- 3.Caridis DT, Liegeois A, Barrett I, Monaco AP. Enhanced survival of canine renal allografts of ALS- treated dogs given bone marrow. Transplant Proc. 1973 Mar;5(1):671–674. [PubMed] [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells.[Reprint in J Immunol. 2010 Jan 1;184(1):5–8; PMID: 20028658] Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978 Oct 12;299(15):799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 6.Salvatierra O, Jr, Vincenti F, Amend W, Potter D, Iwaki Y, Opelz G, et al. Deliberate donor-specific blood transfusions prior to living related renal transplantation. A new approach. Ann Surg. 1980;192(4):543–552. doi: 10.1097/00000658-198010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monaco AP, Clark AW, Wood ML, Sahyoun AI, Codish SD, Brown RW. Possible active enhancement of a human cadaver renal allograft with antilymphocyte serum (ALS) and donor bone marrow: case report of an initial attempt. Surgery. 1976 Apr;79(4):384–392. [PubMed] [Google Scholar]
- 8.Barber WH, Mankin JA, Laskow DA, Deierhoi MH, Julian BA, Curtis JJ, et al. Long-term results of a controlled prospective study with transfusion of donor-specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991 Jan;51(1):70–75. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 9.McDaniel DO, Naftilan J, Hulvey K, Shaneyfelt S, Lemons JA, Lagoo-Deenadayalan S, et al. Peripheral blood chimerism in renal allograft recipients transfused with donor bone marrow. Transplantation. 1994 Mar 27;57(6):852–856. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993 Jun;17(6):1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Demetris AJ, Trucco M, Zeevi A, Ramos H, Terasaki P, et al. Chimerism and donor-specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993 Jun;55(6):1272–1277. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontes P, Rao AS, Demetris AJ, Zeevi A, Trucco M, Carroll P, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994 Jul 16;344(8916):151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricordi C, Karatzas T, Selvaggi G, Nery J, Webb M, Fernandez H, et al. Multiple bone marrow infusions to enhance acceptance of allografts from the same donor. Ann N Y Acad Sci. 1995 Dec 29;770:345–350. doi: 10.1111/j.1749-6632.1995.tb31066.x. [Review]. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Morales R, Carreno M, Mathew J, Zucker K, Cirocco R, Ciancio G, et al. The effects of chimeric cells following donor bone marrow infusions as detected by PCR-flow assays in kidney transplant recipients. J Clin Invest. 1997 Mar 1;99(5):1118–1129. doi: 10.1172/JCI119240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Morales R, Carreno M, Mathew J, Cirocco R, Zucker K, Ciancio G, et al. Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients. Transplantation. 1998 Apr 15;65(7):956–965. doi: 10.1097/00007890-199804150-00016. [DOI] [PubMed] [Google Scholar]
- 16.Miller J, Mathew J, Garcia-Morales R, Zucker KE, Carreno M, Jin Y, et al. The human bone marrow as an immunoregulatory organ. Transplantation. 1999 Oct 27;68(8):1079–1090. doi: 10.1097/00007890-199910270-00001. [Review]. [DOI] [PubMed] [Google Scholar]
- 17.Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke GW, 3rd, Roth D, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation. 2001 Apr 15;71(7):827–835. doi: 10.1097/00007890-200104150-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ciancio G, Burke GW, Garcia-Morales R, Suzart K, Rosen A, Ricordi C, et al. Effect of living-related donor bone marrow infusion on chimerism and in vitro immunoregulatory activity in kidney transplant recipients. Transplantation. 2002;74(4):488–496. doi: 10.1097/00007890-200208270-00010. [DOI] [PubMed] [Google Scholar]
