Abstract
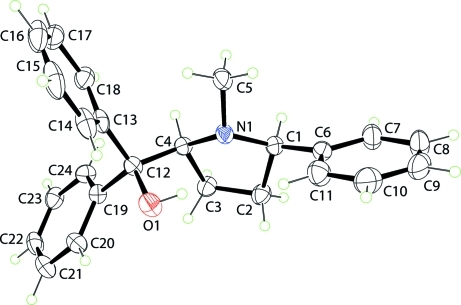
In the title compound, C24H25NO, the phenyl and diphenylmethanol substituents are syn to each other. The pyrrolidine ring has an envelope conformation with the flap atom being the C atom bearing the phenyl substituent. The hydroxy group forms an intramolecular hydrogen bond with the pyrrolidine N atom, and the phenyl rings lie to same side of the molecule. The crystal packing features C—H⋯π interactions. Two slightly displaced co-planar orientations were found for one of the phenyl rings; the major component had a site-occupancy factor of 0.782 (15).
Related literature
For background to the highly enantioselective addition of arylzinc reagents to aldehydes, see: Yoon & Jacobsen (2003 ▶), Taylor, et al. (2011 ▶). For related structures, see: Moro et al. (2010 ▶); Shabbir et al. (2009 ▶). For details of the synthetic protocols, see: Walsh & Kozlowski (2008 ▶); Paixão, et al. (2008 ▶). For ring conformational analysis, see: Cremer & Pople (1975 ▶).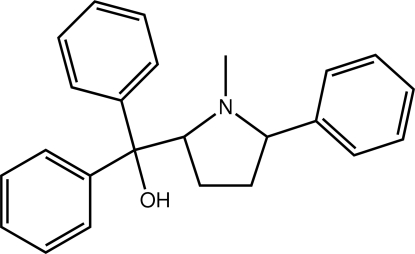
Experimental
Crystal data
C24H25NO
M r = 343.45
Orthorhombic,
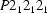
a = 9.9672 (2) Å
b = 13.3376 (2) Å
c = 14.4369 (2) Å
V = 1919.22 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.07 mm−1
T = 100 K
0.22 × 0.15 × 0.15 mm
Data collection
Bruker APEXII CCD diffractometer
24972 measured reflections
2262 independent reflections
1979 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.092
S = 1.05
2262 reflections
255 parameters
H-atom parameters constrained
Δρmax = 0.17 e Å−3
Δρmin = −0.19 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: MarvinSketch (Chemaxon, 2010 ▶) and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811023403/hg5054sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023403/hg5054Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg is the centroid of the C6–C11 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O—H1O⋯N | 0.84 | 2.02 | 2.648 (2) | 132 |
| C28—H28⋯Oi | 0.95 | 2.78 | 3.359 (7) | 120 |
| C17—H17⋯Cgii | 0.95 | 2.92 | 3.776 (3) | 150 |
Symmetry codes: (i) 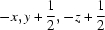 ; (ii)
; (ii) 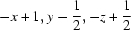 .
.
Acknowledgments
We thank the Brazilian agencies FAPESP, CNPq (research fellowships to JZS, DSL and CRDC) and CAPES (808/2009 to JZS) for financial support.
supplementary crystallographic information
Comment
Chiral β-amino alcohols have found numerous applications in asymmetric catalysis in the past and continue to play a pivotal role in the development of new reactions and chiral ligands (Walsh & Kozlowski, 2008). One asymmetric reaction where chiral β-amino alcohol ligands have found enormous success is the enantioselective addition of organozinc reagents to carbonyl compounds, with particular emphasis in the alkylation of aldehydes by the addition of diethylzinc. A more challenging reaction is the asymmetric arylation reaction, since arylzinc reagents are more reactive than the dialkylzinc and the ligand turnover has to highly efficient in order to circumvent the uncatalyzed background reaction (Paixão et al., 2008). Considering the proline motif as a privileged framework for the development of asymmetric catalysts (Yoon & Jacobsen, 2003) we have recently described a new chiral ligand for the highly enantioselective addition of arylzinc reagents to aldehydes. The ligands were prepared by an straightforward synthetic sequence, with a Heck reaction of arenediazonium salts (Heck–Matsuda reaction) as the key step (Taylor et al., 2011). Herein, we describe the crystal structure analysis of a representative molecule, the title compound, (I).
