Abstract
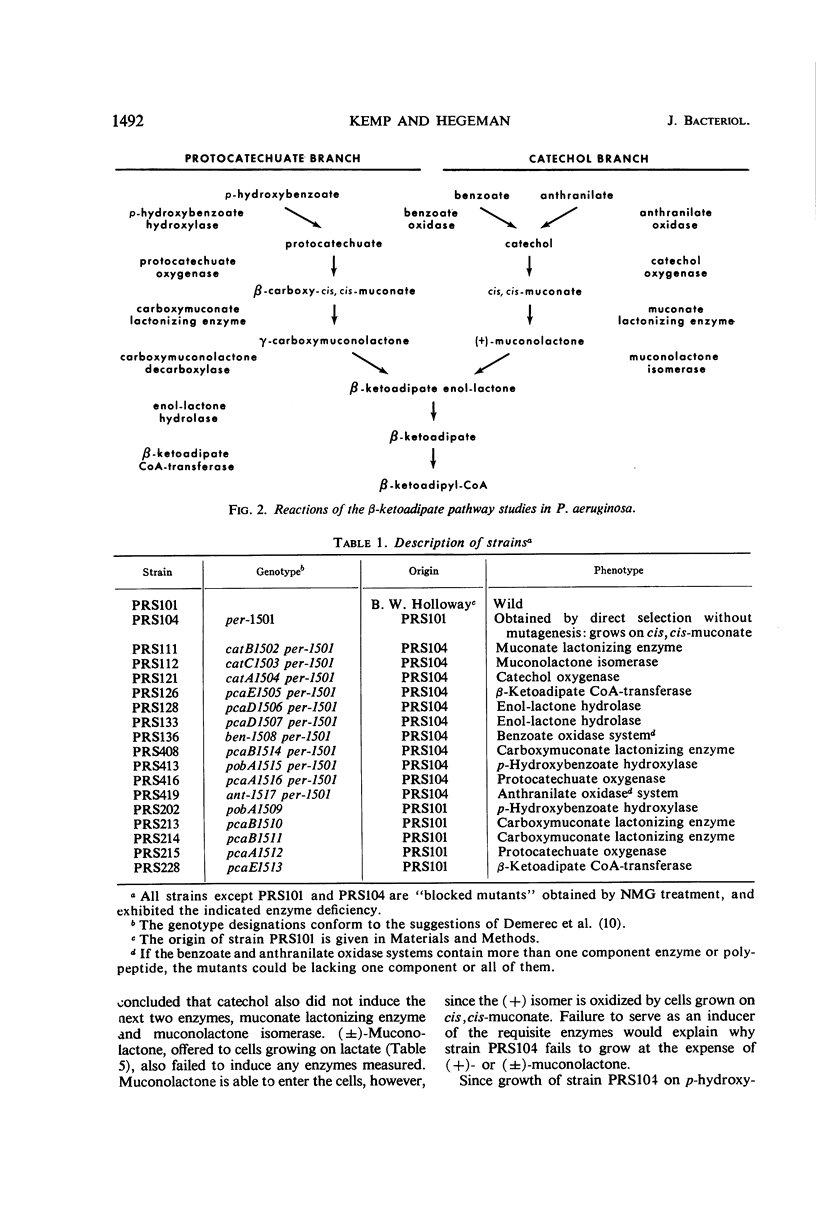
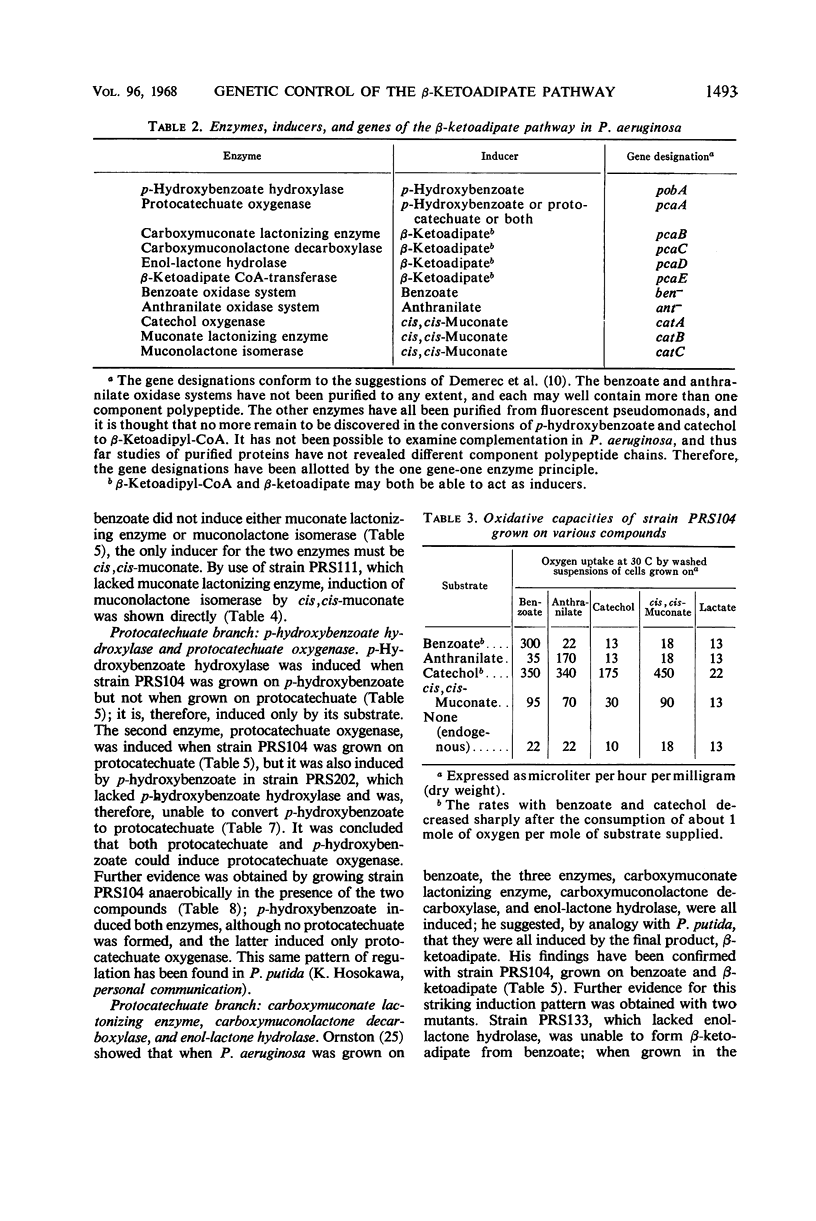
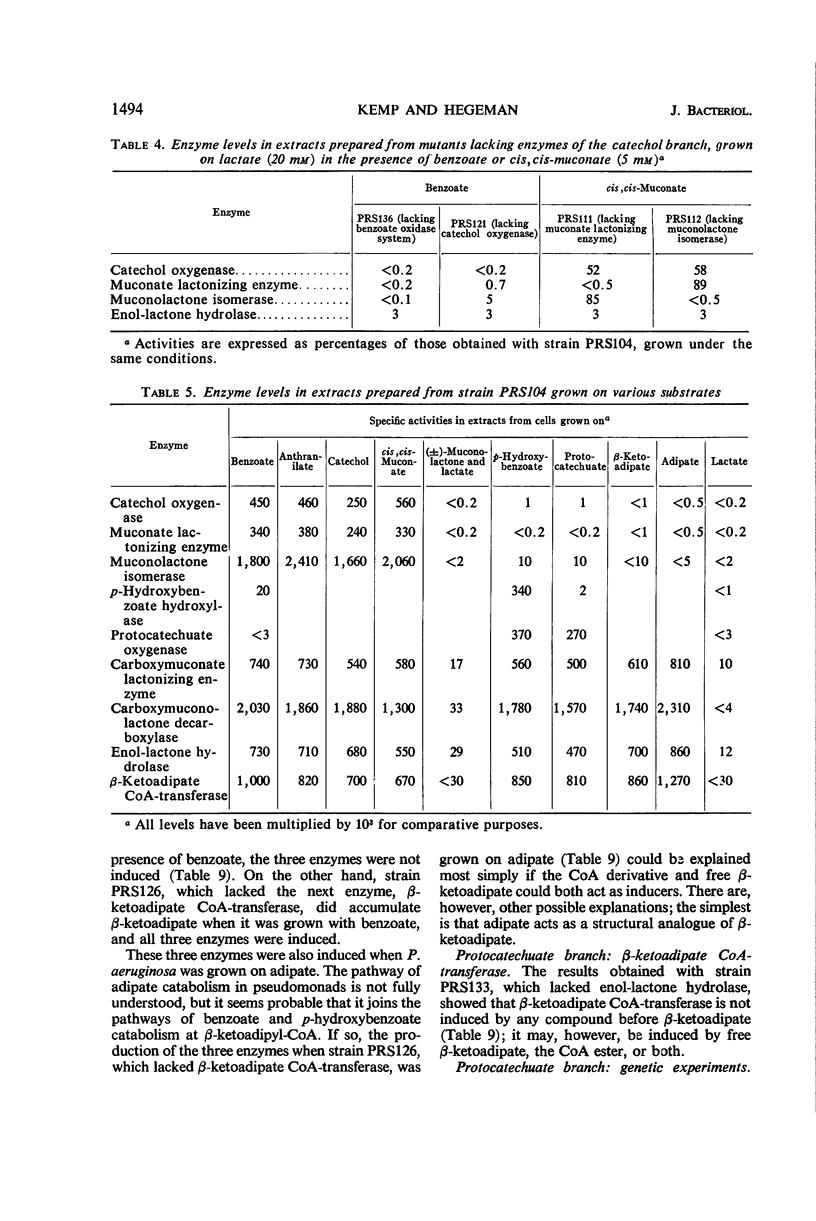
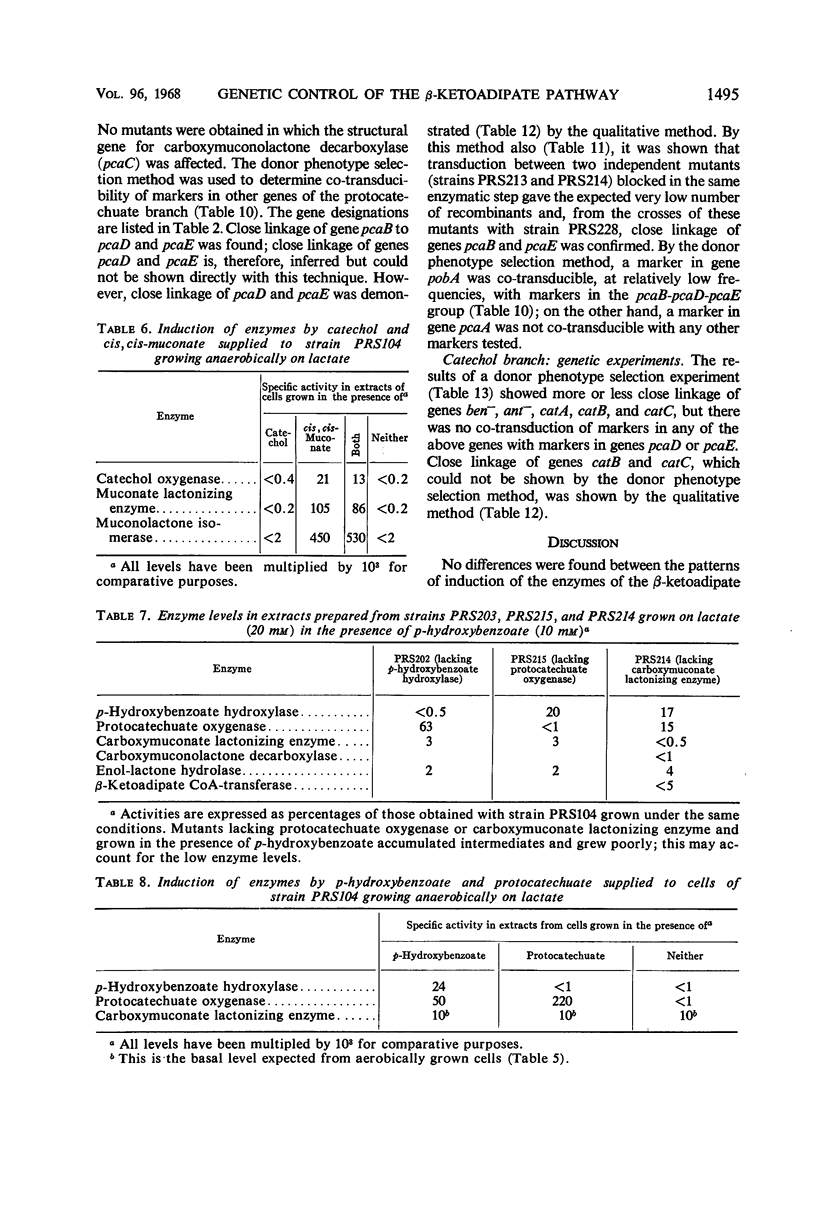
The regulation and genetic control of the β-ketoadipate pathway in Pseudomonas aeruginosa were investigated. The pattern of enzyme induction is apparently the same as in P. putida. Mutants were obtained for all but 1 of the 11 structural genes; the proximity of these genes on the chromosome was examined by transduction of the mutants with phage F116. If a group of enzymes was induced by the same compounds, the corresponding genes were closely clustered. Surprisingly, some locispecifying enzymes not