- 19.Chatzipetrou MA, Mathew JM, Kenyon NS, Esquenazi V, Miller J, Ricordi C, et al. Analysis of post-transplant immune status in recipients of liver/bone marrow allografts. Hum Immunol. 1999;60(12):1281–1288. doi: 10.1016/s0198-8859(99)00115-9. [DOI] [PubMed] [Google Scholar]
- 20.Ciancio G, Burke GW, Moon J, Garcia-Morales R, Rosen A, Esquenazi V, et al. Donor bone marrow infusion in deceased and living donor renal transplantation. Yonsei Medical Journal. 2004;45(6):998–1003. doi: 10.3349/ymj.2004.45.6.998. [DOI] [PubMed] [Google Scholar]
- 21.Mathew JM, Garcia-Morales RO, Carreno M, Jin Y, Fuller L, Blomberg B, et al. Immune responses and their regulation by donor bone marrow cells in clinical organ transplantation. Transpl Immunol. 2003;11(3–4):307–321. doi: 10.1016/S0966-3274(03)00056-X. [DOI] [PubMed] [Google Scholar]
- 22.Cirocco RE, Carreno MR, Mathew JM, Garcia-Morales RO, Fuller L, Esquenazi V, et al. FoxP3 mRNA transcripts and regulatory cells in renal transplant recipients 10 years after donor marrow infusion. Transplantation. 2007;83(12):1611–1619. doi: 10.1097/01.tp.0000266908.37446.02. [DOI] [PubMed] [Google Scholar]
- 23.Gammie JS, Colson YL, Griffith BP, Pham SM. Chimerism and thoracic organ transplantation. Semin Thorac Cardiovasc Surg. 1996 Apr;8(2):149–155. [Review]. [PubMed] [Google Scholar]
- 24.Gammie JS, Pham SM. Simultaneous donor bone marrow and cardiac transplantation: can tolerance be induced with the development of chimerism? Curr Opin Cardiol. 1999;14(2):126–132. doi: 10.1097/00001573-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Salgar SK, Shapiro R, Dodson F, Corry R, McCurry K, Zeevi A, et al. Infusion of donor leukocytes to induce tolerance in organ allograft recipients. J Leukoc Biol. 1999;66(2):310–314. doi: 10.1002/jlb.66.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew JM, Carreno M, Fuller L, Ricordi C, Tzakis A, Esquenazi V, et al. Modulatory effects of human donor bone marrow cells on allogeneic cellular immune responses. Transplantation. 1997;63(5):686–692. doi: 10.1097/00007890-199703150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Mathew JM, Carreno M, Fuller L, Ricordi C, Kenyon N, Tzakis AG, et al. In vitro immunogenicity of cadaver donor bone marrow cells used for the induction of allograft acceptance in clinical transplantation. Transplantation. 1999 Oct 27;68(8):1172–1180. doi: 10.1097/00007890-199910270-00018. [DOI] [PubMed] [Google Scholar]
- 28.Mathew JM, Carreno M, Zucker K, Fuller L, Kenyon N, Esquenazi V, et al. Cellular immune responses of human cadaver donor bone marrow cells and their susceptibility to commonly used immunosuppressive drugs in transplantation. Transplantation. 1998 Apr 15;65(7):947–955. doi: 10.1097/00007890-199804150-00015. [DOI] [PubMed] [Google Scholar]
- 29.Mathew JM, Fuller L, Carreno M, Garcia-Morales R, Burke GW, 3rd, Ricordi C, et al. Involvement of multiple subpopulations of human bone marrow cells in the regulation of allogeneic cellular immune responses. Transplantation. 2000 Dec 27;70(12):1752–1760. doi: 10.1097/00007890-200012270-00015. [DOI] [PubMed] [Google Scholar]
- 30.Mathew JM, Carreno M, Fuller L, Burke GW, 3rd, Ciancio G, Ricordi C, et al. Regulation of alloimmune responses (GvH reactions) in vitro by autologous donor bone marrow cell preparation used in clinical organ transplantation. Transplantation. 2002 Sep 27;74(6):846–855. doi: 10.1097/00007890-200209270-00019. [DOI] [PubMed] [Google Scholar]
- 31.Lagoo-Deenadayalan S, Lagoo AS, Lemons JA, Lorenz HM, Bass JD, McDaniel DO, et al. Donor specific bone marrow cells suppress lymphocyte reactivity to donor antigens and differentially modulate TH1 and TH2 cytokine gene expression in the responder cell population. Transpl Immunol. 1995 Jun;3(2):124–134. doi: 10.1016/0966-3274(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 32.Rachamim N, Gan J, Segall H, Krauthgamer R, Marcus H, Berrebi A, et al. Tolerance induction by "megadose" hematopoietic transplants: donor-type human CD34 stem cells induce potent specific reduction of host anti-donor cytotoxic T lymphocyte precursors in mixed lymphocyte culture. Transplantation. 1998 May 27;65(10):1386–1393. doi: 10.1097/00007890-199805270-00017. [DOI] [PubMed] [Google Scholar]