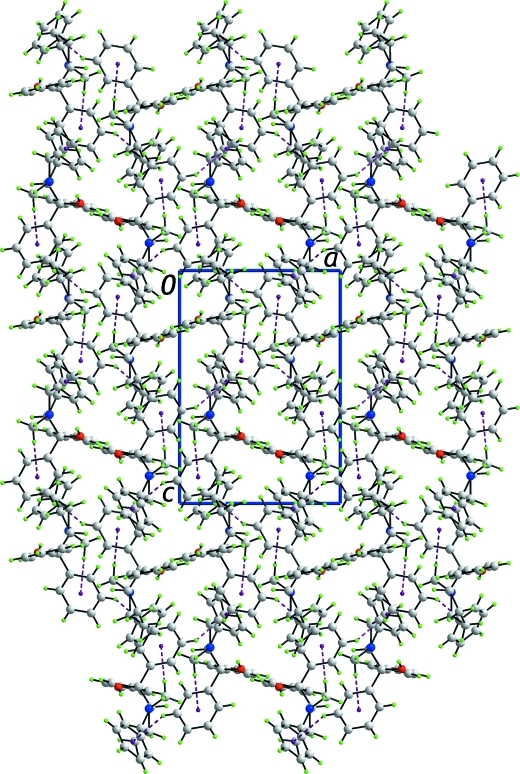
The crystal structure analysis of (I) confirms the structure as having the expected syn relationship between the phenyl and the diphenylmethanol substituents, Fig. 1 (Moro et al., 2010; Shabbir et al., 2009). The pyrrolidine ring is in an envelope conformation with C1 out of the plane formed by the other four atoms, the ring puckering parameters being: q2 = 0.379 (2) ° and φ2 = 32.0 (3) ° (Cremer & Pople, 1975). The hydroxy group is orientated over the five-membered ring to facilitate the formation of an intramolecular O—H···N hydrogen bond, Table 1. The crystal packing is dominated by C—H···π interactions, Table 1. Globally. the pyrrolidine pack in the ab plane and are sandwiched by benzene rings along the c direction, Fig. 2.
Experimental
The starting (2S)-1-tert-butyl 2-methyl 5-argio-1H-pyrrole-1,2(2H,5H)-dicarboxylate was prepared as described in previous work (Moro et al., 2010). To a round-bottomed flask, under a hydrogen atmosphere, were added the Heck adduct ((2S))-1-tert-butyl 2-methyl 5-argio-1H-pyrrole-1,2(2H,5H)-dicarboxylate) (3 mmol) and dry methanol (60 ml), followed by the addition of Pd/C 10% (20% w/w, 0.18 g). The reaction was stirred at room temperature for 24 h. After this time, the crude reaction mixture was filtered in a plug of celite and concentrated under reduced pressure. The resulting product was used without further purification. To a round-bottomed flask, under an argon atmosphere, PhMgBr (5 equiv., 15 mmol) in THF (15 ml, 1 M solution) was added to a THF (10 ml) solution of the (2S)-1-tert-butyl 2-methyl 5-argiopyrrolidine-1,2-dicarboxylate (3 mmol) at room temperature, and the mixture was stirred for 4 h, before being quenched by careful addition of NaOH 2M. The heterogeneous mixture was filtered through a pad of Celite and washed with dichloromethane (3 x 50 ml). The combined organic phases were dried with MgSO4, filtered and the solvent removed under vacuum. The resulting product was used without further purification. To a suspension of lithium aluminium hydride (1.14 g, 30 mmol) in THF (15 ml) in a round-bottomed flask, under an argon atmosphere and cooled to 273 K, a solution of the (2S,5R)-tert-butyl 2-(hydroxydiphenylmethyl)-5-phenylpyrrolidine-1-carboxylate in THF (5 ml) was added. The resulting mixture was refluxed for 12 h. After this time, the mixture was cooled to 273 K and NaOH (4M) was added. The mixture was filtered through a pad of Celite and washed with ethyl acetate. The organic layer was separated, and the filtrate was extracted with ethyl acetate (3 x 50 ml). The combined organic phases were dried with MgSO4, filtered and the solvent removed under vacuum. The crude product was purified by flash chromatography in hexanes/ethyl acetate (95:05), to afford the 0.340 g (33%) of pure ((2S,5R)-1-methyl-5-phenylpyrrolidin-2-yl)diphenylmethanol (cis isomer) and 0.278 g (27%) of pure ((2S,5S)-1-methyl-5-phenylpyrrolidin-2-yl)diphenylmethanol (trans isomer) (60% combined yield). Suitable crystals for X-ray analysis were obtained by vapour diffusion from n-hexane/ethyl ether at 298 K. [α] D20 = +115 (c = 1.02, CHCl3). 1H NMR [CDCl3, 500 MHz, δ (p.p.m.)]: 1.66 (s, 3H, N—CH3), 1.68–1.83 (m, 2H, CH2), 1.92–2.03 (m, 2H, CH2), 3.54 (dd, J1 = 10.8 Hz, J2 = 6.0 Hz, 1H, CH), 3.89 (dd, J1 = 9.8 Hz, J2 = 4.0 Hz, 1H, CH), 4.97 (bs, 1H, OH), 7.09 (t, J = 7.0 Hz, 1H, Ar), 7.13 (t, J = 7.0 Hz, 1H, Ar), 7.19–7.33 (m, 9H, Ar), 7.58 (dd, J1 = 8.5 Hz, J2 = 1.0 Hz, 2H, Ar), 7.68 (dd, J1 = 8.5 Hz, J2 = 1.0 Hz, 2H, Ar). 13C NMR [CDCl3, 125 MHz, δ (p.p.m.)]: 28.2, 34.5, 41.0, 72.5, 73.4, 77.8, 125.3, 125.4, 126.1, 126.2, 126.9, 127.2, 128.0, 128.1, 128.4, 142.6, 146.6, 148.0. IR (film, cm-1): 3428, 3263, 1449. MS (ESI): 209, 167. HRMS (ESI) calc for C24H25NO + H: 344.2014, found: 344.2083.
