Abstract
The asymmetric unit of the title xanthene compound, C25H30O5, contains two molecules in which the pyran ring and the dimethoxyphenyl ring are nearly perpendicular to one another [dihedral angles = 86.81 (8) and 84.45 (9)°]. One of the methoxy groups in one molecule is twisted away from the phenyl ring [C—O—C—C torsion angle = −103.40 (16)°]. The pyran ring adopts a boat conformation whereas the two fused cyclohexane rings adopt envelope conformations in both molecules. In the crystal, molecules are linked into a three-dimensional network by C—H⋯O hydrogen bonds.
Related literature
For applications of xanthene derivatives, see: Lambert et al. (1997 ▶); Hideo (1981 ▶); Poupelin et al. (1978 ▶); Menchen et al. (2003 ▶); Banerjee & Mukherjee (1981 ▶); Ravindranath & Seshadri (1973) ▶. For the synthesis of xanthene and 1,8-dioxooctahydroxanthene derivatives with or without the use of a catalyst, see: Fan et al. (2005 ▶); Jin et al. (2005 ▶); Srihari et al. (2008 ▶). For a related structure, see: Mehdi et al. (2011 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶). For ring conformations, see: Cremer & Pople (1975 ▶).
Experimental
Crystal data
C25H30O5
M r = 410.49
Triclinic,

a = 9.4895 (7) Å
b = 10.2283 (7) Å
c = 23.3218 (16) Å
α = 85.872 (4)°
β = 86.537 (4)°
γ = 74.425 (3)°
V = 2172.9 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 100 K
0.42 × 0.39 × 0.20 mm
Data collection
Bruker SMART APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.965, T max = 0.983
44431 measured reflections
11497 independent reflections
8871 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.127
S = 1.01
11497 reflections
553 parameters
H-atom parameters constrained
Δρmax = 0.42 e Å−3
Δρmin = −0.28 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811023014/hb5885sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023014/hb5885Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811023014/hb5885Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10A—H10A⋯O4Ai | 0.99 | 2.38 | 3.2817 (19) | 152 |
| C18A—H18A⋯O2Aii | 0.95 | 2.35 | 3.2943 (19) | 176 |
| C18B—H18B⋯O2Biii | 0.95 | 2.45 | 3.4000 (19) | 175 |
| C20A—H20A⋯O3Aiv | 0.98 | 2.59 | 3.4144 (19) | 142 |
| C24B—H24D⋯O4Av | 0.98 | 2.48 | 3.453 (2) | 171 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  .
.
Acknowledgments
SHM, OS and RMG would like to acknowledge Universiti Sains Malaysia (USM) for the University Grant 1001/PTEKIND/8140152. HKF and CSY also thank USM for the Research University Grant 1001/PFIZIK/811160.
supplementary crystallographic information
Comment
Synthesis of xanthene derivatives is currently of great interest and has attracted considerable attention by chemists because of their biological and pharmaceutical properties as antiviral (Lambert et al., 1997), antibacterial (Hideo, 1981), and anti-inflammatory (Poupelin et al., 1978). Xanthenes derivatives also find use as dyes, fluorescent material for visualization of biomolecules and in laser technologies (Menchen et al., 2003; Banerjee & Mukherjee, 1981). Several natural occurring polycyclic compounds containing xanthene nucleus are also reported (Ravindranath & Seshadri, 1973). Synthesis of xanthene and 1,8-dioxooctahydroxanthene derivatives have been reported in literature under the different reaction conditions with or without the use of catalyst (Fan et al., 2005; Jin et al., 2005; Srihari et al., 2008). Here we are reporting the synthesis of title compound, (I). The structure of the title compound was established on the basis of its IR, 1H NMR, 13C NMR spectra and finally confirmed by X-ray analysis.
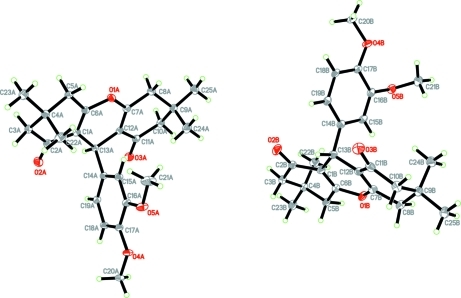
The asymmetric unit of (I) consists of two crystallographically independent molecules, A and B (Fig. 1). The geometric parameters and the conformations are very similar to the previously reported structure (Mehdi et al., 2011). However, only the dimethoxyphenyl of molecule B is different from others. The dimethoxyphenyl grouping in molecule A is almost planar [C20A–O4A–C17A–C18A = -1.9 (2)° and C21A–O5A–C16A–C17A = 4.6 (2)°] whereas for molecule B is not planar [C20B–O4B–C17B–C18B = -6.1 (2)° and C21B–O5B–C16B–C15B = -103.40 (16)°]. Similarly, the mean plane of pyran ring and the dimethoxyphenyl ring for both A and B molecules are nearly perpendicular to one another with the dihedral angles between them being 86.81 (8) and 84.45 (9)°, respectively. For both molecules, the two cyclohexane rings adopt envelope conformations whereas the pyran ring adopts a boat conformation (Cremer & Pople, 1975).
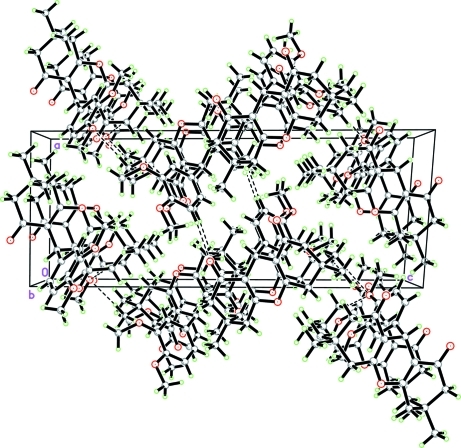
In the crystal, the molecules are linked into a three-dimensional network (Fig. 2) by C—H···O hydrogen bonds (Table 1).