- 33.Schlitt HJ, Kanehiro H, Raddatz G, Steinhoff G, Richter N, Nashan B, et al. Persistence of donor lymphocytes in liver allograft recipients. Transplantation. 1993 Oct;56(4):1001–1007. doi: 10.1097/00007890-199310000-00042. [DOI] [PubMed] [Google Scholar]
- 34.Ayala R, Grande S, Albizua E, Crooke A, Meneu JC, Moreno A, et al. Long-term follow-up of donor chimerism and tolerance after human liver transplantation. Liver Transplantation. 2009 Jun;15(6):581–591. doi: 10.1002/lt.21736. [DOI] [PubMed] [Google Scholar]
- 35.Jonsson JR, Hogan PG, Thomas R, Steadman C, Clouston AD, Balderson GA, et al. Peripheral blood chimerism following human liver transplantation. Hepatology. 1997 May;25(5):1233–1236. doi: 10.1002/hep.510250528. [DOI] [PubMed] [Google Scholar]
- 36.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68(4):480–484. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 37.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006 Sep;6(9):2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 38.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008 Jan 24;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LoCascio SA, Morokata T, Chittenden M, Preffer FI, Dombkowski DM, Andreola G, et al. Mixed chimerism, lymphocyte recovery, and evidence for early donor-specific unresponsiveness in patients receiving combined kidney and bone marrow transplantation to induce tolerance. Transplantation. 2010 Dec 27;90(12):1607–1615. doi: 10.1097/TP.0b013e3181ffbaff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, et al. Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation. 2002;73(9):1386–1391. doi: 10.1097/00007890-200205150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Strober S, Busque S, Dejbakhsh-Jones S, Benike C, Li L, Shizuru J, et al. Inducing Tolerance in Clinical Kidney Transplantation. Transplantation. 2010 July 27;90(2S):323. [Abstract]. [Google Scholar]
- 42.Trivedi H, Shah V, Shah P, Darji P, Sane A, Vanikar A, et al. High dose DBMC associated tolerance in live-related renal allograft recipients. Transplant Proc. 2000 Nov;32(7):2001–2002. doi: 10.1016/s0041-1345(00)01531-1. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi H, Vanikar A, Shah V, Mehta A, Shah S, Shah T, et al. Mega dose unfractionated donor bone marrow-derived cell infusion in thymus and periphery-an integrated clinical approach for tolerance in living related renal allografts. Transplant Proc. 2003;35(1):203–206. doi: 10.1016/s0041-1345(02)03901-5. [DOI] [PubMed] [Google Scholar]
- 44.Trivedi HL, Terasaki PI, Feroz A, Vanikar AV, Trivedi VB, Khemchandani SI, et al. Clonal Deletion With Bortezomib Followed by Low or No Maintenance Immunosuppression in Renal Allograft Recipients. Transplantation. 2010:221–222. doi: 10.1097/TP.0b013e3181dde912. [DOI] [PubMed] [Google Scholar]
- 45.Miller J, Leventhal J, Gallon L, Friedewald J, Levitsky J, Tambur A, et al. Donor Specific Immunoregulation in HLA-Identical Renal Transplant Recipients given Alemtuzimab and Donor Stem Cells. Transplantation. 2010;90:234. [Google Scholar]
- 46.Leventhal J, Gallon L, Miller J, Abecassis M, Ravindra K, Reed E, et al. Induction of Donor Specific Tolerance in Recipients of HLA Disparate Living Donor Kidney Allografts By Donor Stem Cell Infusion. Transplantation. 2010;90:465. [Abstract 2660]. [Google Scholar]