Refinement
All H-atoms were placed in calculated positions (O—H = 0.84 Å, and C—H 0.95 to 1.00 Å) and were included in the refinement in the riding model approximation with Uiso(H) = 1.2Ueq(C) and 1.5Ueq(O; methyl-C). In the absence of significant anomalous scattering effects, 1707 Friedel pairs were averaged in the final refinement. The 2S,5R designation was chosen based on the synthesis (Moro et al., 2010). The C7–C12 benzene ring was found to be disordered with one orientation slightly displaced with respect to the second, co-planar, orientation. In the final refinement, matching C atoms were constrained to have the same anisotropic displacement parameter. The major component of the disordered residue had a site occupancy factor = 0.782 (15).
Figures
Fig. 1.
The molecular structure of compound (I) showing the atom-labelling scheme and displacement ellipsoids at the 35% probability level. Only the major component of the disordered benzene ring is illustrated.
Fig. 2.
A view in projection down the b axis of the unit-cell contents for (I). The C—H···π interactions are shown as purple dashed lines.
Crystal data
| C24H25NO | F(000) = 736 |
| Mr = 343.45 | Dx = 1.189 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 7679 reflections |
| a = 9.9672 (2) Å | θ = 2.5–25.5° |
| b = 13.3376 (2) Å | µ = 0.07 mm−1 |
| c = 14.4369 (2) Å | T = 100 K |
| V = 1919.22 (5) Å3 | Irregular, colourless |
| Z = 4 | 0.22 × 0.15 × 0.15 mm |
Data collection
| Bruker APEXII CCD diffractometer | 1979 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.039 |
| graphite | θmax = 26.5°, θmin = 2.1° |
| φ and ω scans | h = −12→12 |
| 24972 measured reflections | k = −16→16 |
| 2262 independent reflections | l = −18→18 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.038 | H-atom parameters constrained |
| wR(F2) = 0.092 | w = 1/[σ2(Fo2) + (0.0477P)2 + 0.2712P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 2262 reflections | Δρmax = 0.17 e Å−3 |
| 255 parameters | Δρmin = −0.19 e Å−3 |
| 0 restraints | Absolute structure: nd |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O | 0.11866 (14) | 0.86869 (11) | 0.21128 (12) | 0.0486 (4) | |
| H1o | 0.1387 | 0.8166 | 0.1816 | 0.073* | |
| N | 0.30995 (17) | 0.77434 (11) | 0.11848 (11) | 0.0360 (4) | |
| C1 | 0.3091 (2) | 0.74937 (15) | 0.01898 (13) | 0.0364 (4) | |
| H1 | 0.4023 | 0.7558 | −0.0058 | 0.044* | |
| C2 | 0.2230 (2) | 0.83278 (15) | −0.02138 (14) | 0.0422 (5) | |
| H2A | 0.2437 | 0.8432 | −0.0877 | 0.051* | |
| H2B | 0.1264 | 0.8169 | −0.0149 | 0.051* | |
| C3 | 0.2595 (2) | 0.92449 (15) | 0.03493 (14) | 0.0409 (5) | |
| H3A | 0.1786 | 0.9652 | 0.0481 | 0.049* | |
| H3B | 0.3250 | 0.9665 | 0.0010 | 0.049* | |
| C4 | 0.3212 (2) | 0.88486 (13) | 0.12565 (12) | 0.0327 (4) | |
