Abstract
The title compound, C22H16N2OS, is a chalcone analog with a thiazolidinone core that was synthesized as a potential cytotoxic and anticancer agent. The structure is commensurately modulated by unit-cell doubling along the direction of the a axis of the cell. The two crystallographically independent molecules are differerentiated by the dihedral angle between the mean planes of the benzylidene phenyl group against the thiazolidin-4-one moiety, which is 5.01 (7)° in one molecule, and 17.41 (6)° in the other. The two molecules are otherwise close to being indistinguishable and are related by crystallographic pseudo-translation. The two molecules are not planar but are slightly bent with the benzylidene and phenylimino substituents being bent upwards with respect to the center planes of the two molecules. The degree of bending of the two halves of the thiazolidin-4-one moieties (defined as the planes that intersect at the S atom) are 11.08 (7) and 15.88 (7)°. Packing of the molecules is facilitated by C—H⋯π interactions and slipped π–π stacking between one of the phenyl rings and a neighboring ethylene π system [distance between the centroid of the ethylene group and the closest phenyl C atom = 3.267 (2) Å, Cg(phenyl)⋯Cg(ethylene) = 3.926 Å].
Related literature
Abdel-Aziz et al. (2010 ▶), Babu et al. (2011 ▶) and Chavda et al. (2009 ▶) describe the use of conjugated styryl ketones and related compounds as potential cytotoxic and anticancer agents. Satam et al. (2011 ▶) gives background to compounds with a thiazolidinone pharmacophore and describe structures related to the title compound.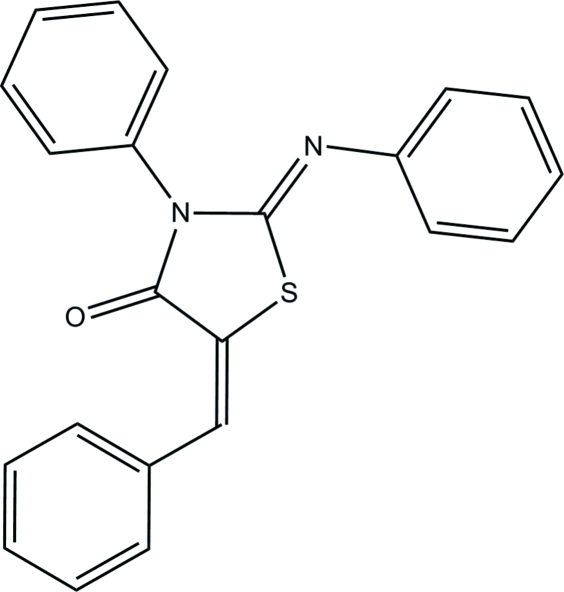
Experimental
Crystal data
C22H16N2OS
M r = 356.43
Monoclinic,

a = 10.7814 (9) Å
b = 32.779 (3) Å
c = 9.8907 (8) Å
β = 98.392 (1)°
V = 3458.0 (5) Å3
Z = 8
Mo Kα radiation
μ = 0.20 mm−1
T = 100 K
0.55 × 0.41 × 0.33 mm
Data collection
Bruker SMART APEX CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.632, T max = 0.746
23712 measured reflections
10081 independent reflections
7732 reflections with I > 2σ(I)
R int = 0.025
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.125
S = 1.03
10081 reflections
469 parameters
H-atom parameters constrained
Δρmax = 0.46 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXTL and publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811023658/gk2385sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023658/gk2385Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811023658/gk2385Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C12A—H12A⋯S1B | 0.95 | 2.92 | 3.6214 (15) | 131 |
| C12A—H12A⋯C2B | 0.95 | 2.85 | 3.7340 (19) | 156 |
| C12A—H12A⋯C3B | 0.95 | 2.59 | 3.5270 (19) | 167 |
| C12B—H12B⋯S1Ai | 0.95 | 2.96 | 3.6118 (15) | 127 |
| C12B—H12B⋯C3Ai | 0.95 | 2.76 | 3.6700 (19) | 162 |
Symmetry code: (i) 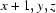 .
.
Acknowledgments
Support from Conjura is gratefully acknowledged. Support from the Department of Chemistry, Sambalpur University for providing facilities for research is also acknowledged. The X-ray diffractometer at Youngstown State University was funded by NSF grant 0087210, Ohio Board of Regents grant CAP-491, and by Youngstown State University.
supplementary crystallographic information
Comment
Continuing our work on the design, syntheses, and evaluation of conjugated styryl ketones and related compounds as potential cytotoxic and anticancer agents (Chavda et al., 2009, Babu et al., 2011, Satam et al., 2011) we synthesized a series of novel chalcone analogs possessing a thiazolidinone core. We envisaged that a combination of the 3-aryl-2-propenoyl unit in chalcones and the thiazolidinone pharmacophore would lead to a series of novel chalcone analogs that may provide a synergistic effect to exhibit interesting cytotoxic activities against malignant cells. A novel series of eleven compounds was assessed for cytotoxic activity against murine B16 and L1210 cancer cell lines (Satam et al., 2011). The title compound belonging to this series was subjected to X-ray crystallographic analysis in order to investigate the geometry about the alkene bond as well as the overall conformation of the compound.
The title compound (3) was synthesized by condensation of 2-phenylimino-3-phenylthiazolidin-4-one (1) with benzaldehyde as shown in Figure 1. Re-crystallization from methanol yielded crystals suitable for X-ray diffraction analysis. The structure crystallizes in P21/c with two crystallographically independent but chemically identical molecules A and B per asymmetric part of the unit cell, Figure 2. Bond distances and angles in both moleucles are in the expected ranges, a Mogul geometry check as implemented in the program Mercury (Macrae et al., 2008) did not indicate any unusual geometric parameters, and double and single bonds are located as expected (Scheme 1, Figure 1). The benzylidene double bond shows the phenyl substituent and the sulfur atoms to be in cis position to each other. The two molecules have very similar conformations and in both molecules the heterocylic ring sections are not planar but are slightly U-shaped with the benzylidene and phenylimino substituents being bent upwards with respect to the center planes of the two molecules. The degree of bending of the thiazolidin-4-one moieties, defined as the angle between the plane formed by the two N atoms, the S atom and C1 and C17 on the one hand and that of the carbonyl group, the sulfur atom and C3 and C4 on the other, is 11.08 (7)° for the A molecule, and 15.88 (7)° for the B molecule. A similar slight deviation from planarity was observed earlier for the 4-methyl benzylidene derivative of the title compound, which has an equivalent bend angle of 15.9 (1)°. The 3-bromo-6 methyl derivative, on the other hand, is essentially planar with a bend angle of only 2.98 (4)° (Satam et al., 2011).