Experimental
A mixture of dimedone (1.40 g m, 10 mmol) and veratraldehyde (1.66 g m, 10 mmol) was heated in 25 ml of glacial acetic acid for three hours. Completion of the reaction was monitored by TLC. The reaction mixture was dried on rotary evaporator under reduced pressure. The crude mixture thus obtained was successively treated with di ethyl ether chloroform and ethanol. The ethanol fraction on crystallization furnished yellow blocks of (I) (m pt. 208 °C, Yield 90%). IR (KBr) νmax: 3085, 3005, 2956, 2929, 2870, 2822, 1667, 1624, 1515, 1467, 1419, 1358, 1227, 1141, 1105, 1023, 851, 830, 751 cm-1. 1H NMR (300 MHz, DMSO-d6): δ 6.75–6.92 (3H, m, Aromatic protons), 4.72 (1H, s), 3.83 (3H, s), 3.78 (3H, s), 2.40 (4H, s), 2.17 (4H, s), 1.05 (6H, s), 1.02 (6H, s). 13C NMR (75 MHz, DMSO-d6): δ 27.2, 36.8, 42.4, 52.2, 56.4, 112.2, 114.6, 115.2, 123.4, 136.8, 146.6, 148.4, 198.2. IR spectrum was taken on Shimadzu IR-408 Perkin Elmer 1800 (FTIR). 1H NMR was recorded on Bruker Avance 300 MHz with TMS as an internal standard and 75 MHz for 13C NMR. Spectrum was recorded in DMSO-d6. The melting point was taken on Thermo Fisher digital melting point apparatus of IA9000 series and is uncorrected.
Refinement
All hydrogen atoms were positioned geomatrically [C–H = 0.95–1.00 Å] and refined using a riding model, with Uiso(H) = 1.2 or 1.5 Ueq(C). A rotating-group model were applied for methyl groups.
Figures
Fig. 1.
The molecular structure of (I) with 50% probability ellipsoids for non-H atoms.
Fig. 2.
The packing of (I), viewed down b axis, showing molecules linked into a three-dimensional network. Hydrogen bonds are shown as dashed lines.
Crystal data
| C25H30O5 | Z = 4 |
| Mr = 410.49 | F(000) = 880 |
| Triclinic, P1 | Dx = 1.255 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.4895 (7) Å | Cell parameters from 9984 reflections |
| b = 10.2283 (7) Å | θ = 2.2–30.0° |
| c = 23.3218 (16) Å | µ = 0.09 mm−1 |
| α = 85.872 (4)° | T = 100 K |
| β = 86.537 (4)° | Block, yellow |
| γ = 74.425 (3)° | 0.42 × 0.39 × 0.20 mm |
| V = 2172.9 (3) Å3 |
Data collection
| Bruker SMART APEXII CCD diffractometer | 11497 independent reflections |
| Radiation source: fine-focus sealed tube | 8871 reflections with I > 2σ(I) |
| graphite | Rint = 0.040 |
| φ and ω scans | θmax = 29.0°, θmin = 0.9° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −12→12 |
| Tmin = 0.965, Tmax = 0.983 | k = −13→13 |
| 44431 measured reflections | l = −31→31 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.127 | H-atom parameters constrained |
| S = 1.01 | w = 1/[σ2(Fo2) + (0.0552P)2 + 1.1161P] where P = (Fo2 + 2Fc2)/3 |
| 11497 reflections | (Δ/σ)max = 0.001 |
| 553 parameters | Δρmax = 0.42 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.51121 (11) | 0.01893 (10) | 0.13242 (4) | 0.0167 (2) | |
| O2A | 0.30935 (12) | 0.27866 (12) | −0.03360 (5) | 0.0222 (2) | |
| O3A | −0.00427 (11) | 0.10581 (11) | 0.13190 (5) | 0.0194 (2) | |
| O4A | 0.03071 (11) | 0.72025 (10) | 0.14408 (5) | 0.0170 (2) | |
| O5A | 0.26685 (12) | 0.56744 (11) | 0.18780 (5) | 0.0212 (2) | |
| C1A | 0.39692 (15) | 0.15892 (14) | 0.05242 (6) | 0.0130 (3) | |
| C2A | 0.41570 (16) | 0.22544 (14) | −0.00480 (6) | 0.0146 (3) | |
| C3A | 0.56930 (16) | 0.22129 (15) | −0.02765 (6) | 0.0163 (3) | |
| H3AA | 0.5662 | 0.3059 | −0.0516 | 0.020* | |
| H3AB | 0.6034 | 0.1442 | −0.0529 | 0.020* | |
| C4A | 0.68098 (16) | 0.20643 (15) | 0.01876 (6) | 0.0156 (3) | |
| C5A | 0.67052 (16) | 0.08554 (15) | 0.06052 (6) | 0.0153 (3) | |