| H4 | 0.4177 | 0.9050 | 0.1300 | 0.039* | |
| C5 | 0.4120 (2) | 0.71912 (17) | 0.16989 (15) | 0.0510 (6) | |
| H5A | 0.3976 | 0.6470 | 0.1619 | 0.076* | |
| H5B | 0.4059 | 0.7362 | 0.2358 | 0.076* | |
| H5C | 0.5011 | 0.7371 | 0.1465 | 0.076* | |
| C12 | 0.24357 (19) | 0.92007 (13) | 0.21337 (13) | 0.0333 (4) | |
| C25 | 0.2101 (7) | 1.0299 (5) | 0.2126 (5) | 0.0299 (10) | 0.782 (15) |
| C26 | 0.0798 (6) | 1.0643 (6) | 0.2313 (5) | 0.0443 (11) | 0.782 (15) |
| H26 | 0.0112 | 1.0168 | 0.2439 | 0.053* | 0.782 (15) |
| C27 | 0.0485 (7) | 1.1642 (4) | 0.2320 (3) | 0.0504 (12) | 0.782 (15) |
| H27 | −0.0405 | 1.1847 | 0.2458 | 0.060* | 0.782 (15) |
| C28 | 0.1454 (8) | 1.2356 (3) | 0.2127 (3) | 0.0462 (14) | 0.782 (15) |
| H28 | 0.1232 | 1.3049 | 0.2128 | 0.055* | 0.782 (15) |
| C29 | 0.2743 (7) | 1.2049 (4) | 0.1933 (3) | 0.0441 (15) | 0.782 (15) |
| H29 | 0.3421 | 1.2532 | 0.1810 | 0.053* | 0.782 (15) |
| C30 | 0.3055 (7) | 1.1029 (6) | 0.1918 (5) | 0.0383 (11) | 0.782 (15) |
| H30 | 0.3940 | 1.0827 | 0.1762 | 0.046* | 0.782 (15) |
| C13 | 0.3186 (2) | 0.89712 (14) | 0.30368 (13) | 0.0396 (5) | |
| C14 | 0.2559 (3) | 0.84209 (16) | 0.37346 (15) | 0.0563 (7) | |
| H14 | 0.1668 | 0.8185 | 0.3648 | 0.068* | |
| C15 | 0.3229 (5) | 0.8218 (2) | 0.45513 (17) | 0.0781 (10) | |
| H15 | 0.2799 | 0.7831 | 0.5017 | 0.094* | |
| C16 | 0.4509 (4) | 0.8566 (2) | 0.46997 (18) | 0.0800 (11) | |
| H16 | 0.4959 | 0.8426 | 0.5265 | 0.096* | |
| C17 | 0.5129 (3) | 0.91177 (18) | 0.40224 (18) | 0.0656 (8) | |
| H17 | 0.6011 | 0.9366 | 0.4121 | 0.079* | |
| C18 | 0.4475 (2) | 0.93152 (15) | 0.31956 (16) | 0.0479 (5) | |
| H18 | 0.4920 | 0.9693 | 0.2730 | 0.057* | |
| C6 | 0.2582 (2) | 0.64512 (15) | −0.00095 (14) | 0.0399 (5) | |
| C7 | 0.3065 (3) | 0.59257 (18) | −0.07668 (16) | 0.0548 (6) | |
| H7 | 0.3759 | 0.6207 | −0.1136 | 0.066* | |
| C8 | 0.2548 (4) | 0.4995 (2) | −0.0990 (2) | 0.0711 (8) | |
| H8 | 0.2877 | 0.4650 | −0.1519 | 0.085* | |
| C9 | 0.1568 (3) | 0.45654 (19) | −0.0460 (2) | 0.0705 (8) | |
| H9 | 0.1223 | 0.3923 | −0.0616 | 0.085* | |
| C10 | 0.1091 (3) | 0.50665 (18) | 0.0295 (2) | 0.0645 (7) | |
| H10 | 0.0417 | 0.4768 | 0.0670 | 0.077* | |
| C11 | 0.1583 (3) | 0.60091 (17) | 0.05168 (18) | 0.0544 (6) | |
| H11 | 0.1230 | 0.6356 | 0.1037 | 0.065* | |
| C19 | 0.235 (3) | 1.043 (2) | 0.204 (2) | 0.0299 (10) | 0.218 (15) |
| C20 | 0.116 (2) | 1.072 (3) | 0.238 (2) | 0.0443 (11) | 0.218 (15) |
| H20 | 0.0495 | 1.0280 | 0.2623 | 0.053* | 0.218 (15) |
| C21 | 0.101 (3) | 1.1852 (17) | 0.2343 (15) | 0.0504 (12) | 0.218 (15) |
| H21 | 0.0187 | 1.2167 | 0.2509 | 0.060* | 0.218 (15) |
| C22 | 0.207 (3) | 1.2397 (15) | 0.2064 (13) | 0.0462 (14) | 0.218 (15) |
| H22 | 0.1991 | 1.3107 | 0.2057 | 0.055* | 0.218 (15) |
