Abstract
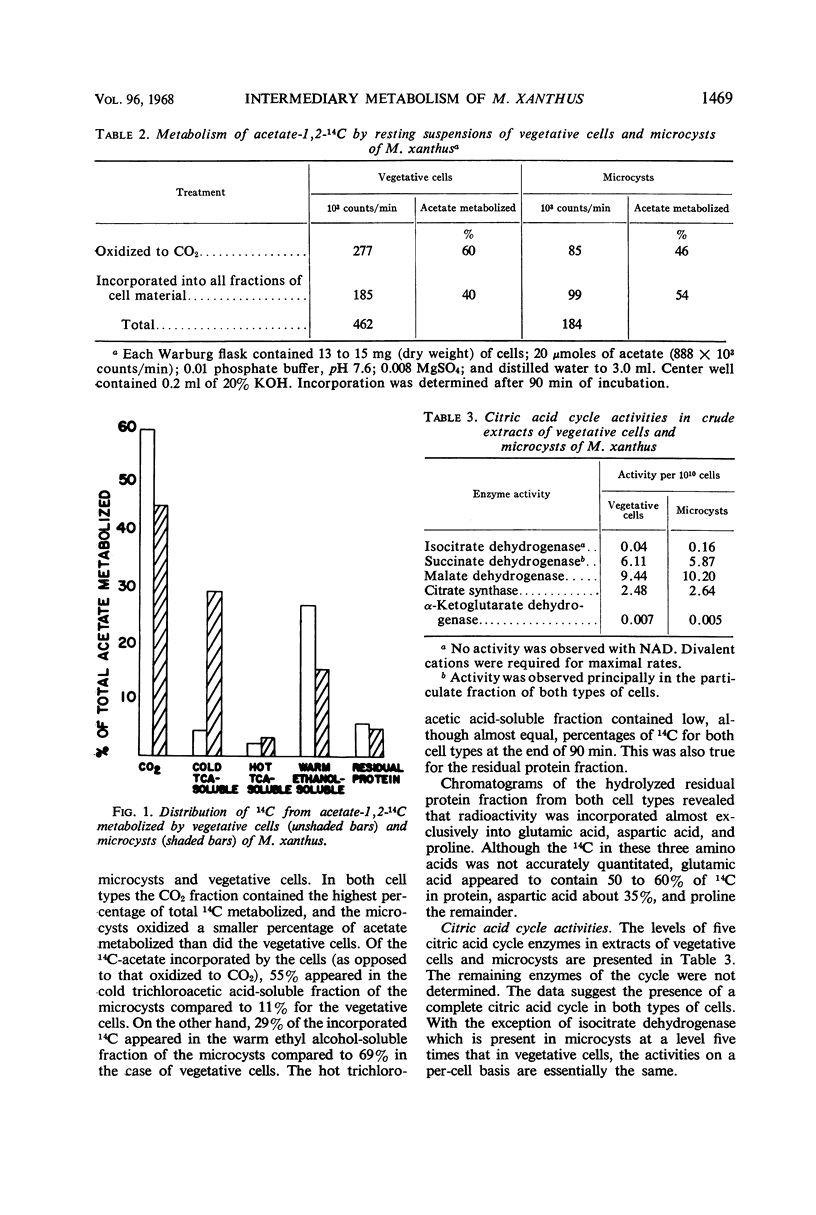
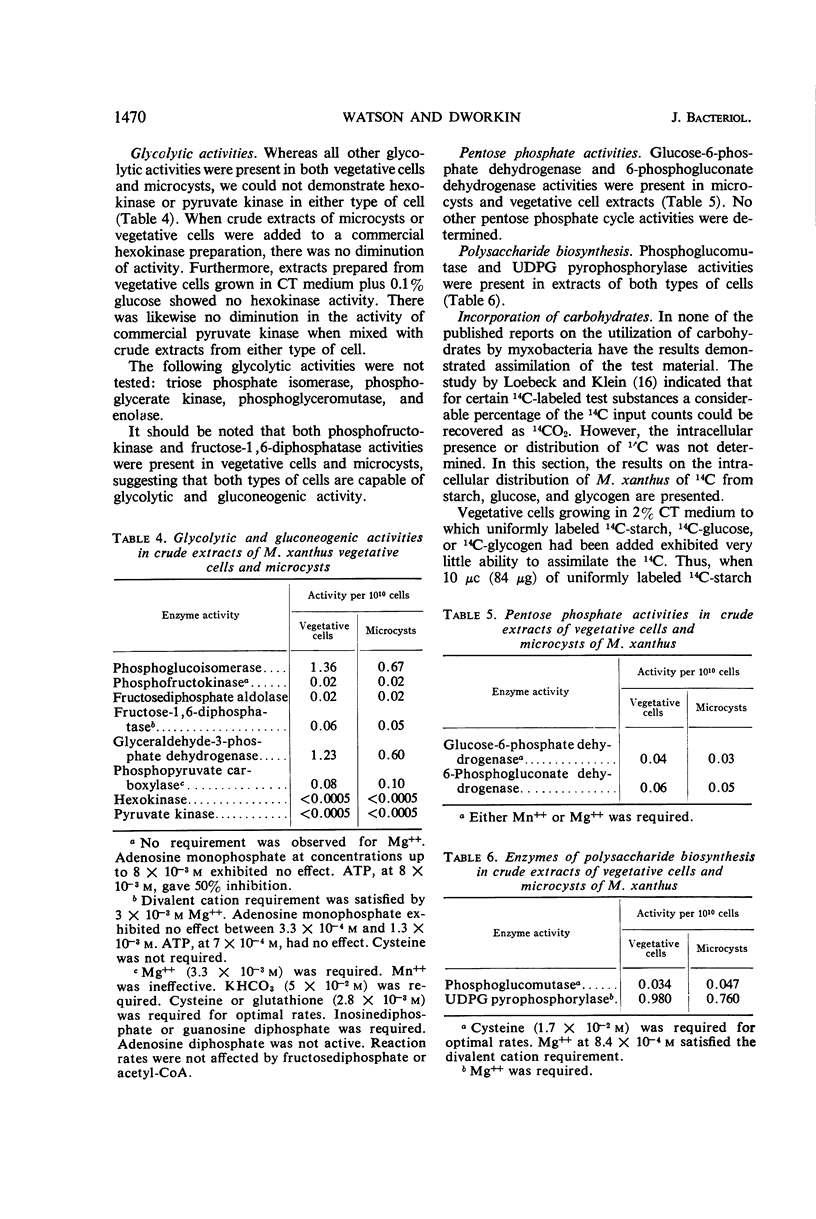
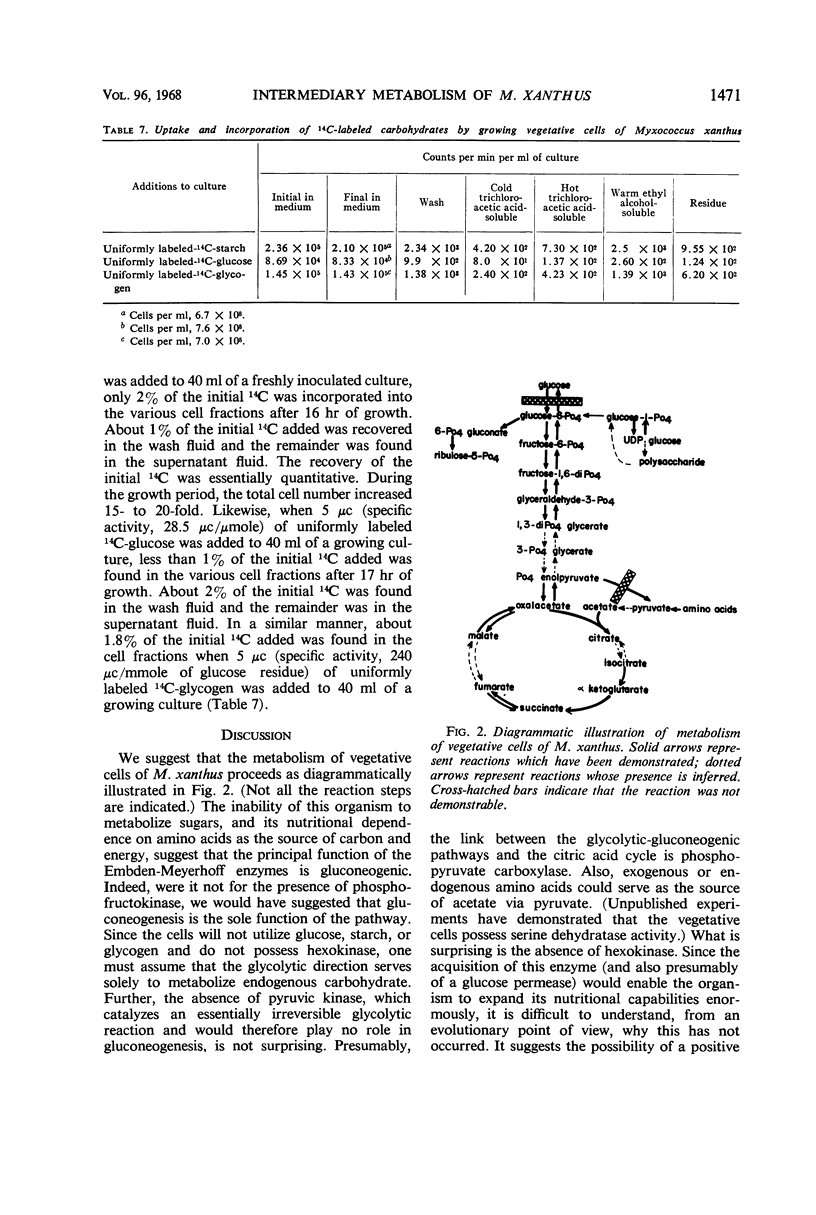
Crude extracts of both vegetative cells and glycerol-induced microcysts of Myxococcus xanthus contained the following enzyme activities: phosphofructokinase, phosphoglucoisomerase, fructose-1,6-diphosphatase, fructosediphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, phosphopyruvate carboxylase, citrate synthase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinate dehydrogenase, malate dehydrogenase, glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, phosphoglucomutase, and uridine diphosphate glucose pyrophosphorylase. With the exception of isocitrate dehydrogenase, which was present at a fivefold higher concentration in microcysts, all activities in extracts from both types of cells were essentially equal. Hexokinase and pyruvate kinase could not be detected in extracts from either type of cell. Microcysts metabolized acetate at a lower rate than did vegetative cells. Most of this decrease was reflected in a substantial decrease in ability of