| H5AA | 0.7132 | −0.0002 | 0.0412 | 0.018* | |
| H5AB | 0.7289 | 0.0847 | 0.0944 | 0.018* | |
| C6A | 0.51680 (16) | 0.09159 (14) | 0.08028 (6) | 0.0137 (3) | |
| C7A | 0.37572 (16) | 0.00791 (14) | 0.15400 (6) | 0.0147 (3) | |
| C8A | 0.38861 (16) | −0.08773 (15) | 0.20637 (6) | 0.0176 (3) | |
| H8AA | 0.4737 | −0.0831 | 0.2280 | 0.021* | |
| H8AB | 0.4064 | −0.1818 | 0.1943 | 0.021* | |
| C9A | 0.24950 (16) | −0.05339 (15) | 0.24603 (6) | 0.0167 (3) | |
| C10A | 0.11824 (17) | −0.04029 (15) | 0.20912 (6) | 0.0173 (3) | |
| H10A | 0.1195 | −0.1320 | 0.1977 | 0.021* | |
| H10B | 0.0276 | −0.0067 | 0.2330 | 0.021* | |
| C11A | 0.11168 (16) | 0.05299 (14) | 0.15528 (6) | 0.0150 (3) | |
| C12A | 0.25000 (16) | 0.07503 (14) | 0.12990 (6) | 0.0133 (3) | |
| C13A | 0.24385 (15) | 0.17523 (14) | 0.07831 (6) | 0.0129 (3) | |
| H13A | 0.1811 | 0.1549 | 0.0490 | 0.015* | |
| C14A | 0.18013 (15) | 0.32153 (14) | 0.09553 (6) | 0.0132 (3) | |
| C15A | 0.25380 (16) | 0.37360 (14) | 0.13478 (6) | 0.0151 (3) | |
| H15A | 0.3402 | 0.3170 | 0.1511 | 0.018* | |
| C16A | 0.20193 (16) | 0.50682 (15) | 0.15011 (6) | 0.0150 (3) | |
| C17A | 0.07316 (15) | 0.59013 (14) | 0.12625 (6) | 0.0140 (3) | |
| C18A | −0.00021 (16) | 0.53859 (14) | 0.08766 (6) | 0.0151 (3) | |
| H18A | −0.0873 | 0.5945 | 0.0716 | 0.018* | |
| C19A | 0.05405 (15) | 0.40429 (14) | 0.07237 (6) | 0.0143 (3) | |
| H19A | 0.0037 | 0.3694 | 0.0457 | 0.017* | |
| C20A | −0.09712 (16) | 0.80929 (15) | 0.11921 (7) | 0.0169 (3) | |
| H20A | −0.1143 | 0.9000 | 0.1337 | 0.025* | |
| H20B | −0.1820 | 0.7738 | 0.1296 | 0.025* | |
| H20C | −0.0825 | 0.8150 | 0.0772 | 0.025* | |
| C21A | 0.4031 (2) | 0.48823 (19) | 0.20905 (9) | 0.0326 (4) | |
| H21A | 0.4398 | 0.5414 | 0.2351 | 0.049* | |
| H21B | 0.4740 | 0.4631 | 0.1767 | 0.049* | |
| H21C | 0.3891 | 0.4057 | 0.2300 | 0.049* | |
| C22A | 0.65038 (18) | 0.33737 (16) | 0.05122 (7) | 0.0223 (3) | |
| H22A | 0.6608 | 0.4127 | 0.0244 | 0.033* | |
| H22B | 0.7203 | 0.3249 | 0.0817 | 0.033* | |
| H22C | 0.5505 | 0.3580 | 0.0683 | 0.033* | |
| C23A | 0.83548 (17) | 0.17712 (16) | −0.00895 (7) | 0.0203 (3) | |
| H23A | 0.8439 | 0.2542 | −0.0352 | 0.031* | |
| H23B | 0.8548 | 0.0949 | −0.0305 | 0.031* | |
| H23C | 0.9068 | 0.1634 | 0.0211 | 0.031* | |
| C24A | 0.23732 (19) | 0.07937 (17) | 0.27513 (7) | 0.0244 (3) | |
| H24A | 0.1489 | 0.1000 | 0.3004 | 0.037* | |
| H24B | 0.2317 | 0.1537 | 0.2457 | 0.037* | |
| H24C | 0.3236 | 0.0692 | 0.2979 | 0.037* | |
| C25A | 0.25761 (18) | −0.16875 (17) | 0.29255 (7) | 0.0235 (3) | |
| H25A | 0.1710 | −0.1456 | 0.3188 | 0.035* | |
| H25B | 0.3460 | −0.1813 | 0.3142 | 0.035* | |
| H25C | 0.2610 | −0.2531 | 0.2744 | 0.035* | |
| O1B | −0.06753 (11) | 0.41491 (11) | 0.63022 (4) | 0.0175 (2) | |
| O2B | 0.17623 (12) | 0.22238 (12) | 0.46132 (5) | 0.0258 (3) | |
| O3B | 0.43753 (12) | 0.39314 (12) | 0.61832 (5) | 0.0246 (3) | |
| O4B | 0.48890 (11) | −0.23710 (10) | 0.66652 (5) | 0.0195 (2) | |
| O5B | 0.23066 (11) | −0.09300 (11) | 0.71319 (4) | 0.0182 (2) | |
| C1B | 0.06703 (16) | 0.30810 (15) | 0.54858 (6) | 0.0156 (3) | |
| C2B | 0.06302 (17) | 0.26311 (15) | 0.49034 (6) | 0.0177 (3) | |
| C3B | −0.08492 (17) | 0.27615 (16) | 0.46638 (6) | 0.0195 (3) | |
| H3BA | −0.0750 | 0.2027 | 0.4398 | 0.023* | |
| H3BB | −0.1144 | 0.3638 | 0.4436 | 0.023* | |
| C4B | −0.20776 (16) | 0.26930 (15) | 0.51161 (6) | 0.0167 (3) | |
| C5B | −0.20940 (16) | 0.37152 (15) | 0.55704 (6) | 0.0173 (3) | |
| H5BA | −0.2512 | 0.4648 | 0.5405 | 0.021* | |