| C23 | 0.323 (2) | 1.1984 (16) | 0.1794 (14) | 0.0441 (15) | 0.218 (15) |
| H23 | 0.3949 | 1.2404 | 0.1602 | 0.053* | 0.218 (15) |
| C24 | 0.340 (2) | 1.098 (2) | 0.179 (2) | 0.0383 (11) | 0.218 (15) |
| H24 | 0.4225 | 1.0679 | 0.1620 | 0.046* | 0.218 (15) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O | 0.0386 (8) | 0.0402 (8) | 0.0671 (10) | −0.0100 (7) | 0.0144 (7) | −0.0061 (7) |
| N | 0.0446 (9) | 0.0298 (8) | 0.0337 (8) | 0.0063 (8) | −0.0033 (8) | −0.0016 (6) |
| C1 | 0.0357 (10) | 0.0407 (10) | 0.0327 (10) | 0.0019 (9) | 0.0008 (8) | −0.0048 (8) |
| C2 | 0.0501 (12) | 0.0411 (11) | 0.0355 (10) | −0.0006 (10) | −0.0064 (9) | 0.0013 (9) |
| C3 | 0.0459 (12) | 0.0377 (10) | 0.0392 (11) | −0.0011 (10) | −0.0040 (9) | 0.0025 (8) |
| C4 | 0.0331 (10) | 0.0304 (9) | 0.0344 (9) | −0.0005 (8) | −0.0009 (8) | 0.0012 (7) |
| C5 | 0.0668 (15) | 0.0439 (12) | 0.0423 (12) | 0.0218 (11) | −0.0111 (11) | −0.0075 (9) |
| C12 | 0.0318 (9) | 0.0277 (9) | 0.0405 (10) | −0.0023 (8) | 0.0049 (8) | −0.0005 (8) |
| C25 | 0.034 (3) | 0.026 (2) | 0.0295 (18) | −0.0063 (17) | 0.0048 (17) | 0.0031 (14) |
| C26 | 0.042 (3) | 0.0436 (18) | 0.047 (2) | 0.008 (3) | 0.005 (3) | −0.0021 (14) |
| C27 | 0.055 (3) | 0.042 (2) | 0.0541 (15) | 0.013 (2) | 0.011 (2) | −0.0058 (15) |
| C28 | 0.068 (4) | 0.0304 (12) | 0.0402 (14) | 0.012 (2) | 0.002 (2) | −0.0049 (11) |
| C29 | 0.059 (4) | 0.0318 (14) | 0.042 (2) | −0.005 (3) | −0.006 (2) | 0.0003 (14) |
| C30 | 0.035 (3) | 0.0340 (13) | 0.046 (2) | −0.003 (2) | −0.002 (2) | 0.0041 (16) |
| C13 | 0.0591 (13) | 0.0253 (9) | 0.0344 (10) | 0.0074 (9) | 0.0060 (9) | −0.0043 (7) |
| C14 | 0.0913 (18) | 0.0380 (11) | 0.0395 (12) | 0.0125 (13) | 0.0202 (12) | 0.0008 (9) |
| C15 | 0.151 (3) | 0.0489 (15) | 0.0343 (13) | 0.030 (2) | 0.0228 (17) | 0.0036 (11) |
| C16 | 0.149 (3) | 0.0556 (16) | 0.0352 (13) | 0.042 (2) | −0.0182 (17) | −0.0086 (12) |
| C17 | 0.091 (2) | 0.0513 (13) | 0.0544 (15) | 0.0220 (15) | −0.0274 (14) | −0.0158 (12) |
| C18 | 0.0645 (14) | 0.0357 (11) | 0.0434 (12) | 0.0060 (11) | −0.0100 (11) | −0.0020 (9) |
| C6 | 0.0434 (11) | 0.0362 (10) | 0.0401 (11) | 0.0066 (9) | −0.0071 (9) | −0.0035 (8) |
| C7 | 0.0678 (15) | 0.0495 (13) | 0.0473 (12) | 0.0054 (13) | −0.0030 (12) | −0.0123 (10) |
| C8 | 0.090 (2) | 0.0554 (14) | 0.0674 (17) | 0.0117 (15) | −0.0144 (17) | −0.0271 (13) |
| C9 | 0.0798 (19) | 0.0372 (12) | 0.094 (2) | 0.0030 (13) | −0.0359 (18) | −0.0136 (14) |
| C10 | 0.0615 (15) | 0.0442 (13) | 0.088 (2) | −0.0066 (12) | −0.0111 (15) | 0.0042 (14) |
| C11 | 0.0562 (14) | 0.0433 (12) | 0.0637 (15) | −0.0025 (11) | 0.0020 (12) | −0.0047 (11) |
| C19 | 0.034 (3) | 0.026 (2) | 0.0295 (18) | −0.0063 (17) | 0.0048 (17) | 0.0031 (14) |
| C20 | 0.042 (3) | 0.0436 (18) | 0.047 (2) | 0.008 (3) | 0.005 (3) | −0.0021 (14) |