The major difference between the two molecules in the structure of the title compound is the rotation angle of the benzylidene phenyl rings with respect to the remainder of the molecules. The torsion angle of the C—H bonded phenyl group against the thiazolidin-4-one moiety is 5.01 (7)° in molecule A, and 17.41 (6)° in molecule B. All other torsion angles, that of the phenylimino and of the phenyl rings, differ only marginally between the two molecules as can be seen in an overlay of the two molecules, Figure 3.
The two crystallographically independent molecules are not only conformationally very similar, they are also related by a crystallographic pseudotranslation along the a-axis of the unit cell as shown in Figure 4. The structure can indeed also be successfully refined in a smaller cell with the a-axis length cut in half. If refined in this smaller volume setting the benzylidene phenyl ring has to be refined as being disordered over two equally occupied mutually incompatible sites closely resembling the overlays shown in Figures 3 and 4. R values and figures of merit for this disordered structure are actually lower (R1 = 0.0436 and wR2 = 0.1157) than those of the actual structure. Reflections due to the unit cell doubling are however clearly visible in the diffraction pattern and the lower R value can be readily explained by the omission of the weaker less accurately determined reflections in the average structure. Satellite reflections caused by the unit cell doubling have an average intensity of 5.8 σ, while all other data average to 18.9 times σ. The structure can thus be seen as a commensurately modulated structure with a q-vector of 0.5 along the a-axis direction with the phenyl torsion angles as the only major modulation parameter. Packing of the molecules is unexceptional and partially facilitated by C—H···π interactions (Figure 5) and some slipped π–π stacking, e.g. between the ring of C5A through C10A and the double bond of C3B and C4B. The closest contacts, from C10A towards C3Bi and C4Bi, are with 3.374 (2) and 3.295 (2) Å well within the range of substantial π–π interactions (symmetry operator (i): 1 - x,-y,-z). The distance between C10A and the centroid of the double bond is 3.267 Å.
Experimental
To a solution of 2-phenylimino-3-phenylthiazolidin-4-one (Abdel-Aziz et al., 2010) (1, 0.27 g, 1.0 mmol) and benzaldehyde (2, 0.11 g, 1.0 mmol) in ethanol (5.0 ml) was added aqueous potassium carbonate (15%, 3.0 ml) at room temperature. The reaction mixture was stirred at room temperature for 10 h. The progress of the reaction was monitored by TLC using 50% ethyl acetate in hexane as the eluent system. The precipitated solid was filtered, washed with water and dried. The crude product was purified by column chromatography using silica gel and 30% ethyl acetate in hexane as the eluent system to obtain pure product as a white solid. The compound was dissolved in hot methanol and the clear solution was left at room temperature for two days. The crystals formed were filtered off, washed with cold methanol and dried in vacuum. (Yield: 0.21 g, 58.3%; m.p.: 384–386 K; IR (KBr) cm-11680, 1640, 1591, 1370, 1264, 739, 695. 1H NMR (CDCl3): δ 7.83 (s, 1H), 7.57–7.53 (m, 2H), 7.50–7.34 (m, 10H), 7.19–7.15 (m, 1H), 6.99–6.96 (m, 2H); MS: ESI (m/z) 357.3 (M+H)+.
Refinement
All hydrogen atoms were added in calculated positions with a C—H bond distance of 0.95 Å and were refined with an isotropic displacement parameters of 1.2 times that of the equivalent isotropic displacement parameter of the adjacent carbon atom.
Figures
Fig. 1.
Synthetic pathway towards 5-benzylidene-3-phenyl-2-(phenylimino)thiazolidin-4-one (3)
Fig. 2.
Thermal ellipsoid style plot of the molecules of the title compound with atom numbering scheme. Probability levels for non-H atoms are at 50%.
Fig. 3.
Least squares overlay of the two crystallographically independent molecules. Red: molecule A, orange: molecule B.
Fig. 4.
View down the a-axis showing the pseudotranslation along this axis. Red: molecule A, orange: molecule B.
Fig. 5.
Packing view of (3). Blue dashed lines indicate significant C—H···π interactions.