| H5BB | −0.2736 | 0.3555 | 0.5904 | 0.021* | |
| C6B | −0.06074 (16) | 0.36086 (15) | 0.57727 (6) | 0.0157 (3) | |
| C7B | 0.05787 (16) | 0.44058 (15) | 0.64789 (6) | 0.0160 (3) | |
| C8B | 0.02695 (17) | 0.52491 (16) | 0.69919 (7) | 0.0187 (3) | |
| H8BA | −0.0426 | 0.4924 | 0.7262 | 0.022* | |
| H8BB | −0.0201 | 0.6205 | 0.6867 | 0.022* | |
| C9B | 0.16583 (17) | 0.51902 (15) | 0.73048 (6) | 0.0180 (3) | |
| C10B | 0.28224 (17) | 0.54196 (16) | 0.68493 (7) | 0.0195 (3) | |
| H10C | 0.2476 | 0.6339 | 0.6659 | 0.023* | |
| H10D | 0.3736 | 0.5379 | 0.7042 | 0.023* | |
| C11B | 0.31460 (16) | 0.43762 (16) | 0.63990 (7) | 0.0185 (3) | |
| C12B | 0.18952 (16) | 0.39419 (14) | 0.62110 (6) | 0.0158 (3) | |
| C13B | 0.21341 (16) | 0.29660 (15) | 0.57359 (6) | 0.0156 (3) | |
| H13B | 0.2789 | 0.3248 | 0.5427 | 0.019* | |
| C14B | 0.28762 (16) | 0.15174 (15) | 0.59614 (6) | 0.0153 (3) | |
| C15B | 0.22690 (16) | 0.09184 (15) | 0.64401 (6) | 0.0165 (3) | |
| H15B | 0.1366 | 0.1405 | 0.6613 | 0.020* | |
| C16B | 0.29577 (16) | −0.03643 (15) | 0.66644 (6) | 0.0154 (3) | |
| C17B | 0.42943 (16) | −0.11082 (15) | 0.64140 (6) | 0.0150 (3) | |
| C18B | 0.49040 (16) | −0.05217 (15) | 0.59372 (6) | 0.0163 (3) | |
| H18B | 0.5805 | −0.1007 | 0.5763 | 0.020* | |
| C19B | 0.41944 (16) | 0.07738 (15) | 0.57161 (6) | 0.0164 (3) | |
| H19B | 0.4621 | 0.1159 | 0.5390 | 0.020* | |
| C20B | 0.61663 (17) | −0.31935 (16) | 0.63789 (7) | 0.0215 (3) | |
| H20D | 0.6444 | −0.4098 | 0.6577 | 0.032* | |
| H20E | 0.6974 | −0.2766 | 0.6385 | 0.032* | |
| H20F | 0.5954 | −0.3278 | 0.5979 | 0.032* | |
| C21B | 0.29838 (18) | −0.08858 (17) | 0.76608 (7) | 0.0221 (3) | |
| H21D | 0.2459 | −0.1256 | 0.7979 | 0.033* | |
| H21E | 0.2950 | 0.0058 | 0.7727 | 0.033* | |
| H21F | 0.4006 | −0.1429 | 0.7638 | 0.033* | |
| C22B | −0.18128 (19) | 0.12487 (16) | 0.53976 (7) | 0.0226 (3) | |
| H22D | −0.1820 | 0.0612 | 0.5104 | 0.034* | |
| H22E | −0.2588 | 0.1223 | 0.5692 | 0.034* | |
| H22F | −0.0861 | 0.0989 | 0.5576 | 0.034* | |
| C23B | −0.35524 (18) | 0.30927 (17) | 0.48314 (7) | 0.0227 (3) | |
| H23D | −0.3562 | 0.2436 | 0.4547 | 0.034* | |
| H23E | −0.3704 | 0.4002 | 0.4640 | 0.034* | |
| H23G | −0.4338 | 0.3098 | 0.5125 | 0.034* | |
| C24B | 0.22219 (19) | 0.37987 (17) | 0.76248 (7) | 0.0246 (4) | |
| H24D | 0.1452 | 0.3623 | 0.7895 | 0.037* | |
| H24G | 0.3083 | 0.3795 | 0.7838 | 0.037* | |
| H24E | 0.2491 | 0.3089 | 0.7347 | 0.037* | |
| C25B | 0.13179 (19) | 0.63007 (17) | 0.77360 (7) | 0.0226 (3) | |
| H25D | 0.0522 | 0.6180 | 0.8005 | 0.034* | |
| H25E | 0.1020 | 0.7194 | 0.7530 | 0.034* | |
| H25F | 0.2193 | 0.6241 | 0.7950 | 0.034* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0117 (5) | 0.0192 (5) | 0.0173 (5) | −0.0019 (4) | −0.0001 (4) | 0.0038 (4) |
| O2A | 0.0161 (5) | 0.0282 (6) | 0.0198 (5) | −0.0020 (4) | −0.0034 (4) | 0.0031 (5) |
| O3A | 0.0142 (5) | 0.0211 (5) | 0.0228 (5) | −0.0046 (4) | −0.0010 (4) | −0.0011 (4) |
| O4A | 0.0151 (5) | 0.0109 (5) | 0.0230 (5) | 0.0012 (4) | −0.0043 (4) | −0.0027 (4) |
| O5A | 0.0169 (5) | 0.0177 (5) | 0.0284 (6) | 0.0006 (4) | −0.0105 (5) | −0.0084 (5) |
| C1A | 0.0122 (7) | 0.0116 (6) | 0.0153 (7) | −0.0027 (5) | 0.0003 (5) | −0.0035 (5) |
| C2A | 0.0158 (7) | 0.0120 (6) | 0.0157 (7) | −0.0026 (5) | −0.0010 (6) | −0.0036 (5) |
| C3A | 0.0155 (7) | 0.0180 (7) | 0.0140 (7) | −0.0027 (5) | 0.0004 (5) | 0.0003 (6) |
| C4A | 0.0137 (7) | 0.0157 (7) | 0.0168 (7) | −0.0035 (5) | 0.0008 (5) | −0.0004 (5) |
| C5A | 0.0125 (7) | 0.0148 (7) | 0.0166 (7) | −0.0005 (5) | −0.0002 (5) | −0.0002 (5) |