sharing a common inducer were also clustered. It is suggested that this latter finding may indicate a degree of chromosomal specialization.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., MARTIN R. G. BIOCHEMICAL ASPECTS OF GENETICS: THE OPERON. Annu Rev Biochem. 1964;33:235–258. doi: 10.1146/annurev.bi.33.070164.001315. [DOI] [PubMed] [Google Scholar]
- BURTON J. F., CAIN B. F. Antileukaemic activity of polyporic acid. Nature. 1959 Oct 24;184(Suppl 17):1326–1327. doi: 10.1038/1841326a0. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
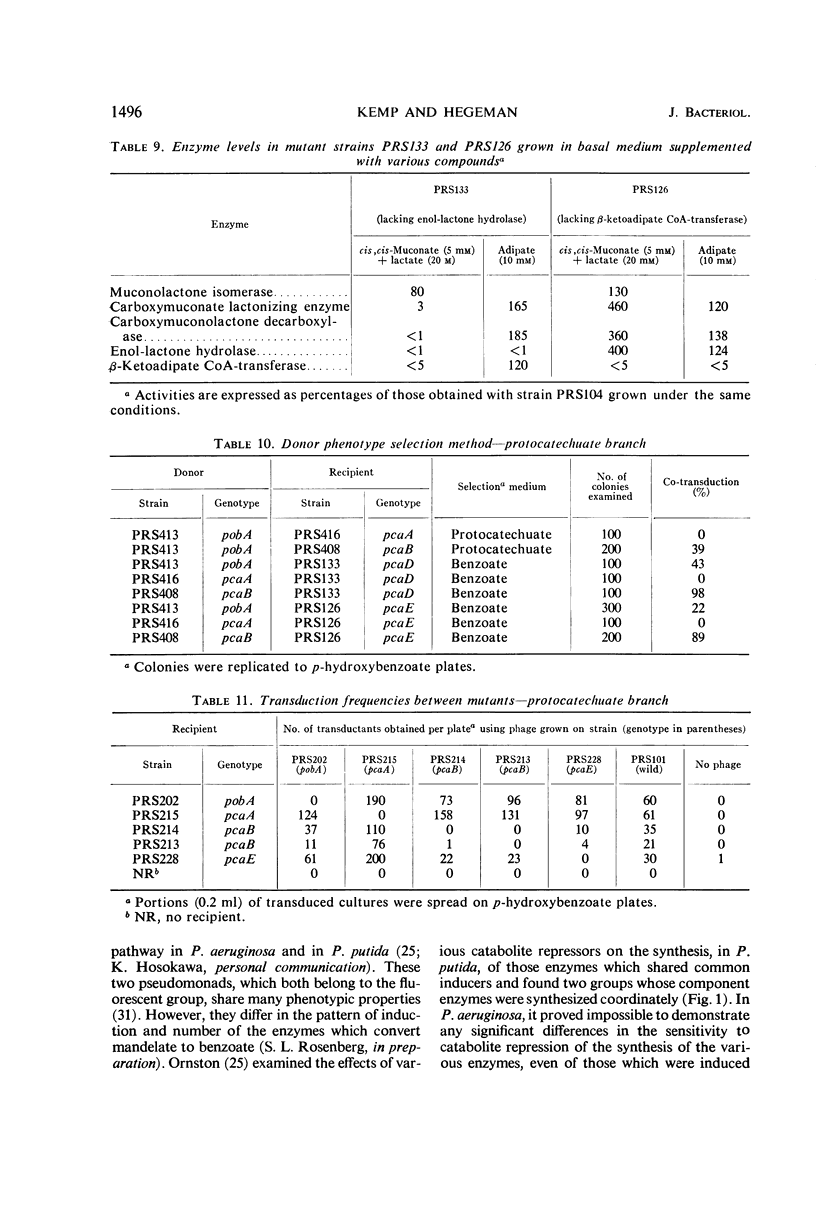