Crystal data
| C22H16N2OS | F(000) = 1488 |
| Mr = 356.43 | Dx = 1.369 Mg m−3 |
| Monoclinic, P21/c | Melting point: 385 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.7814 (9) Å | Cell parameters from 6817 reflections |
| b = 32.779 (3) Å | θ = 2.3–30.7° |
| c = 9.8907 (8) Å | µ = 0.20 mm−1 |
| β = 98.392 (1)° | T = 100 K |
| V = 3458.0 (5) Å3 | Block, colourless |
| Z = 8 | 0.55 × 0.41 × 0.33 mm |
Data collection
| Bruker SMART APEX CCD diffractometer | 10081 independent reflections |
| Radiation source: fine-focus sealed tube | 7732 reflections with I > 2σ(I) |
| graphite | Rint = 0.025 |
| ω scans | θmax = 31.2°, θmin = 2.0° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −15→15 |
| Tmin = 0.632, Tmax = 0.746 | k = −47→47 |
| 23712 measured reflections | l = −14→8 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.125 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0616P)2 + 1.0979P] where P = (Fo2 + 2Fc2)/3 |
| 10081 reflections | (Δ/σ)max < 0.001 |
| 469 parameters | Δρmax = 0.46 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1A | 0.37254 (3) | 0.113655 (9) | 0.05977 (3) | 0.01578 (8) | |
| O1A | 0.20268 (10) | 0.06597 (3) | 0.33830 (10) | 0.0250 (2) | |
| N1A | 0.45402 (11) | 0.17270 (3) | 0.24759 (11) | 0.0165 (2) | |
| N2A | 0.34340 (10) | 0.11725 (3) | 0.31666 (11) | 0.0153 (2) | |
| C1A | 0.39888 (12) | 0.13899 (4) | 0.22017 (12) | 0.0146 (2) | |
| C2A | 0.26620 (13) | 0.08498 (4) | 0.26862 (13) | 0.0172 (2) | |
| C3A | 0.27051 (12) | 0.07864 (4) | 0.12030 (13) | 0.0162 (2) | |
| C4A | 0.19911 (13) | 0.04982 (4) | 0.05026 (13) | 0.0185 (3) | |
| H4A | 0.1531 | 0.0332 | 0.1041 | 0.022* | |
| C5A | 0.18112 (12) | 0.03998 (4) | −0.09501 (13) | 0.0168 (2) | |
| C6A | 0.23784 (14) | 0.06091 (4) | −0.19309 (14) | 0.0212 (3) | |
| H6A | 0.2930 | 0.0829 | −0.1656 | 0.025* | |
| C7A | 0.21457 (14) | 0.04992 (5) | −0.32984 (14) | 0.0236 (3) | |
| H7A | 0.2545 | 0.0643 | −0.3949 | 0.028* | |
| C8A | 0.13337 (13) | 0.01802 (4) | −0.37254 (14) | 0.0215 (3) | |
| H8A | 0.1172 | 0.0108 | −0.4665 | 0.026* | |
| C9A | 0.07626 (14) | −0.00315 (4) | −0.27756 (14) | 0.0235 (3) | |
| H9A | 0.0203 | −0.0249 | −0.3061 | 0.028* | |
| C10A | 0.10086 (13) | 0.00749 (4) | −0.14036 (14) | 0.0211 (3) | |
| H10A | 0.0625 | −0.0076 | −0.0756 | 0.025* | |
| C11A | 0.50187 (12) | 0.19422 (4) | 0.14188 (12) | 0.0147 (2) | |
| C12A | 0.59772 (13) | 0.17841 (4) | 0.07749 (14) | 0.0199 (3) | |
| H12A | 0.6301 | 0.1520 | 0.1011 | 0.024* | |
| C13A | 0.64633 (13) | 0.20112 (4) | −0.02127 (14) | 0.0223 (3) | |
| H13A | 0.7124 | 0.1903 | −0.0642 | 0.027* | |
| C14A | 0.59849 (14) | 0.23952 (4) | −0.05721 (14) | 0.0221 (3) | |
| H14A | 0.6312 | 0.2549 | −0.1253 | 0.026* | |
| C15A | 0.50274 (14) | 0.25535 (4) | 0.00649 (15) | 0.0237 (3) | |
| H15A | 0.4694 | 0.2815 | −0.0188 | 0.028* | |
| C16A | 0.45523 (13) | 0.23312 (4) | 0.10705 (14) | 0.0204 (3) | |
| H16A | 0.3911 | 0.2444 | 0.1520 | 0.024* | |
| C17A | 0.35528 (12) | 0.13144 (4) | 0.45589 (12) | 0.0152 (2) | |
| C18A | 0.46121 (13) | 0.12049 (4) | 0.54559 (13) | 0.0180 (3) | |
| H18A | 0.5230 | 0.1032 | 0.5166 | 0.022* | |
| C19A | 0.47534 (13) | 0.13529 (4) | 0.67901 (13) | 0.0207 (3) | |
| H19A | 0.5470 | 0.1279 | 0.7420 | 0.025* | |
| C20A | 0.38492 (13) | 0.16079 (4) | 0.72006 (13) | 0.0200 (3) | |
| H20A | 0.3958 | 0.1713 | 0.8105 | 0.024* | |
| C21A | 0.27842 (13) | 0.17101 (4) | 0.62922 (14) | 0.0206 (3) | |
| H21A | 0.2161 | 0.1880 | 0.6583 | 0.025* | |
| C22A | 0.26323 (12) | 0.15630 (4) | 0.49588 (13) | 0.0176 (2) | |
| H22A | 0.1909 | 0.1632 | 0.4332 | 0.021* | |
| S1B | 0.87010 (3) | 0.114166 (10) | 0.07134 (3) | 0.01700 (8) | |