| C6A | 0.0147 (7) | 0.0117 (6) | 0.0145 (6) | −0.0029 (5) | 0.0002 (5) | −0.0016 (5) |
| C7A | 0.0140 (7) | 0.0136 (7) | 0.0166 (7) | −0.0036 (5) | 0.0015 (5) | −0.0026 (5) |
| C8A | 0.0159 (7) | 0.0159 (7) | 0.0186 (7) | −0.0013 (5) | −0.0004 (6) | 0.0021 (6) |
| C9A | 0.0178 (7) | 0.0170 (7) | 0.0150 (7) | −0.0047 (6) | 0.0019 (6) | −0.0015 (6) |
| C10A | 0.0183 (7) | 0.0172 (7) | 0.0176 (7) | −0.0072 (6) | 0.0021 (6) | −0.0021 (6) |
| C11A | 0.0156 (7) | 0.0138 (7) | 0.0164 (7) | −0.0042 (5) | −0.0002 (6) | −0.0051 (5) |
| C12A | 0.0144 (7) | 0.0107 (6) | 0.0148 (6) | −0.0028 (5) | 0.0005 (5) | −0.0031 (5) |
| C13A | 0.0111 (6) | 0.0119 (6) | 0.0148 (6) | −0.0011 (5) | −0.0009 (5) | −0.0019 (5) |
| C14A | 0.0117 (7) | 0.0132 (6) | 0.0140 (6) | −0.0023 (5) | 0.0014 (5) | −0.0005 (5) |
| C15A | 0.0118 (7) | 0.0146 (7) | 0.0173 (7) | −0.0005 (5) | −0.0022 (5) | −0.0005 (5) |
| C16A | 0.0134 (7) | 0.0155 (7) | 0.0159 (7) | −0.0031 (5) | −0.0015 (5) | −0.0022 (5) |
| C17A | 0.0125 (7) | 0.0121 (6) | 0.0162 (7) | −0.0017 (5) | 0.0009 (5) | 0.0000 (5) |
| C18A | 0.0121 (7) | 0.0139 (7) | 0.0174 (7) | −0.0003 (5) | −0.0015 (5) | 0.0007 (5) |
| C19A | 0.0123 (7) | 0.0155 (7) | 0.0153 (7) | −0.0036 (5) | −0.0014 (5) | −0.0023 (5) |
| C20A | 0.0130 (7) | 0.0129 (7) | 0.0219 (7) | 0.0016 (5) | −0.0030 (6) | −0.0001 (6) |
| C21A | 0.0249 (9) | 0.0268 (9) | 0.0440 (11) | 0.0035 (7) | −0.0212 (8) | −0.0127 (8) |
| C22A | 0.0210 (8) | 0.0189 (8) | 0.0279 (8) | −0.0064 (6) | 0.0011 (7) | −0.0056 (6) |
| C23A | 0.0152 (7) | 0.0239 (8) | 0.0215 (8) | −0.0056 (6) | 0.0026 (6) | 0.0004 (6) |
| C24A | 0.0281 (9) | 0.0249 (8) | 0.0209 (8) | −0.0067 (7) | −0.0017 (7) | −0.0075 (6) |
| C25A | 0.0228 (8) | 0.0261 (8) | 0.0203 (8) | −0.0062 (6) | 0.0020 (6) | 0.0033 (6) |
| O1B | 0.0123 (5) | 0.0217 (5) | 0.0192 (5) | −0.0047 (4) | 0.0028 (4) | −0.0084 (4) |
| O2B | 0.0199 (6) | 0.0333 (7) | 0.0213 (6) | −0.0025 (5) | 0.0077 (5) | −0.0068 (5) |
| O3B | 0.0141 (5) | 0.0300 (6) | 0.0296 (6) | −0.0065 (5) | 0.0039 (5) | −0.0022 (5) |
| O4B | 0.0171 (5) | 0.0149 (5) | 0.0229 (5) | 0.0007 (4) | 0.0059 (4) | −0.0018 (4) |
| O5B | 0.0169 (5) | 0.0196 (5) | 0.0184 (5) | −0.0065 (4) | 0.0052 (4) | −0.0013 (4) |
| C1B | 0.0147 (7) | 0.0142 (7) | 0.0171 (7) | −0.0026 (5) | 0.0014 (6) | −0.0010 (5) |
| C2B | 0.0197 (7) | 0.0156 (7) | 0.0163 (7) | −0.0032 (6) | 0.0036 (6) | −0.0003 (6) |
| C3B | 0.0215 (8) | 0.0205 (7) | 0.0152 (7) | −0.0035 (6) | 0.0005 (6) | −0.0016 (6) |
| C4B | 0.0169 (7) | 0.0176 (7) | 0.0161 (7) | −0.0050 (6) | 0.0010 (6) | −0.0039 (6) |
| C5B | 0.0141 (7) | 0.0180 (7) | 0.0190 (7) | −0.0027 (5) | 0.0015 (6) | −0.0046 (6) |
| C6B | 0.0158 (7) | 0.0160 (7) | 0.0154 (7) | −0.0042 (5) | 0.0003 (6) | −0.0024 (5) |
| C7B | 0.0139 (7) | 0.0152 (7) | 0.0192 (7) | −0.0042 (5) | 0.0004 (6) | −0.0016 (6) |
| C8B | 0.0164 (7) | 0.0185 (7) | 0.0213 (7) | −0.0043 (6) | 0.0018 (6) | −0.0058 (6) |
| C9B | 0.0183 (7) | 0.0189 (7) | 0.0174 (7) | −0.0062 (6) | 0.0015 (6) | −0.0019 (6) |
| C10B | 0.0177 (7) | 0.0223 (8) | 0.0212 (7) | −0.0103 (6) | −0.0015 (6) | 0.0003 (6) |
| C11B | 0.0157 (7) | 0.0199 (7) | 0.0195 (7) | −0.0054 (6) | 0.0000 (6) | 0.0044 (6) |
| C12B | 0.0157 (7) | 0.0138 (7) | 0.0169 (7) | −0.0027 (5) | 0.0004 (6) | 0.0005 (5) |
| C13B | 0.0113 (7) | 0.0169 (7) | 0.0176 (7) | −0.0028 (5) | 0.0043 (5) | −0.0011 (6) |
| C14B | 0.0122 (7) | 0.0164 (7) | 0.0177 (7) | −0.0037 (5) | 0.0007 (5) | −0.0041 (6) |