- 47.Leventhal JR, Gallon L, Miller J, Abecassis M, Ravindra K, Reed E, et al. Facilitating Cell Enriched Stem Cell Infusion Results in Durable Chimerism, Donor Specific Tolerance, and Allows For Immunosuppressive Drug Withdrawal in Recipients of HLA Disparate Living Donor Kidney Allografts: 3375. Transplantation. 2010;90:183. Supplement 23rd International Meeting (Abstracts) [Google Scholar]
- 48.Kaufman C, Colson Y, Wren S, Watkins S, Simmons R, Ildstad S. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994 October 15;84(8):2436–2446. 1994. [PubMed] [Google Scholar]
- 49.Ildstad ST, Crilley PA, Kaufman CL, et al. Graft engineering (II): A multivariate analysis of the facilitating cell protocol in advanced hematologic malignancy for mismatched donors. ASH Abstract. 1999 [Google Scholar]
- 50.Ildstad ST, Crilley PA, Kaufman CL, et al. A graft engineering method to allow bone marrow transplantation (BMT) for more hightly mismatched recipients with leukemia: Multivariate analysis of a Phase I/II protocol. [AST/ASTS Abstract] 2000 [Google Scholar]
- 51.Ravindra K, Herzig R, Paris K, Buell J, Ildstad S. Donor marrow infusion in kidney transplantation: Results of preliminary study. Am J Transplant. 2008;8(s2):214. [Abstract]. [Google Scholar]
- 52.Anderson D, Billingham RE, Lampkin GH, Medawar PB. The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity. 1952;6:201–221. [Google Scholar]
- 53.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;(102):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 54.Drexler C, Wagner T. Blood group chimerism. Curr Opin Hematol. 2006 Nov;13(6):484–489. doi: 10.1097/01.moh.0000245690.54956.f3. [Review]. [DOI] [PubMed] [Google Scholar]
- 55.Sivasai KS, Alevy YG, Duffy BF, Brennan DC, Singer GG, Shenoy S, et al. Peripheral blood microchimerism in human liver and renal transplant recipients: rejection despite donor-specific chimerism [erratum appears in Transplantation 1997 Dec 15;64(11):1636] Transplantation. 1997 Aug 15;64(3):427–432. doi: 10.1097/00007890-199708150-00010. [DOI] [PubMed] [Google Scholar]
- 56.Schlitt HJ, Hundrieser J, Ringe B, Pichlmayr R. Donor-type microchimerism associated with graft rejection eight years after liver transplantation. N Engl J Med. 1994 Mar 3;330(9):646–647. doi: 10.1056/NEJM199403033300919. [Letter]. [DOI] [PubMed] [Google Scholar]
- 57.van Tol MJ, Langlois van den Bergh R, Mesker W, Ouwerkerk-van Velzen MC, Vossen JM, Tanke HJ. Simultaneous detection of X and Y chromosomes by two-colour fluorescence in situ hybridization in combination with immunophenotyping of single cells to document chimaerism after sex-mismatched bone marrow transplantation. Bone Marrow Transplant. 1998 Mar;21(5):497–503. doi: 10.1038/sj.bmt.1701122. [DOI] [PubMed] [Google Scholar]
- 58.Pei R, Chen T, Orpilla J, Lee JH. A simultaneous negative and positive selection method that can detect chimerism at a frequency of 1 per 10,000 by flow cytometry. Tissue Antigens. 1997 Aug;50(2):197–201. doi: 10.1111/j.1399-0039.1997.tb02859.x. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Morales R, Esquenazi V, Zucker K, Gomez CI, Fuller L, Carreno M, et al. An assessment of the effects of cadaver donor bone marrow on kidney allograft recipient blood cell chimerism by a novel technique combining PCR and flow cytometry. Transplantation. 1996 Oct 27;62(8):1149–1160. doi: 10.1097/00007890-199610270-00021. [DOI] [PubMed] [Google Scholar]
- 60. Gineikiene E, Stoskus M, Griskevicius L. Recent advances in quantitative chimerism analysis. Expert Review of Molecular Diagnostics. 2009 Nov;9(8):817–832. doi: 10.1586/erm.09.66. [Review].. This paper reviews the recent advances and technology-specific imperfections of the two most widely used chimerism quantitation techniques, PCR amplification of short tandem repeats and single-nucleotide polymorphism-specific quantitative real-time PCR.