| O1B | 0.68006 (10) | 0.07316 (3) | 0.34400 (10) | 0.0245 (2) | |
| N1B | 0.95837 (11) | 0.17253 (3) | 0.25832 (11) | 0.0172 (2) | |
| N2B | 0.83667 (10) | 0.11966 (3) | 0.32700 (11) | 0.0162 (2) | |
| C1B | 0.89758 (12) | 0.13981 (4) | 0.23112 (12) | 0.0150 (2) | |
| C2B | 0.74959 (13) | 0.09027 (4) | 0.27595 (13) | 0.0174 (2) | |
| C3B | 0.75375 (12) | 0.08386 (4) | 0.12809 (13) | 0.0168 (2) | |
| C4B | 0.67166 (13) | 0.05884 (4) | 0.05426 (14) | 0.0195 (3) | |
| H4B | 0.6119 | 0.0465 | 0.1030 | 0.023* | |
| C5B | 0.66074 (13) | 0.04779 (4) | −0.08999 (14) | 0.0195 (3) | |
| C6B | 0.75221 (14) | 0.05608 (4) | −0.17333 (14) | 0.0233 (3) | |
| H6B | 0.8259 | 0.0706 | −0.1370 | 0.028* | |
| C7B | 0.73604 (16) | 0.04329 (5) | −0.30865 (15) | 0.0276 (3) | |
| H7B | 0.7981 | 0.0494 | −0.3646 | 0.033* | |
| C8B | 0.62962 (16) | 0.02168 (5) | −0.36239 (15) | 0.0299 (3) | |
| H8B | 0.6199 | 0.0124 | −0.4543 | 0.036* | |
| C9B | 0.53774 (16) | 0.01368 (5) | −0.28224 (15) | 0.0323 (4) | |
| H9B | 0.4645 | −0.0009 | −0.3193 | 0.039* | |
| C10B | 0.55234 (14) | 0.02700 (4) | −0.14759 (15) | 0.0254 (3) | |
| H10B | 0.4879 | 0.0219 | −0.0938 | 0.031* | |
| C11B | 1.00800 (12) | 0.19307 (4) | 0.15183 (13) | 0.0162 (2) | |
| C12B | 1.10058 (13) | 0.17597 (4) | 0.08498 (14) | 0.0197 (3) | |
| H12B | 1.1308 | 0.1493 | 0.1081 | 0.024* | |
| C13B | 1.14876 (13) | 0.19803 (4) | −0.01569 (14) | 0.0225 (3) | |
| H13B | 1.2124 | 0.1864 | −0.0606 | 0.027* | |
| C14B | 1.10450 (14) | 0.23687 (4) | −0.05078 (14) | 0.0237 (3) | |
| H14B | 1.1374 | 0.2518 | −0.1199 | 0.028* | |
| C15B | 1.01203 (15) | 0.25394 (4) | 0.01530 (15) | 0.0251 (3) | |
| H15B | 0.9811 | 0.2805 | −0.0092 | 0.030* | |
| C16B | 0.96464 (14) | 0.23236 (4) | 0.11700 (14) | 0.0214 (3) | |
| H16B | 0.9024 | 0.2444 | 0.1632 | 0.026* | |
| C17B | 0.84998 (12) | 0.13379 (4) | 0.46609 (13) | 0.0164 (2) | |
| C18B | 0.95529 (13) | 0.12222 (4) | 0.55533 (14) | 0.0203 (3) | |
| H18B | 1.0158 | 0.1045 | 0.5260 | 0.024* | |
| C19B | 0.97085 (13) | 0.13698 (4) | 0.68874 (14) | 0.0229 (3) | |
| H19B | 1.0423 | 0.1293 | 0.7514 | 0.027* | |
| C20B | 0.88194 (13) | 0.16298 (4) | 0.73020 (14) | 0.0216 (3) | |
| H20B | 0.8935 | 0.1733 | 0.8209 | 0.026* | |
| C21B | 0.77625 (13) | 0.17394 (4) | 0.63987 (14) | 0.0215 (3) | |
| H21B | 0.7153 | 0.1915 | 0.6691 | 0.026* | |
| C22B | 0.75968 (13) | 0.15919 (4) | 0.50639 (14) | 0.0192 (3) | |
| H22B | 0.6875 | 0.1664 | 0.4440 | 0.023* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1A | 0.01872 (16) | 0.01662 (15) | 0.01241 (15) | −0.00329 (11) | 0.00371 (11) | −0.00114 (10) |
| O1A | 0.0353 (6) | 0.0255 (5) | 0.0152 (5) | −0.0138 (4) | 0.0071 (4) | −0.0009 (4) |
| N1A | 0.0200 (6) | 0.0162 (5) | 0.0136 (5) | −0.0024 (4) | 0.0030 (4) | 0.0006 (4) |
| N2A | 0.0190 (5) | 0.0150 (5) | 0.0122 (5) | −0.0037 (4) | 0.0030 (4) | 0.0003 (4) |
| C1A | 0.0159 (6) | 0.0158 (5) | 0.0121 (6) | 0.0010 (4) | 0.0019 (4) | 0.0009 (4) |
| C2A | 0.0212 (6) | 0.0158 (5) | 0.0145 (6) | −0.0023 (5) | 0.0023 (5) | 0.0004 (4) |
| C3A | 0.0194 (6) | 0.0152 (5) | 0.0142 (6) | −0.0018 (5) | 0.0032 (5) | 0.0009 (4) |
| C4A | 0.0229 (7) | 0.0184 (6) | 0.0146 (6) | −0.0044 (5) | 0.0034 (5) | 0.0004 (5) |
| C5A | 0.0182 (6) | 0.0174 (6) | 0.0146 (6) | −0.0008 (5) | 0.0016 (5) | −0.0010 (4) |
| C6A | 0.0262 (7) | 0.0214 (6) | 0.0159 (6) | −0.0055 (5) | 0.0025 (5) | −0.0007 (5) |
| C7A | 0.0270 (7) | 0.0292 (7) | 0.0149 (6) | −0.0051 (6) | 0.0038 (5) | 0.0006 (5) |
| C8A | 0.0227 (7) | 0.0274 (7) | 0.0134 (6) | 0.0015 (5) | −0.0010 (5) | −0.0030 (5) |
| C9A | 0.0247 (7) | 0.0238 (7) | 0.0211 (7) | −0.0040 (5) | 0.0003 (5) | −0.0054 (5) |