- Brammar W. J., Clarke P. H., Skinner A. J. Biochemical and genetic studies with regulator mutants of the Pseudomonas aeruginosa 8602 amidase system. J Gen Microbiol. 1967 Apr;47(1):87–102. doi: 10.1099/00221287-47-1-87. [DOI] [PubMed] [Google Scholar]
- CLOWES R. C. Investigation of the genetics of cysteineless mutants of Salmonella typhimurium by transduction. J Gen Microbiol. 1958 Feb;18(1):154–172. doi: 10.1099/00221287-18-1-154. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Gunsalus C. F., Gunsalus I. C. Transduction and the clustering of genes in fluorescent Pseudomonads. Proc Natl Acad Sci U S A. 1968 May;60(1):168–175. doi: 10.1073/pnas.60.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
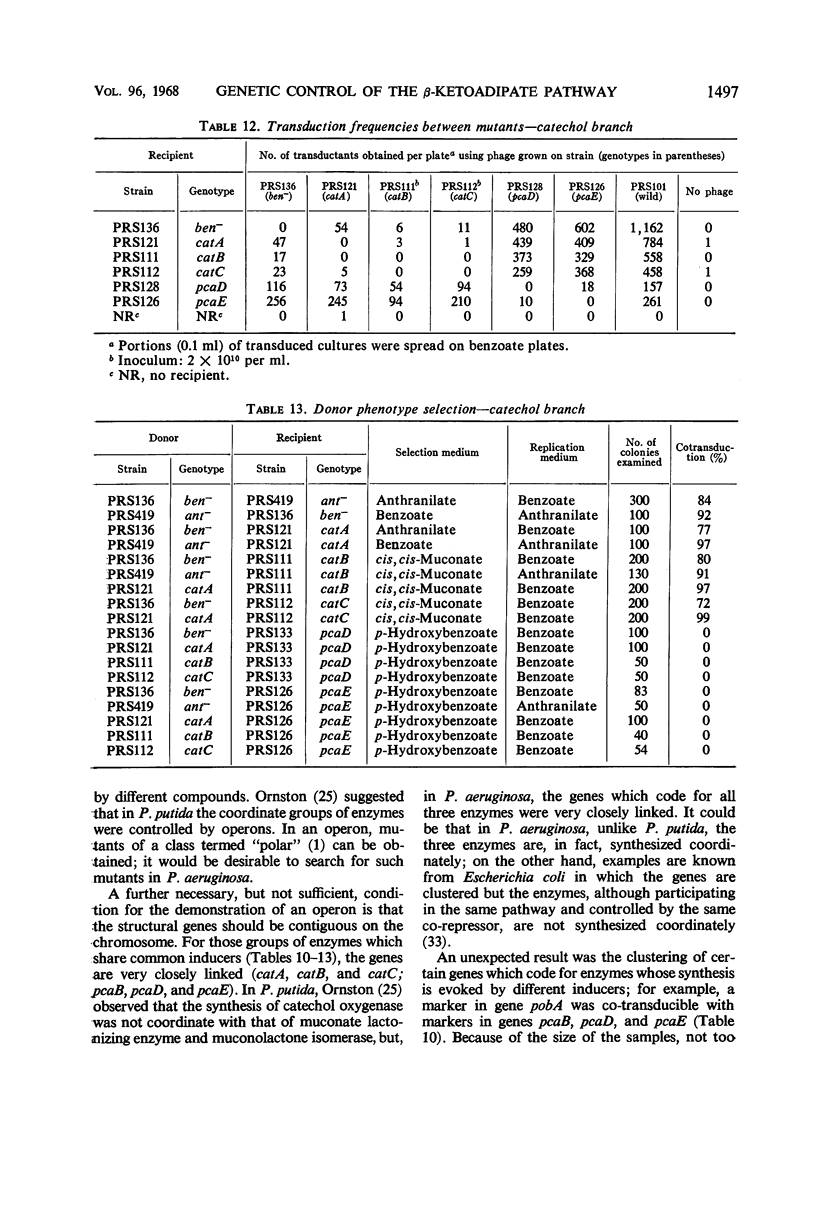
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Wheelis M. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 2. The role of protocatechuate as inducer. Eur J Biochem. 1968 Jan;3(3):293–304. doi: 10.1111/j.1432-1033.1968.tb19529.x. [DOI] [PubMed] [Google Scholar]
- DEMEREC M. CLUSTERING OF FUNCTIONALLY RELATED GENES IN SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1964 Jun;51:1057–1060. doi: 10.1073/pnas.51.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955 Dec;13(3):572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LOUTIT J. S. A transduction-like process within a single strain of Pseudomonas aeruginosa. J Gen Microbiol. 1958 Apr;18(2):315–319. doi: 10.1099/00221287-18-2-315. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mee B. J., Lee B. T. An analysis of histidine requiring mutants in Pseudomonas aeruginosa. Genetics. 1967 Apr;55(4):709–722. doi: 10.1093/genetics/55.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- PEARCE L. E., LOUTIT J. S. BIOCHEMICAL AND GENETIC GROUPING OF ISOLEUCINE-VALINE MUTANTS OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1965 Jan;89:58–63. doi: 10.1128/jb.89.1.58-63.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., INGRAHAM J. L. Protocatechuic acid oxidase. J Biol Chem. 1954 Oct;210(2):799–808. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