- 61.Sahota A, Gao S, Hayes J, Jindal RM. Microchimerism and rejection: a meta-analysis. Clinical Transplantation. 2000 Aug;14(4 Pt 1):345–350. doi: 10.1034/j.1399-0012.2000.140411.x. [DOI] [PubMed] [Google Scholar]
- 62.Saraji A, Pourmand G, Mehrsai A, Taherimahmodi M, Nikoobakht M, Asadpour A, et al. Microchimerism and renal transplantation: doubt still persists. Transplant Proc. 2007 May;39(4):948–950. doi: 10.1016/j.transproceed.2007.03.082. [DOI] [PubMed] [Google Scholar]
- 63.Elwood ET, Larsen CP, Maurer DH, Routenberg KL, Neylan JF, Whelchel JD, et al. Microchimerism and rejection in clinical transplantation. Lancet. 1997 May 10;349(9062):1358–1360. doi: 10.1016/s0140-6736(96)09105-2. [DOI] [PubMed] [Google Scholar]
- 64.SivaSai KS, Jendrisak M, Duffy BF, Phelan D, Ravenscraft M, Howard T, et al. Chimerism in peripheral blood of sensitized patients waiting for renal transplantation: clinical implications. Transplantation. 2000 Feb 27;69(4):538–544. doi: 10.1097/00007890-200002270-00013. [DOI] [PubMed] [Google Scholar]
- 65.Pollack MS, Speeg KV, Callander NS, Freytes CO, Espinoza AA, Esterl RM, et al. Severe, late-onset graft-versus-host disease in a liver transplant recipient documented by chimerism analysis. Hum Immunol. 2005 Jan;66(1):28–31. doi: 10.1016/j.humimm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Ruiz P. Solid organ transplant-associated acute graft-versus-host disease. Arch Pathol Lab Med. 2010 Aug;134(8):1220–1224. doi: 10.5858/2008-0679-RS.1. [Review]. [DOI] [PubMed] [Google Scholar]
- 67.Mathew JM, Garcia-Morales R, Fuller L, Rosen A, Ciancio G, Burke GW, et al. Donor bone marrow-derived chimeric cells present in renal transplant recipients infused with donor marrow I. Potent regulators of recipient antidonor immune responses. Transplantation. 2000;70(12):1675–1682. doi: 10.1097/00007890-200012270-00003. [DOI] [PubMed] [Google Scholar]
- 68. Mathew JM, Ciancio G, Burke GW, Garcia-Morales RO, Rosen A, Wang E, et al. Immune "tolerance profiles" in donor bone marrow infused kidney transplant patients using multiple ex vivo functional assays. Hum Immunol. 2010;71(6):566–576. doi: 10.1016/j.humimm.2010.02.008.. This paper suggests that patients who have achieved donor-specific unresponsiveness in a cluster-analysis combining multiple immune assays are possible candidates for immunosuppression minimization and that such a condition appears to have occurred in many immunosuppressed donor bone marrow cell infused renal transplant recipients.
- 69.Austyn JM, Larsen CP. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 1990 Jan;49(1):1–7. doi: 10.1097/00007890-199001000-00001. [Review]. [DOI] [PubMed] [Google Scholar]
- 70.Larsen CP, Austyn JM, Morris PJ. The role of graft-derived dendritic leukocytes in the rejection of vascularized organ allografts. Recent findings on the migration and function of dendritic leukocytes after transplantation. Ann Surg. 1990 Sep;212(3):308–315. doi: 10.1097/00000658-199009000-00009. [Review]. discussion 316-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ringden O, Soderdahl G, Mattsson J, Uzunel M, Remberger M, Hentschke P, et al. Transplantation of autologous and allogeneic bone marrow with liver from a cadaveric donor for primary liver cancer. Transplantation. 2000;69(10):2043–2048. doi: 10.1097/00007890-200005270-00012. [DOI] [PubMed] [Google Scholar]
- 72.Nierhoff D, Horvath HC, Mytilineos J, Golling M, Bud O, Klar E, et al. Microchimerism in bone marrow-derived CD34(+) cells of patients after liver transplantation. Blood. 2002 Jul 15;96(2):763–767. [PubMed] [Google Scholar]