| C10A | 0.0233 (7) | 0.0214 (6) | 0.0187 (7) | −0.0043 (5) | 0.0038 (5) | −0.0023 (5) |
| C11A | 0.0164 (6) | 0.0150 (5) | 0.0123 (6) | −0.0035 (4) | 0.0009 (4) | −0.0006 (4) |
| C12A | 0.0196 (6) | 0.0200 (6) | 0.0203 (7) | 0.0013 (5) | 0.0036 (5) | 0.0028 (5) |
| C13A | 0.0201 (7) | 0.0265 (7) | 0.0212 (7) | −0.0006 (5) | 0.0058 (5) | 0.0010 (5) |
| C14A | 0.0246 (7) | 0.0244 (7) | 0.0171 (7) | −0.0063 (5) | 0.0025 (5) | 0.0045 (5) |
| C15A | 0.0279 (7) | 0.0180 (6) | 0.0251 (7) | −0.0002 (5) | 0.0034 (6) | 0.0055 (5) |
| C16A | 0.0229 (7) | 0.0178 (6) | 0.0211 (7) | 0.0001 (5) | 0.0057 (5) | 0.0004 (5) |
| C17A | 0.0194 (6) | 0.0155 (5) | 0.0107 (6) | −0.0041 (5) | 0.0023 (4) | −0.0002 (4) |
| C18A | 0.0179 (6) | 0.0192 (6) | 0.0168 (6) | −0.0003 (5) | 0.0024 (5) | 0.0019 (5) |
| C19A | 0.0199 (7) | 0.0267 (7) | 0.0146 (6) | −0.0033 (5) | −0.0007 (5) | 0.0019 (5) |
| C20A | 0.0253 (7) | 0.0227 (6) | 0.0123 (6) | −0.0071 (5) | 0.0034 (5) | −0.0006 (5) |
| C21A | 0.0220 (7) | 0.0229 (6) | 0.0180 (7) | −0.0021 (5) | 0.0070 (5) | −0.0024 (5) |
| C22A | 0.0173 (6) | 0.0192 (6) | 0.0162 (6) | −0.0015 (5) | 0.0018 (5) | 0.0007 (5) |
| S1B | 0.01907 (17) | 0.01822 (15) | 0.01406 (16) | −0.00326 (11) | 0.00352 (12) | −0.00196 (11) |
| O1B | 0.0306 (6) | 0.0250 (5) | 0.0192 (5) | −0.0105 (4) | 0.0082 (4) | −0.0019 (4) |
| N1B | 0.0190 (5) | 0.0175 (5) | 0.0152 (5) | −0.0015 (4) | 0.0025 (4) | 0.0002 (4) |
| N2B | 0.0185 (5) | 0.0175 (5) | 0.0130 (5) | −0.0028 (4) | 0.0031 (4) | −0.0006 (4) |
| C1B | 0.0151 (6) | 0.0171 (5) | 0.0126 (6) | 0.0011 (4) | 0.0016 (4) | 0.0000 (4) |
| C2B | 0.0198 (6) | 0.0153 (5) | 0.0169 (6) | −0.0017 (5) | 0.0027 (5) | −0.0011 (4) |
| C3B | 0.0191 (6) | 0.0161 (5) | 0.0156 (6) | −0.0014 (5) | 0.0039 (5) | −0.0009 (4) |
| C4B | 0.0214 (7) | 0.0178 (6) | 0.0193 (7) | −0.0028 (5) | 0.0035 (5) | −0.0006 (5) |
| C5B | 0.0236 (7) | 0.0170 (6) | 0.0172 (6) | −0.0012 (5) | 0.0003 (5) | −0.0004 (5) |
| C6B | 0.0258 (7) | 0.0234 (6) | 0.0208 (7) | −0.0048 (5) | 0.0035 (5) | −0.0043 (5) |
| C7B | 0.0349 (9) | 0.0298 (7) | 0.0188 (7) | −0.0031 (6) | 0.0065 (6) | −0.0029 (6) |
| C8B | 0.0370 (9) | 0.0350 (8) | 0.0156 (7) | −0.0032 (7) | −0.0028 (6) | −0.0031 (6) |
| C9B | 0.0336 (9) | 0.0403 (9) | 0.0202 (7) | −0.0108 (7) | −0.0054 (6) | −0.0034 (6) |
| C10B | 0.0266 (8) | 0.0277 (7) | 0.0207 (7) | −0.0041 (6) | −0.0009 (6) | 0.0011 (5) |
| C11B | 0.0169 (6) | 0.0171 (5) | 0.0139 (6) | −0.0042 (5) | 0.0004 (5) | −0.0004 (4) |
| C12B | 0.0181 (6) | 0.0201 (6) | 0.0208 (7) | 0.0008 (5) | 0.0021 (5) | 0.0028 (5) |
| C13B | 0.0184 (7) | 0.0284 (7) | 0.0209 (7) | −0.0006 (5) | 0.0040 (5) | 0.0012 (5) |
| C14B | 0.0243 (7) | 0.0271 (7) | 0.0190 (7) | −0.0066 (6) | 0.0012 (5) | 0.0054 (5) |
| C15B | 0.0302 (8) | 0.0189 (6) | 0.0257 (7) | −0.0014 (5) | 0.0020 (6) | 0.0046 (5) |
| C16B | 0.0233 (7) | 0.0189 (6) | 0.0222 (7) | 0.0002 (5) | 0.0043 (5) | 0.0001 (5) |
| C17B | 0.0196 (6) | 0.0171 (6) | 0.0126 (6) | −0.0041 (5) | 0.0027 (5) | −0.0001 (4) |
| C18B | 0.0198 (7) | 0.0226 (6) | 0.0182 (7) | 0.0003 (5) | 0.0019 (5) | 0.0000 (5) |
| C19B | 0.0213 (7) | 0.0291 (7) | 0.0171 (7) | −0.0021 (5) | −0.0012 (5) | 0.0021 (5) |
| C20B | 0.0235 (7) | 0.0270 (7) | 0.0142 (6) | −0.0068 (5) | 0.0028 (5) | −0.0019 (5) |
| C21B | 0.0212 (7) | 0.0260 (7) | 0.0180 (7) | −0.0008 (5) | 0.0056 (5) | −0.0022 (5) |
| C22B | 0.0181 (6) | 0.0221 (6) | 0.0171 (6) | −0.0008 (5) | 0.0023 (5) | −0.0001 (5) |
Geometric parameters (Å, °)
| S1A—C3A | 1.7544 (13) | S1B—C3B | 1.7553 (13) |
| S1A—C1A | 1.7764 (13) | S1B—C1B | 1.7762 (13) |
| O1A—C2A | 1.2124 (15) | O1B—C2B | 1.2151 (16) |
| N1A—C1A | 1.2653 (16) | N1B—C1B | 1.2654 (16) |