| C21 | 0.055 (3) | 0.042 (2) | 0.0541 (15) | 0.013 (2) | 0.011 (2) | −0.0058 (15) |
| C22 | 0.068 (4) | 0.0304 (12) | 0.0402 (14) | 0.012 (2) | 0.002 (2) | −0.0049 (11) |
| C23 | 0.059 (4) | 0.0318 (14) | 0.042 (2) | −0.005 (3) | −0.006 (2) | 0.0003 (14) |
| C24 | 0.035 (3) | 0.0340 (13) | 0.046 (2) | −0.003 (2) | −0.002 (2) | 0.0041 (16) |
Geometric parameters (Å, °)
| O—C12 | 1.421 (2) | C13—C18 | 1.383 (3) |
| O—H1o | 0.8401 | C13—C14 | 1.394 (3) |
| N—C5 | 1.459 (3) | C14—C15 | 1.382 (4) |
| N—C1 | 1.475 (2) | C14—H14 | 0.9500 |
| N—C4 | 1.482 (2) | C15—C16 | 1.374 (5) |
| C1—C6 | 1.508 (3) | C15—H15 | 0.9500 |
| C1—C2 | 1.521 (3) | C16—C17 | 1.371 (4) |
| C1—H1 | 1.0000 | C16—H16 | 0.9500 |
| C2—C3 | 1.513 (3) | C17—C18 | 1.385 (3) |
| C2—H2A | 0.9900 | C17—H17 | 0.9500 |
| C2—H2B | 0.9900 | C18—H18 | 0.9500 |
| C3—C4 | 1.541 (3) | C6—C11 | 1.384 (3) |
| C3—H3A | 0.9900 | C6—C7 | 1.385 (3) |
| C3—H3B | 0.9900 | C7—C8 | 1.383 (4) |
| C4—C12 | 1.557 (3) | C7—H7 | 0.9500 |
| C4—H4 | 1.0000 | C8—C9 | 1.366 (5) |
| C5—H5A | 0.9800 | C8—H8 | 0.9500 |
| C5—H5B | 0.9800 | C9—C10 | 1.364 (4) |
| C5—H5C | 0.9800 | C9—H9 | 0.9500 |
| C12—C25 | 1.503 (7) | C10—C11 | 1.387 (3) |
| C12—C13 | 1.534 (3) | C10—H10 | 0.9500 |
| C12—C19 | 1.65 (3) | C11—H11 | 0.9500 |
| C25—C30 | 1.394 (7) | C19—C24 | 1.32 (3) |
| C25—C26 | 1.403 (9) | C19—C20 | 1.34 (4) |
| C26—C27 | 1.368 (9) | C20—C21 | 1.51 (4) |
| C26—H26 | 0.9500 | C20—H20 | 0.9500 |
| C27—C28 | 1.385 (6) | C21—C22 | 1.34 (2) |
| C27—H27 | 0.9500 | C21—H21 | 0.9500 |
| C28—C29 | 1.378 (5) | C22—C23 | 1.34 (2) |
| C28—H28 | 0.9500 | C22—H22 | 0.9500 |
| C29—C30 | 1.395 (9) | C23—C24 | 1.35 (4) |
| C29—H29 | 0.9500 | C23—H23 | 0.9500 |
| C30—H30 | 0.9500 | C24—H24 | 0.9500 |
| C12—O—H1o | 101.6 | C25—C30—C29 | 121.7 (5) |
| C5—N—C1 | 112.69 (16) | C25—C30—H30 | 119.1 |
| C5—N—C4 | 114.43 (16) | C29—C30—H30 | 119.1 |
| C1—N—C4 | 107.05 (14) | C18—C13—C14 | 118.1 (2) |
| N—C1—C6 | 113.34 (16) | C18—C13—C12 | 121.84 (18) |
| N—C1—C2 | 102.20 (16) | C14—C13—C12 | 120.0 (2) |
| C6—C1—C2 | 114.29 (17) | C15—C14—C13 | 120.2 (3) |
| N—C1—H1 | 108.9 | C15—C14—H14 | 119.9 |
| C6—C1—H1 | 108.9 | C13—C14—H14 | 119.9 |
| C2—C1—H1 | 108.9 | C16—C15—C14 | 121.0 (3) |
| C3—C2—C1 | 104.46 (16) | C16—C15—H15 | 119.5 |
| C3—C2—H2A | 110.9 | C14—C15—H15 | 119.5 |
| C1—C2—H2A | 110.9 | C17—C16—C15 | 119.3 (3) |
| C3—C2—H2B | 110.9 | C17—C16—H16 | 120.4 |
| C1—C2—H2B | 110.9 | C15—C16—H16 | 120.4 |
| H2A—C2—H2B | 108.9 | C16—C17—C18 | 120.3 (3) |
| C2—C3—C4 | 105.99 (16) | C16—C17—H17 | 119.8 |
| C2—C3—H3A | 110.5 | C18—C17—H17 | 119.8 |
| C4—C3—H3A | 110.5 | C13—C18—C17 | 121.1 (2) |
| C2—C3—H3B | 110.5 | C13—C18—H18 | 119.5 |
| C4—C3—H3B | 110.5 | C17—C18—H18 | 119.5 |
| H3A—C3—H3B | 108.7 | C11—C6—C7 | 117.9 (2) |