- 73.Starzl TE. Chimerism and tolerance in transplantation. Proc Natl Acad Sci U S A. 2004 Oct 5;101 Suppl 2:14607–14614. doi: 10.1073/pnas.0404829101. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rugeles MT, Aitouche A, Zeevi A, Fung JJ, Watkins SC, Starzl TE, et al. Evidence for the presence of multilineage chimerism and progenitors of donor dendritic cells in the peripheral blood of bone marrow-augmented organ transplant recipients. Transplantation. 1997 Sep 15;64(5):735–741. doi: 10.1097/00007890-199709150-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moroso V, Metselaar HJ, Mancham S, Tilanus HW, Eissens D, van der Meer A, et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transplantation. 2010 Jul;16(7):895–908. doi: 10.1002/lt.22080. [DOI] [PubMed] [Google Scholar]
- 76.Burlingham WJ, Grailer AP, Fechner JH, Jr, Kusaka S, Trucco M, Kocova M, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995 Apr 27;59(8):1147–1155. [PubMed] [Google Scholar]
- 77.Demirkiran A, Bosma BM, Kok A, Baan CC, Metselaar HJ, Ijzermans JNM, et al. Allosuppressive donor CD4+CD25+ regulatory T cells detach from the graft and circulate in recipients after liver transplantation. J Immunol. 2007 May 15;178(10):6066–6072. doi: 10.4049/jimmunol.178.10.6066. [DOI] [PubMed] [Google Scholar]
- 78.Game DS, Hernandez-Fuentes MP, Chaudhry AN, Lechler RI. CD4+CD25+ regulatory T cells do not significantly contribute to direct pathway hyporesponsiveness in stable renal transplant patients. Journal of the American Society of Nephrology. 2003 Jun;14(6):1652–1661. doi: 10.1097/01.asn.0000067411.03024.a9. [DOI] [PubMed] [Google Scholar]
- 79.Kreijveld E, Koenen HJPM, Klasen IS, Hilbrands LB, Joosten I. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant. 2007 Jan;7(1):249–255. doi: 10.1111/j.1600-6143.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 80.Sewgobind VDKD, van der Laan LJW, Klepper M, Ijzermans JNM, Tilanus HW, Weimar W, et al. Functional analysis of CD4+ CD25bright T cells in kidney transplant patients: improving suppression of donor-directed responses after transplantation. Clinical Transplantation. 2008 Sep–Oct;22(5):579–586. doi: 10.1111/j.1399-0012.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 81.Baan CC, Velthuis JHL, van Gurp EAFJ, Mol WM, Klepper M, Ijzermans JNM, et al. Functional CD25(bright+) alloresponsive T cells in fully immunosuppressed renal allograft recipients. Clinical Transplantation. 2007 Jan–Feb;21(1):63–71. doi: 10.1111/j.1399-0012.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 82.Levitsky J, Miller J, Leventhal J, Huang X, Flaa C, Wang E, et al. The human "Treg MLR": immune monitoring for FOXP3+ T regulatory cell generation. Transplantation. 2009 Dec 15;88(11):1303–1311. doi: 10.1097/TP.0b013e3181bbee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levitsky J, Gallon L, Miller J, Tambur AR, Leventhal J, Flaa C, et al. Allospecific Regulatory Effects of Sirolimus and Tacrolimus in the Human Mixed Lymphocyte Reaction. Transplantation. 2011;91(2):199–206. doi: 10.1097/TP.0b013e318200e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant. 2006 May;6(5 Pt 1):844–893. doi: 10.1111/j.1600-6143.2006.01260.x. [Review]. [DOI] [PubMed] [Google Scholar]
- 85.Chauhan B, Mathew JM, Shenoy S, Flye MW, Howard T, Mohanakumar T. Donor human leukocyte antigens in the circulation of liver allograft recipients. Clinical Transplantation. 1995;9(1):14–19. [PubMed] [Google Scholar]
- 86.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010 Jun 1;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010 Jun 1;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pallier A, Hillion S, Danger R, Giral M, Racape M, Degauque N, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010 Sep;78(5):503–513. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 89.Mathew JM, Miller J. Immunoregulatory role of chimerism in clinical organ transplantation. Bone Marrow Transplant. 2001;28(2):115–119. doi: 10.1038/sj.bmt.1703110. [DOI] [PubMed] [Google Scholar]