| N1A—C11A | 1.4192 (16) | N1B—C11B | 1.4189 (16) |
| N2A—C2A | 1.3863 (16) | N2B—C2B | 1.3879 (16) |
| N2A—C1A | 1.3940 (16) | N2B—C1B | 1.3960 (16) |
| N2A—C17A | 1.4414 (15) | N2B—C17B | 1.4387 (16) |
| C2A—C3A | 1.4891 (17) | C2B—C3B | 1.4846 (18) |
| C3A—C4A | 1.3452 (17) | C3B—C4B | 1.3418 (18) |
| C4A—C5A | 1.4577 (18) | C4B—C5B | 1.4600 (18) |
| C4A—H4A | 0.9500 | C4B—H4B | 0.9500 |
| C5A—C6A | 1.3997 (18) | C5B—C10B | 1.4003 (19) |
| C5A—C10A | 1.4039 (18) | C5B—C6B | 1.401 (2) |
| C6A—C7A | 1.3867 (19) | C6B—C7B | 1.389 (2) |
| C6A—H6A | 0.9500 | C6B—H6B | 0.9500 |
| C7A—C8A | 1.3893 (19) | C7B—C8B | 1.387 (2) |
| C7A—H7A | 0.9500 | C7B—H7B | 0.9500 |
| C8A—C9A | 1.383 (2) | C8B—C9B | 1.381 (2) |
| C8A—H8A | 0.9500 | C8B—H8B | 0.9500 |
| C9A—C10A | 1.3887 (19) | C9B—C10B | 1.388 (2) |
| C9A—H9A | 0.9500 | C9B—H9B | 0.9500 |
| C10A—H10A | 0.9500 | C10B—H10B | 0.9500 |
| C11A—C12A | 1.3909 (18) | C11B—C12B | 1.3930 (19) |
| C11A—C16A | 1.3952 (18) | C11B—C16B | 1.3960 (18) |
| C12A—C13A | 1.3902 (19) | C12B—C13B | 1.3912 (19) |
| C12A—H12A | 0.9500 | C12B—H12B | 0.9500 |
| C13A—C14A | 1.387 (2) | C13B—C14B | 1.386 (2) |
| C13A—H13A | 0.9500 | C13B—H13B | 0.9500 |
| C14A—C15A | 1.386 (2) | C14B—C15B | 1.387 (2) |
| C14A—H14A | 0.9500 | C14B—H14B | 0.9500 |
| C15A—C16A | 1.3898 (19) | C15B—C16B | 1.3868 (19) |
| C15A—H15A | 0.9500 | C15B—H15B | 0.9500 |
| C16A—H16A | 0.9500 | C16B—H16B | 0.9500 |
| C17A—C22A | 1.3855 (18) | C17B—C22B | 1.3831 (19) |
| C17A—C18A | 1.3873 (18) | C17B—C18B | 1.3854 (18) |
| C18A—C19A | 1.3933 (18) | C18B—C19B | 1.3925 (19) |
| C18A—H18A | 0.9500 | C18B—H18B | 0.9500 |
| C19A—C20A | 1.389 (2) | C19B—C20B | 1.389 (2) |
| C19A—H19A | 0.9500 | C19B—H19B | 0.9500 |
| C20A—C21A | 1.3917 (19) | C20B—C21B | 1.389 (2) |
| C20A—H20A | 0.9500 | C20B—H20B | 0.9500 |
| C21A—C22A | 1.3914 (18) | C21B—C22B | 1.3928 (18) |
| C21A—H21A | 0.9500 | C21B—H21B | 0.9500 |
| C22A—H22A | 0.9500 | C22B—H22B | 0.9500 |
| C3A—S1A—C1A | 91.58 (6) | C3B—S1B—C1B | 91.01 (6) |
| C1A—N1A—C11A | 119.08 (11) | C1B—N1B—C11B | 118.95 (11) |
| C2A—N2A—C1A | 116.85 (10) | C2B—N2B—C1B | 116.21 (10) |
| C2A—N2A—C17A | 122.60 (10) | C2B—N2B—C17B | 122.62 (11) |
| C1A—N2A—C17A | 120.13 (10) | C1B—N2B—C17B | 120.41 (10) |
| N1A—C1A—N2A | 122.34 (11) | N1B—C1B—N2B | 122.48 (11) |
| N1A—C1A—S1A | 127.49 (10) | N1B—C1B—S1B | 127.18 (10) |
| N2A—C1A—S1A | 110.10 (9) | N2B—C1B—S1B | 110.28 (9) |
| O1A—C2A—N2A | 123.92 (12) | O1B—C2B—N2B | 123.92 (12) |
| O1A—C2A—C3A | 126.13 (12) | O1B—C2B—C3B | 126.16 (12) |
| N2A—C2A—C3A | 109.91 (11) | N2B—C2B—C3B | 109.91 (11) |
| C4A—C3A—C2A | 120.50 (12) | C4B—C3B—C2B | 120.68 (12) |
| C4A—C3A—S1A | 128.61 (10) | C4B—C3B—S1B | 128.12 (10) |
| C2A—C3A—S1A | 110.86 (9) | C2B—C3B—S1B | 111.14 (9) |
| C3A—C4A—C5A | 130.30 (12) | C3B—C4B—C5B | 129.51 (13) |
| C3A—C4A—H4A | 114.8 | C3B—C4B—H4B | 115.2 |
| C5A—C4A—H4A | 114.8 | C5B—C4B—H4B | 115.2 |
| C6A—C5A—C10A | 117.50 (12) | C10B—C5B—C6B | 118.19 (13) |
| C6A—C5A—C4A | 124.47 (12) | C10B—C5B—C4B | 117.50 (13) |
| C10A—C5A—C4A | 118.03 (12) | C6B—C5B—C4B | 124.30 (13) |
| C7A—C6A—C5A | 120.90 (13) | C7B—C6B—C5B | 120.54 (14) |
| C7A—C6A—H6A | 119.6 | C7B—C6B—H6B | 119.7 |
| C5A—C6A—H6A | 119.6 | C5B—C6B—H6B | 119.7 |
| C6A—C7A—C8A | 120.55 (13) | C8B—C7B—C6B | 120.27 (14) |
| C6A—C7A—H7A | 119.7 | C8B—C7B—H7B | 119.9 |
| C8A—C7A—H7A | 119.7 | C6B—C7B—H7B | 119.9 |
| C9A—C8A—C7A | 119.64 (12) | C9B—C8B—C7B | 119.97 (14) |
| C9A—C8A—H8A | 120.2 | C9B—C8B—H8B | 120.0 |
| C7A—C8A—H8A | 120.2 | C7B—C8B—H8B | 120.0 |
| C8A—C9A—C10A | 119.83 (13) | C8B—C9B—C10B | 120.04 (14) |
| C8A—C9A—H9A | 120.1 | C8B—C9B—H9B | 120.0 |