| C15B | 0.0113 (7) | 0.0184 (7) | 0.0191 (7) | −0.0030 (5) | 0.0047 (6) | −0.0047 (6) |
| C16B | 0.0134 (7) | 0.0179 (7) | 0.0161 (7) | −0.0066 (5) | 0.0034 (5) | −0.0034 (6) |
| C17B | 0.0117 (7) | 0.0162 (7) | 0.0178 (7) | −0.0040 (5) | −0.0005 (5) | −0.0047 (6) |
| C18B | 0.0116 (7) | 0.0194 (7) | 0.0174 (7) | −0.0028 (5) | 0.0037 (5) | −0.0068 (6) |
| C19B | 0.0143 (7) | 0.0191 (7) | 0.0156 (7) | −0.0046 (6) | 0.0035 (6) | −0.0030 (6) |
| C20B | 0.0174 (8) | 0.0180 (7) | 0.0254 (8) | 0.0016 (6) | 0.0036 (6) | −0.0052 (6) |
| C21B | 0.0216 (8) | 0.0251 (8) | 0.0196 (7) | −0.0067 (6) | 0.0037 (6) | −0.0024 (6) |
| C22B | 0.0246 (8) | 0.0186 (8) | 0.0256 (8) | −0.0075 (6) | −0.0003 (7) | −0.0016 (6) |
| C23B | 0.0199 (8) | 0.0283 (9) | 0.0210 (8) | −0.0070 (6) | −0.0019 (6) | −0.0054 (7) |
| C24B | 0.0256 (9) | 0.0243 (8) | 0.0211 (8) | −0.0036 (7) | 0.0016 (7) | 0.0028 (6) |
| C25B | 0.0243 (8) | 0.0249 (8) | 0.0211 (8) | −0.0100 (7) | −0.0022 (6) | −0.0045 (6) |
Geometric parameters (Å, °)
| O1A—C7A | 1.3822 (17) | O1B—C7B | 1.3774 (18) |
| O1A—C6A | 1.3847 (16) | O1B—C6B | 1.3800 (18) |
| O2A—C2A | 1.2248 (18) | O2B—C2B | 1.2240 (18) |
| O3A—C11A | 1.2291 (18) | O3B—C11B | 1.2240 (18) |
| O4A—C17A | 1.3704 (17) | O4B—C17B | 1.3673 (17) |
| O4A—C20A | 1.4329 (17) | O4B—C20B | 1.4346 (17) |
| O5A—C16A | 1.3733 (18) | O5B—C16B | 1.3875 (16) |
| O5A—C21A | 1.4247 (19) | O5B—C21B | 1.434 (2) |
| C1A—C6A | 1.340 (2) | C1B—C6B | 1.346 (2) |
| C1A—C2A | 1.4768 (19) | C1B—C2B | 1.471 (2) |
| C1A—C13A | 1.5102 (19) | C1B—C13B | 1.511 (2) |
| C2A—C3A | 1.512 (2) | C2B—C3B | 1.512 (2) |
| C3A—C4A | 1.532 (2) | C3B—C4B | 1.537 (2) |
| C3A—H3AA | 0.9900 | C3B—H3BA | 0.9900 |
| C3A—H3AB | 0.9900 | C3B—H3BB | 0.9900 |
| C4A—C23A | 1.528 (2) | C4B—C23B | 1.527 (2) |
| C4A—C22A | 1.537 (2) | C4B—C22B | 1.535 (2) |
| C4A—C5A | 1.5390 (19) | C4B—C5B | 1.538 (2) |
| C5A—C6A | 1.489 (2) | C5B—C6B | 1.488 (2) |
| C5A—H5AA | 0.9900 | C5B—H5BA | 0.9900 |
| C5A—H5AB | 0.9900 | C5B—H5BB | 0.9900 |
| C7A—C12A | 1.340 (2) | C7B—C12B | 1.344 (2) |
| C7A—C8A | 1.4983 (19) | C7B—C8B | 1.494 (2) |
| C8A—C9A | 1.540 (2) | C8B—C9B | 1.530 (2) |
| C8A—H8AA | 0.9900 | C8B—H8BA | 0.9900 |
| C8A—H8AB | 0.9900 | C8B—H8BB | 0.9900 |
| C9A—C10A | 1.528 (2) | C9B—C25B | 1.528 (2) |
| C9A—C25A | 1.534 (2) | C9B—C24B | 1.535 (2) |
| C9A—C24A | 1.535 (2) | C9B—C10B | 1.538 (2) |
| C10A—C11A | 1.515 (2) | C10B—C11B | 1.511 (2) |
| C10A—H10A | 0.9900 | C10B—H10C | 0.9900 |
| C10A—H10B | 0.9900 | C10B—H10D | 0.9900 |
| C11A—C12A | 1.4730 (19) | C11B—C12B | 1.474 (2) |
| C12A—C13A | 1.5167 (19) | C12B—C13B | 1.511 (2) |
| C13A—C14A | 1.5289 (19) | C13B—C14B | 1.530 (2) |
| C13A—H13A | 1.0000 | C13B—H13B | 1.0000 |
| C14A—C19A | 1.3813 (19) | C14B—C19B | 1.391 (2) |
| C14A—C15A | 1.399 (2) | C14B—C15B | 1.4026 (19) |
| C15A—C16A | 1.384 (2) | C15B—C16B | 1.380 (2) |
| C15A—H15A | 0.9500 | C15B—H15B | 0.9500 |
| C16A—C17A | 1.408 (2) | C16B—C17B | 1.4090 (19) |
| C17A—C18A | 1.383 (2) | C17B—C18B | 1.394 (2) |
| C18A—C19A | 1.396 (2) | C18B—C19B | 1.393 (2) |
| C18A—H18A | 0.9500 | C18B—H18B | 0.9500 |
| C19A—H19A | 0.9500 | C19B—H19B | 0.9500 |
| C20A—H20A | 0.9800 | C20B—H20D | 0.9800 |
| C20A—H20B | 0.9800 | C20B—H20E | 0.9800 |
| C20A—H20C | 0.9800 | C20B—H20F | 0.9800 |
| C21A—H21A | 0.9800 | C21B—H21D | 0.9800 |
| C21A—H21B | 0.9800 | C21B—H21E | 0.9800 |
| C21A—H21C | 0.9800 | C21B—H21F | 0.9800 |
| C22A—H22A | 0.9800 | C22B—H22D | 0.9800 |
| C22A—H22B | 0.9800 | C22B—H22E | 0.9800 |
| C22A—H22C | 0.9800 | C22B—H22F | 0.9800 |