microcysts to oxidize acetate to CO2. In addition, microcysts and vegetative cells showed a different distribution of 14C-label from incorporated acetate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., WALTON G. M. KINETICS OF REGULATORY ENZYMES. ESCHERICHIA COLI PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 Feb;240:757–763. [PubMed] [Google Scholar]
- BECK W. S. Determination of triose phosphates and proposed modifications in the aldolase method of Sibley and Lehninger. J Biol Chem. 1955 Feb;212(2):847–857. [PubMed] [Google Scholar]
- COOPER J., SRERE P. A., TABACHNICK M., RACKER E. The oxidative pentose phosphate cycle. II. Quantitative determination of intermediates and enzymes. Arch Biochem Biophys. 1958 Apr;74(2):306–314. doi: 10.1016/0003-9861(58)90002-x. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., GIBSON S. M. A SYSTEM FOR STUDYING MICROBIAL MORPHOGENESIS: RAPID FORMATION OF MICROCYSTS IN MYXOCOCCUS XANTHUS. Science. 1964 Oct 9;146(3641):243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- DWORKIN M., NIEDERPRUEM D. J. ELECTRON TRANSPORT SYSTEM IN VEGETATIVE CELLS AND MICROCYSTS OF MYXOCOCCUS XANTHUS. J Bacteriol. 1964 Feb;87:316–322. doi: 10.1128/jb.87.2.316-322.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Fraenkel D. G., Horecker B. L. Fructose-1, 6-diphosphatase and acid hexose phosphatase of Escherichia coli. J Bacteriol. 1965 Oct;90(4):837–842. doi: 10.1128/jb.90.4.837-842.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Chilton W. S. Galactosamine glycan of Chondrococcus columnaris. Science. 1966 May 27;152(3726):1247–1248. doi: 10.1126/science.152.3726.1247. [DOI] [PubMed] [Google Scholar]
- LOEBECK M. E., KLEIN H. P. Substrates for Myxococcus virescens with special reference to eubacterial fractions. J Gen Microbiol. 1956 Apr;14(2):281–289. doi: 10.1099/00221287-14-2-281. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SANADI D. R., LITTLEFIELD J. W. Studies on alpha-ketoglutaric oxidase. I. Formation of "active" succinate. J Biol Chem. 1951 Dec;193(2):683–689. [PubMed] [Google Scholar]
- UTTER M. F., KURAHASHI K. Mechanism of action of oxalacetic carboxylase. J Biol Chem. 1954 Apr;207(2):821–841. [PubMed] [Google Scholar]