| N—C4—C3 | 104.56 (15) | C11—C6—C1 | 122.02 (19) |
| N—C4—C12 | 108.61 (15) | C7—C6—C1 | 120.0 (2) |
| C3—C4—C12 | 112.90 (15) | C8—C7—C6 | 120.6 (3) |
| N—C4—H4 | 110.2 | C8—C7—H7 | 119.7 |
| C3—C4—H4 | 110.2 | C6—C7—H7 | 119.7 |
| C12—C4—H4 | 110.2 | C9—C8—C7 | 120.8 (3) |
| N—C5—H5A | 109.5 | C9—C8—H8 | 119.6 |
| N—C5—H5B | 109.5 | C7—C8—H8 | 119.6 |
| H5A—C5—H5B | 109.5 | C10—C9—C8 | 119.4 (3) |
| N—C5—H5C | 109.5 | C10—C9—H9 | 120.3 |
| H5A—C5—H5C | 109.5 | C8—C9—H9 | 120.3 |
| H5B—C5—H5C | 109.5 | C9—C10—C11 | 120.3 (3) |
| O—C12—C25 | 106.0 (3) | C9—C10—H10 | 119.8 |
| O—C12—C13 | 110.43 (16) | C11—C10—H10 | 119.8 |
| C25—C12—C13 | 108.0 (3) | C6—C11—C10 | 120.9 (2) |
| O—C12—C4 | 105.84 (15) | C6—C11—H11 | 119.5 |
| C25—C12—C4 | 113.5 (3) | C10—C11—H11 | 119.5 |
| C13—C12—C4 | 112.86 (15) | C24—C19—C20 | 130 (3) |
| O—C12—C19 | 115.5 (11) | C24—C19—C12 | 122 (2) |
| C13—C12—C19 | 107.2 (12) | C20—C19—C12 | 108 (2) |
| C4—C12—C19 | 105.0 (10) | C19—C20—C21 | 111 (2) |
| C30—C25—C26 | 116.4 (6) | C19—C20—H20 | 124.5 |
| C30—C25—C12 | 122.1 (5) | C21—C20—H20 | 124.5 |
| C26—C25—C12 | 121.5 (5) | C22—C21—C20 | 118.2 (18) |
| C27—C26—C25 | 122.1 (5) | C22—C21—H21 | 120.9 |
| C27—C26—H26 | 119.0 | C20—C21—H21 | 120.9 |
| C25—C26—H26 | 119.0 | C23—C22—C21 | 123.0 (18) |
| C26—C27—C28 | 120.6 (4) | C23—C22—H22 | 118.5 |
| C26—C27—H27 | 119.7 | C21—C22—H22 | 118.5 |
| C28—C27—H27 | 119.7 | C22—C23—C24 | 121 (2) |
| C29—C28—C27 | 119.1 (4) | C22—C23—H23 | 119.5 |
| C29—C28—H28 | 120.4 | C24—C23—H23 | 119.5 |
| C27—C28—H28 | 120.4 | C19—C24—C23 | 117 (2) |
| C28—C29—C30 | 120.0 (4) | C19—C24—H24 | 121.7 |
| C28—C29—H29 | 120.0 | C23—C24—H24 | 121.7 |
| C30—C29—H29 | 120.0 | ||
| C5—N—C1—C6 | 70.2 (2) | O—C12—C13—C14 | −6.8 (2) |
| C4—N—C1—C6 | −163.11 (17) | C25—C12—C13—C14 | 108.7 (3) |
| C5—N—C1—C2 | −166.29 (18) | C4—C12—C13—C14 | −124.99 (18) |
| C4—N—C1—C2 | −39.6 (2) | C19—C12—C13—C14 | 119.8 (10) |
| N—C1—C2—C3 | 36.6 (2) | C18—C13—C14—C15 | −1.0 (3) |
| C6—C1—C2—C3 | 159.41 (17) | C12—C13—C14—C15 | −179.62 (19) |
| C1—C2—C3—C4 | −20.7 (2) | C13—C14—C15—C16 | 1.2 (4) |
| C5—N—C4—C3 | 152.32 (17) | C14—C15—C16—C17 | −0.4 (4) |
| C1—N—C4—C3 | 26.7 (2) | C15—C16—C17—C18 | −0.4 (4) |
| C5—N—C4—C12 | −86.9 (2) | C14—C13—C18—C17 | 0.2 (3) |
| C1—N—C4—C12 | 147.48 (16) | C12—C13—C18—C17 | 178.72 (19) |
| C2—C3—C4—N | −3.0 (2) | C16—C17—C18—C13 | 0.6 (3) |
| C2—C3—C4—C12 | −120.85 (18) | N—C1—C6—C11 | 34.1 (3) |
| N—C4—C12—O | −45.89 (19) | C2—C1—C6—C11 | −82.4 (2) |
| C3—C4—C12—O | 69.60 (19) | N—C1—C6—C7 | −149.2 (2) |
| N—C4—C12—C25 | −161.7 (3) | C2—C1—C6—C7 | 94.2 (2) |
| C3—C4—C12—C25 | −46.2 (4) | C11—C6—C7—C8 | 0.8 (3) |
| N—C4—C12—C13 | 75.00 (19) | C1—C6—C7—C8 | −176.0 (2) |
| C3—C4—C12—C13 | −169.51 (16) | C6—C7—C8—C9 | −1.3 (4) |