| C23A—H23A | 0.9800 | C23B—H23D | 0.9800 |
| C23A—H23B | 0.9800 | C23B—H23E | 0.9800 |
| C23A—H23C | 0.9800 | C23B—H23G | 0.9800 |
| C24A—H24A | 0.9800 | C24B—H24D | 0.9800 |
| C24A—H24B | 0.9800 | C24B—H24G | 0.9800 |
| C24A—H24C | 0.9800 | C24B—H24E | 0.9800 |
| C25A—H25A | 0.9800 | C25B—H25D | 0.9800 |
| C25A—H25B | 0.9800 | C25B—H25E | 0.9800 |
| C25A—H25C | 0.9800 | C25B—H25F | 0.9800 |
| C7A—O1A—C6A | 117.69 (11) | C7B—O1B—C6B | 117.46 (11) |
| C17A—O4A—C20A | 116.72 (12) | C17B—O4B—C20B | 116.61 (11) |
| C16A—O5A—C21A | 116.35 (12) | C16B—O5B—C21B | 112.54 (11) |
| C6A—C1A—C2A | 118.52 (12) | C6B—C1B—C2B | 118.46 (14) |
| C6A—C1A—C13A | 122.70 (12) | C6B—C1B—C13B | 122.33 (14) |
| C2A—C1A—C13A | 118.71 (12) | C2B—C1B—C13B | 119.20 (12) |
| O2A—C2A—C1A | 120.48 (13) | O2B—C2B—C1B | 120.67 (14) |
| O2A—C2A—C3A | 121.12 (13) | O2B—C2B—C3B | 121.21 (14) |
| C1A—C2A—C3A | 118.36 (12) | C1B—C2B—C3B | 118.04 (13) |
| C2A—C3A—C4A | 114.67 (12) | C2B—C3B—C4B | 115.09 (12) |
| C2A—C3A—H3AA | 108.6 | C2B—C3B—H3BA | 108.5 |
| C4A—C3A—H3AA | 108.6 | C4B—C3B—H3BA | 108.5 |
| C2A—C3A—H3AB | 108.6 | C2B—C3B—H3BB | 108.5 |
| C4A—C3A—H3AB | 108.6 | C4B—C3B—H3BB | 108.5 |
| H3AA—C3A—H3AB | 107.6 | H3BA—C3B—H3BB | 107.5 |
| C23A—C4A—C3A | 109.81 (12) | C23B—C4B—C22B | 109.54 (13) |
| C23A—C4A—C22A | 109.05 (13) | C23B—C4B—C3B | 109.74 (12) |
| C3A—C4A—C22A | 110.56 (12) | C22B—C4B—C3B | 110.21 (12) |
| C23A—C4A—C5A | 108.77 (12) | C23B—C4B—C5B | 108.79 (12) |
| C3A—C4A—C5A | 108.24 (12) | C22B—C4B—C5B | 110.42 (12) |
| C22A—C4A—C5A | 110.40 (12) | C3B—C4B—C5B | 108.10 (12) |
| C6A—C5A—C4A | 112.43 (12) | C6B—C5B—C4B | 112.54 (12) |
| C6A—C5A—H5AA | 109.1 | C6B—C5B—H5BA | 109.1 |
| C4A—C5A—H5AA | 109.1 | C4B—C5B—H5BA | 109.1 |
| C6A—C5A—H5AB | 109.1 | C6B—C5B—H5BB | 109.1 |
| C4A—C5A—H5AB | 109.1 | C4B—C5B—H5BB | 109.1 |
| H5AA—C5A—H5AB | 107.9 | H5BA—C5B—H5BB | 107.8 |
| C1A—C6A—O1A | 123.02 (12) | C1B—C6B—O1B | 122.38 (14) |
| C1A—C6A—C5A | 125.53 (12) | C1B—C6B—C5B | 126.03 (14) |
| O1A—C6A—C5A | 111.45 (12) | O1B—C6B—C5B | 111.58 (12) |
| C12A—C7A—O1A | 123.02 (12) | C12B—C7B—O1B | 122.74 (14) |
| C12A—C7A—C8A | 125.29 (13) | C12B—C7B—C8B | 125.82 (14) |
| O1A—C7A—C8A | 111.68 (12) | O1B—C7B—C8B | 111.44 (12) |
| C7A—C8A—C9A | 111.83 (12) | C7B—C8B—C9B | 112.29 (12) |
| C7A—C8A—H8AA | 109.3 | C7B—C8B—H8BA | 109.1 |
| C9A—C8A—H8AA | 109.3 | C9B—C8B—H8BA | 109.1 |
| C7A—C8A—H8AB | 109.3 | C7B—C8B—H8BB | 109.1 |
| C9A—C8A—H8AB | 109.3 | C9B—C8B—H8BB | 109.1 |
| H8AA—C8A—H8AB | 107.9 | H8BA—C8B—H8BB | 107.9 |
| C10A—C9A—C25A | 109.77 (13) | C25B—C9B—C8B | 109.18 (13) |
| C10A—C9A—C24A | 110.97 (13) | C25B—C9B—C24B | 109.33 (13) |
| C25A—C9A—C24A | 108.99 (13) | C8B—C9B—C24B | 110.65 (13) |
| C10A—C9A—C8A | 107.73 (12) | C25B—C9B—C10B | 110.53 (13) |
| C25A—C9A—C8A | 109.27 (12) | C8B—C9B—C10B | 107.64 (12) |
| C24A—C9A—C8A | 110.09 (13) | C24B—C9B—C10B | 109.49 (13) |
| C11A—C10A—C9A | 115.36 (12) | C11B—C10B—C9B | 112.29 (12) |
| C11A—C10A—H10A | 108.4 | C11B—C10B—H10C | 109.1 |
| C9A—C10A—H10A | 108.4 | C9B—C10B—H10C | 109.1 |
| C11A—C10A—H10B | 108.4 | C11B—C10B—H10D | 109.1 |
| C9A—C10A—H10B | 108.4 | C9B—C10B—H10D | 109.1 |
| H10A—C10A—H10B | 107.5 | H10C—C10B—H10D | 107.9 |
| O3A—C11A—C12A | 120.24 (13) | O3B—C11B—C12B | 120.67 (15) |
| O3A—C11A—C10A | 121.64 (13) | O3B—C11B—C10B | 122.39 (14) |
| C12A—C11A—C10A | 118.08 (13) | C12B—C11B—C10B | 116.91 (13) |
| C7A—C12A—C11A | 118.74 (12) | C7B—C12B—C11B | 118.63 (14) |