| C10A—C9A—H9A | 120.1 | C10B—C9B—H9B | 120.0 |
| C9A—C10A—C5A | 121.57 (13) | C9B—C10B—C5B | 120.95 (14) |
| C9A—C10A—H10A | 119.2 | C9B—C10B—H10B | 119.5 |
| C5A—C10A—H10A | 119.2 | C5B—C10B—H10B | 119.5 |
| C12A—C11A—C16A | 119.51 (12) | C12B—C11B—C16B | 119.44 (12) |
| C12A—C11A—N1A | 121.76 (11) | C12B—C11B—N1B | 122.37 (12) |
| C16A—C11A—N1A | 118.65 (11) | C16B—C11B—N1B | 118.16 (12) |
| C13A—C12A—C11A | 120.24 (12) | C13B—C12B—C11B | 119.92 (13) |
| C13A—C12A—H12A | 119.9 | C13B—C12B—H12B | 120.0 |
| C11A—C12A—H12A | 119.9 | C11B—C12B—H12B | 120.0 |
| C14A—C13A—C12A | 120.13 (13) | C14B—C13B—C12B | 120.39 (13) |
| C14A—C13A—H13A | 119.9 | C14B—C13B—H13B | 119.8 |
| C12A—C13A—H13A | 119.9 | C12B—C13B—H13B | 119.8 |
| C15A—C14A—C13A | 119.81 (13) | C13B—C14B—C15B | 119.84 (13) |
| C15A—C14A—H14A | 120.1 | C13B—C14B—H14B | 120.1 |
| C13A—C14A—H14A | 120.1 | C15B—C14B—H14B | 120.1 |
| C14A—C15A—C16A | 120.37 (13) | C16B—C15B—C14B | 120.13 (13) |
| C14A—C15A—H15A | 119.8 | C16B—C15B—H15B | 119.9 |
| C16A—C15A—H15A | 119.8 | C14B—C15B—H15B | 119.9 |
| C15A—C16A—C11A | 119.92 (13) | C15B—C16B—C11B | 120.27 (13) |
| C15A—C16A—H16A | 120.0 | C15B—C16B—H16B | 119.9 |
| C11A—C16A—H16A | 120.0 | C11B—C16B—H16B | 119.9 |
| C22A—C17A—C18A | 121.78 (12) | C22B—C17B—C18B | 121.81 (12) |
| C22A—C17A—N2A | 119.68 (11) | C22B—C17B—N2B | 119.55 (12) |
| C18A—C17A—N2A | 118.52 (11) | C18B—C17B—N2B | 118.63 (12) |
| C17A—C18A—C19A | 118.80 (12) | C17B—C18B—C19B | 118.86 (13) |
| C17A—C18A—H18A | 120.6 | C17B—C18B—H18B | 120.6 |
| C19A—C18A—H18A | 120.6 | C19B—C18B—H18B | 120.6 |
| C20A—C19A—C18A | 120.13 (13) | C20B—C19B—C18B | 120.02 (13) |
| C20A—C19A—H19A | 119.9 | C20B—C19B—H19B | 120.0 |
| C18A—C19A—H19A | 119.9 | C18B—C19B—H19B | 120.0 |
| C19A—C20A—C21A | 120.29 (12) | C19B—C20B—C21B | 120.38 (13) |
| C19A—C20A—H20A | 119.9 | C19B—C20B—H20B | 119.8 |
| C21A—C20A—H20A | 119.9 | C21B—C20B—H20B | 119.8 |
| C22A—C21A—C20A | 120.02 (13) | C20B—C21B—C22B | 119.97 (13) |
| C22A—C21A—H21A | 120.0 | C20B—C21B—H21B | 120.0 |
| C20A—C21A—H21A | 120.0 | C22B—C21B—H21B | 120.0 |
| C17A—C22A—C21A | 118.97 (12) | C17B—C22B—C21B | 118.94 (13) |
| C17A—C22A—H22A | 120.5 | C17B—C22B—H22B | 120.5 |
| C21A—C22A—H22A | 120.5 | C21B—C22B—H22B | 120.5 |
| C11A—N1A—C1A—N2A | −176.35 (11) | C11B—N1B—C1B—N2B | −174.35 (11) |
| C11A—N1A—C1A—S1A | 0.28 (18) | C11B—N1B—C1B—S1B | 2.64 (18) |
| C2A—N2A—C1A—N1A | 168.24 (12) | C2B—N2B—C1B—N1B | 164.86 (12) |
| C17A—N2A—C1A—N1A | −4.49 (19) | C17B—N2B—C1B—N1B | −5.38 (19) |
| C2A—N2A—C1A—S1A | −8.91 (14) | C2B—N2B—C1B—S1B | −12.58 (14) |
| C17A—N2A—C1A—S1A | 178.36 (9) | C17B—N2B—C1B—S1B | 177.18 (9) |
| C3A—S1A—C1A—N1A | −169.45 (13) | C3B—S1B—C1B—N1B | −166.12 (13) |
| C3A—S1A—C1A—N2A | 7.52 (10) | C3B—S1B—C1B—N2B | 11.17 (10) |
| C1A—N2A—C2A—O1A | −172.66 (13) | C1B—N2B—C2B—O1B | −172.17 (13) |
| C17A—N2A—C2A—O1A | −0.1 (2) | C17B—N2B—C2B—O1B | −2.2 (2) |
| C1A—N2A—C2A—C3A | 5.38 (16) | C1B—N2B—C2B—C3B | 6.89 (16) |
| C17A—N2A—C2A—C3A | 177.92 (11) | C17B—N2B—C2B—C3B | 176.89 (11) |
| O1A—C2A—C3A—C4A | 0.5 (2) | O1B—C2B—C3B—C4B | 3.7 (2) |
| N2A—C2A—C3A—C4A | −177.54 (12) | N2B—C2B—C3B—C4B | −175.32 (12) |
| O1A—C2A—C3A—S1A | 178.68 (12) | O1B—C2B—C3B—S1B | −178.94 (12) |
| N2A—C2A—C3A—S1A | 0.69 (14) | N2B—C2B—C3B—S1B | 2.02 (14) |
| C1A—S1A—C3A—C4A | 173.38 (13) | C1B—S1B—C3B—C4B | 169.61 (13) |
| C1A—S1A—C3A—C2A | −4.67 (10) | C1B—S1B—C3B—C2B | −7.49 (10) |
| C2A—C3A—C4A—C5A | 175.30 (13) | C2B—C3B—C4B—C5B | −178.83 (13) |
| S1A—C3A—C4A—C5A | −2.6 (2) | S1B—C3B—C4B—C5B | 4.3 (2) |
| C3A—C4A—C5A—C6A | −1.2 (2) | C3B—C4B—C5B—C10B | −168.33 (14) |