| N—C4—C12—C19 | −168.5 (12) | C7—C8—C9—C10 | 0.5 (4) |
| C3—C4—C12—C19 | −53.1 (12) | C8—C9—C10—C11 | 0.7 (4) |
| O—C12—C25—C30 | −164.1 (6) | C7—C6—C11—C10 | 0.4 (3) |
| C13—C12—C25—C30 | 77.5 (7) | C1—C6—C11—C10 | 177.2 (2) |
| C4—C12—C25—C30 | −48.4 (8) | C9—C10—C11—C6 | −1.2 (4) |
| C19—C12—C25—C30 | −10 (7) | O—C12—C19—C24 | −161 (3) |
| O—C12—C25—C26 | 14.7 (7) | C25—C12—C19—C24 | 172 (10) |
| C13—C12—C25—C26 | −103.7 (7) | C13—C12—C19—C24 | 76 (3) |
| C4—C12—C25—C26 | 130.4 (6) | C4—C12—C19—C24 | −45 (3) |
| C19—C12—C25—C26 | 169 (8) | O—C12—C19—C20 | 28 (3) |
| C30—C25—C26—C27 | −2.0 (11) | C25—C12—C19—C20 | 1(6) |
| C12—C25—C26—C27 | 179.2 (6) | C13—C12—C19—C20 | −95 (2) |
| C25—C26—C27—C28 | 0.9 (10) | C4—C12—C19—C20 | 144 (2) |
| C26—C27—C28—C29 | −0.4 (7) | C24—C19—C20—C21 | 9(5) |
| C27—C28—C29—C30 | 1.0 (7) | C12—C19—C20—C21 | 179 (2) |
| C26—C25—C30—C29 | 2.6 (11) | C19—C20—C21—C22 | −6(4) |
| C12—C25—C30—C29 | −178.5 (5) | C20—C21—C22—C23 | 2(4) |
| C28—C29—C30—C25 | −2.2 (9) | C21—C22—C23—C24 | 0(4) |
| O—C12—C13—C18 | 174.73 (17) | C20—C19—C24—C23 | −7(5) |
| C25—C12—C13—C18 | −69.8 (3) | C12—C19—C24—C23 | −176 (2) |
| C4—C12—C13—C18 | 56.5 (2) | C22—C23—C24—C19 | 2(4) |
| C19—C12—C13—C18 | −58.7 (10) |
Hydrogen-bond geometry (Å, °)
| Cg is the centroid of the C6–C11 ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| O—H1O···N | 0.84 | 2.02 | 2.648 (2) | 132 |
| C28—H28···Oi | 0.95 | 2.78 | 3.359 (7) | 120 |
| C17—H17···Cgii | 0.95 | 2.92 | 3.776 (3) | 150 |
Symmetry codes: (i) −x, y+1/2, −z+1/2; (ii) −x+1, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5054).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, 115–119.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chemaxon (2010). Marvinsketch. http://www.chemaxon.com.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Moro, A. V., Tiekink, E. R. T., Zukerman-Schpector, J., Lüdtke, D. S. & Correia, C. R. D. (2010). Eur. J. Org. Chem. pp. 3696–3703.
- Paixão, M. W., Braga, A. L. & Lüdtke, D. S. (2008). J. Braz. Chem. Soc. 19, 813–830.
- Shabbir, S. H., Joyce, L. A., da Cruz, G. M., Lynch, V. M., Sorey, S. & Anslyn, E. V. (2009). J. Am. Chem. Soc. 131, 13125–13131. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Taylor, J. G., Moro, A. V. & Correia, C. R. D. (2011). Eur. J. Org. Chem. pp. 1403–1428.
- Walsh, P. J. & Kozlowski, M. C. (2008). Fundamentals of Asymmetric Catalysis Sausalito, CA: University Science Books Sausalito.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Yoon, T. P. & Jacobsen, E. N. (2003). Science, 299, 1691–1693. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811023403/hg5054sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023403/hg5054Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report