| C3A—C4A—C5A—C10A | 179.25 (14) | C3B—C4B—C5B—C6B | 12.7 (2) |
| C10A—C5A—C6A—C7A | 0.4 (2) | C10B—C5B—C6B—C7B | −1.1 (2) |
| C4A—C5A—C6A—C7A | −179.19 (13) | C4B—C5B—C6B—C7B | 177.88 (13) |
| C5A—C6A—C7A—C8A | 0.6 (2) | C5B—C6B—C7B—C8B | −0.8 (2) |
| C6A—C7A—C8A—C9A | −0.6 (2) | C6B—C7B—C8B—C9B | 1.6 (2) |
| C7A—C8A—C9A—C10A | −0.3 (2) | C7B—C8B—C9B—C10B | −0.5 (3) |
| C8A—C9A—C10A—C5A | 1.2 (2) | C8B—C9B—C10B—C5B | −1.5 (2) |
| C6A—C5A—C10A—C9A | −1.3 (2) | C6B—C5B—C10B—C9B | 2.2 (2) |
| C4A—C5A—C10A—C9A | 178.32 (13) | C4B—C5B—C10B—C9B | −176.82 (14) |
| C1A—N1A—C11A—C12A | −64.30 (17) | C1B—N1B—C11B—C12B | −64.65 (17) |
| C1A—N1A—C11A—C16A | 118.93 (14) | C1B—N1B—C11B—C16B | 117.54 (14) |
| C16A—C11A—C12A—C13A | −0.3 (2) | C16B—C11B—C12B—C13B | −0.1 (2) |
| N1A—C11A—C12A—C13A | −177.02 (12) | N1B—C11B—C12B—C13B | −177.89 (12) |
| C11A—C12A—C13A—C14A | −0.7 (2) | C11B—C12B—C13B—C14B | −0.5 (2) |
| C12A—C13A—C14A—C15A | 0.6 (2) | C12B—C13B—C14B—C15B | 0.3 (2) |
| C13A—C14A—C15A—C16A | 0.6 (2) | C13B—C14B—C15B—C16B | 0.5 (2) |
| C14A—C15A—C16A—C11A | −1.6 (2) | C14B—C15B—C16B—C11B | −1.1 (2) |
| C12A—C11A—C16A—C15A | 1.4 (2) | C12B—C11B—C16B—C15B | 0.9 (2) |
| N1A—C11A—C16A—C15A | 178.27 (12) | N1B—C11B—C16B—C15B | 178.82 (12) |
| C2A—N2A—C17A—C22A | −79.01 (16) | C2B—N2B—C17B—C22B | −73.31 (16) |
| C1A—N2A—C17A—C22A | 93.29 (15) | C1B—N2B—C17B—C22B | 96.28 (15) |
| C2A—N2A—C17A—C18A | 102.61 (15) | C2B—N2B—C17B—C18B | 108.23 (15) |
| C1A—N2A—C17A—C18A | −85.09 (15) | C1B—N2B—C17B—C18B | −82.17 (16) |
| C22A—C17A—C18A—C19A | −0.57 (19) | C22B—C17B—C18B—C19B | −0.7 (2) |
| N2A—C17A—C18A—C19A | 177.78 (11) | N2B—C17B—C18B—C19B | 177.73 (12) |
| C17A—C18A—C19A—C20A | −0.5 (2) | C17B—C18B—C19B—C20B | −0.3 (2) |
| C18A—C19A—C20A—C21A | 1.4 (2) | C18B—C19B—C20B—C21B | 1.0 (2) |
| C19A—C20A—C21A—C22A | −1.2 (2) | C19B—C20B—C21B—C22B | −0.7 (2) |
| C18A—C17A—C22A—C21A | 0.78 (19) | C18B—C17B—C22B—C21B | 0.9 (2) |
| N2A—C17A—C22A—C21A | −177.56 (11) | N2B—C17B—C22B—C21B | −177.47 (12) |
| C20A—C21A—C22A—C17A | 0.1 (2) | C20B—C21B—C22B—C17B | −0.2 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C12A—H12A···S1B | 0.95 | 2.92 | 3.6214 (15) | 131 |
| C12A—H12A···C2B | 0.95 | 2.85 | 3.7340 (19) | 156 |
| C12A—H12A···C3B | 0.95 | 2.59 | 3.5270 (19) | 167 |
| C12B—H12B···S1Ai | 0.95 | 2.96 | 3.6118 (15) | 127 |
| C12B—H12B···C3Ai | 0.95 | 2.76 | 3.6700 (19) | 162 |
Symmetry codes: (i) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2385).
References
- Abdel-Aziz, H. A., El-Zahabi, H. S. A. & Dawood, K. M. (2010). Eur. J. Med. Chem. 45, 2427–2432. [DOI] [PubMed]
- Babu, B., Lee, M., Lee, L., Strobel, R., Brockway, O., Nickols, A., Sjoholm, R., Tzou, S., Chavda, S., Desta, D., Fraley, G., Siegfried, A., Pennington, W., Hartley, H. M., Westbrook, C., Mooberry, S. L., Kiakos, K., Hartley, J. A. & Lee, M. (2011). Bioorg. Med. Chem. 19, 2359–2367. [DOI] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chavda, S., Davis, R., Ferguson, A., Riddering, C., Dittenhafer, K., Mackay, H., Babu, B., Lee, M., Siegfried, A., Pennington, W., Shadfan, M., Mooberry, S., Mishra, B. K. & Pati, H. N. (2009). Lett. Drug Des. Discov. pp. 531–537.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Satam, V. S., Bandi, R. K., Behera, A. K., Mishra, B. K., Brockway, O., Tzou, S., Zeller, M., Pati, H. N. & Lee, M. (2011). Lett. Drug. Design Discov. In the press. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811023658/gk2385sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023658/gk2385Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811023658/gk2385Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report