| C7A—C12A—C13A | 122.70 (13) | C7B—C12B—C13B | 122.00 (14) |
| C11A—C12A—C13A | 118.55 (12) | C11B—C12B—C13B | 119.35 (13) |
| C1A—C13A—C12A | 108.84 (11) | C12B—C13B—C1B | 108.13 (12) |
| C1A—C13A—C14A | 109.54 (11) | C12B—C13B—C14B | 110.72 (12) |
| C12A—C13A—C14A | 111.20 (11) | C1B—C13B—C14B | 112.55 (12) |
| C1A—C13A—H13A | 109.1 | C12B—C13B—H13B | 108.4 |
| C12A—C13A—H13A | 109.1 | C1B—C13B—H13B | 108.4 |
| C14A—C13A—H13A | 109.1 | C14B—C13B—H13B | 108.4 |
| C19A—C14A—C15A | 119.31 (13) | C19B—C14B—C15B | 117.90 (13) |
| C19A—C14A—C13A | 121.78 (13) | C19B—C14B—C13B | 121.54 (13) |
| C15A—C14A—C13A | 118.89 (12) | C15B—C14B—C13B | 120.51 (12) |
| C16A—C15A—C14A | 120.59 (13) | C16B—C15B—C14B | 121.26 (13) |
| C16A—C15A—H15A | 119.7 | C16B—C15B—H15B | 119.4 |
| C14A—C15A—H15A | 119.7 | C14B—C15B—H15B | 119.4 |
| O5A—C16A—C15A | 124.95 (13) | C15B—C16B—O5B | 119.43 (13) |
| O5A—C16A—C17A | 115.49 (13) | C15B—C16B—C17B | 120.43 (13) |
| C15A—C16A—C17A | 119.56 (14) | O5B—C16B—C17B | 120.12 (13) |
| O4A—C17A—C18A | 124.90 (13) | O4B—C17B—C18B | 125.18 (13) |
| O4A—C17A—C16A | 115.18 (13) | O4B—C17B—C16B | 116.13 (12) |
| C18A—C17A—C16A | 119.91 (13) | C18B—C17B—C16B | 118.69 (13) |
| C17A—C18A—C19A | 119.82 (13) | C19B—C18B—C17B | 120.14 (13) |
| C17A—C18A—H18A | 120.1 | C19B—C18B—H18B | 119.9 |
| C19A—C18A—H18A | 120.1 | C17B—C18B—H18B | 119.9 |
| C14A—C19A—C18A | 120.80 (14) | C14B—C19B—C18B | 121.58 (13) |
| C14A—C19A—H19A | 119.6 | C14B—C19B—H19B | 119.2 |
| C18A—C19A—H19A | 119.6 | C18B—C19B—H19B | 119.2 |
| O4A—C20A—H20A | 109.5 | O4B—C20B—H20D | 109.5 |
| O4A—C20A—H20B | 109.5 | O4B—C20B—H20E | 109.5 |
| H20A—C20A—H20B | 109.5 | H20D—C20B—H20E | 109.5 |
| O4A—C20A—H20C | 109.5 | O4B—C20B—H20F | 109.5 |
| H20A—C20A—H20C | 109.5 | H20D—C20B—H20F | 109.5 |
| H20B—C20A—H20C | 109.5 | H20E—C20B—H20F | 109.5 |
| O5A—C21A—H21A | 109.5 | O5B—C21B—H21D | 109.5 |
| O5A—C21A—H21B | 109.5 | O5B—C21B—H21E | 109.5 |
| H21A—C21A—H21B | 109.5 | H21D—C21B—H21E | 109.5 |
| O5A—C21A—H21C | 109.5 | O5B—C21B—H21F | 109.5 |
| H21A—C21A—H21C | 109.5 | H21D—C21B—H21F | 109.5 |
| H21B—C21A—H21C | 109.5 | H21E—C21B—H21F | 109.5 |
| C4A—C22A—H22A | 109.5 | C4B—C22B—H22D | 109.5 |
| C4A—C22A—H22B | 109.5 | C4B—C22B—H22E | 109.5 |
| H22A—C22A—H22B | 109.5 | H22D—C22B—H22E | 109.5 |
| C4A—C22A—H22C | 109.5 | C4B—C22B—H22F | 109.5 |
| H22A—C22A—H22C | 109.5 | H22D—C22B—H22F | 109.5 |
| H22B—C22A—H22C | 109.5 | H22E—C22B—H22F | 109.5 |
| C4A—C23A—H23A | 109.5 | C4B—C23B—H23D | 109.5 |
| C4A—C23A—H23B | 109.5 | C4B—C23B—H23E | 109.5 |
| H23A—C23A—H23B | 109.5 | H23D—C23B—H23E | 109.5 |
| C4A—C23A—H23C | 109.5 | C4B—C23B—H23G | 109.5 |
| H23A—C23A—H23C | 109.5 | H23D—C23B—H23G | 109.5 |
| H23B—C23A—H23C | 109.5 | H23E—C23B—H23G | 109.5 |
| C9A—C24A—H24A | 109.5 | C9B—C24B—H24D | 109.5 |
| C9A—C24A—H24B | 109.5 | C9B—C24B—H24G | 109.5 |
| H24A—C24A—H24B | 109.5 | H24D—C24B—H24G | 109.5 |
| C9A—C24A—H24C | 109.5 | C9B—C24B—H24E | 109.5 |
| H24A—C24A—H24C | 109.5 | H24D—C24B—H24E | 109.5 |
| H24B—C24A—H24C | 109.5 | H24G—C24B—H24E | 109.5 |
| C9A—C25A—H25A | 109.5 | C9B—C25B—H25D | 109.5 |
| C9A—C25A—H25B | 109.5 | C9B—C25B—H25E | 109.5 |
| H25A—C25A—H25B | 109.5 | H25D—C25B—H25E | 109.5 |
| C9A—C25A—H25C | 109.5 | C9B—C25B—H25F | 109.5 |
| H25A—C25A—H25C | 109.5 | H25D—C25B—H25F | 109.5 |
| H25B—C25A—H25C | 109.5 | H25E—C25B—H25F | 109.5 |
| C6A—C1A—C2A—O2A | 174.17 (14) | C6B—C1B—C2B—O2B | 175.45 (14) |
| C13A—C1A—C2A—O2A | −8.7 (2) | C13B—C1B—C2B—O2B | −3.2 (2) |
| C6A—C1A—C2A—C3A | −3.5 (2) | C6B—C1B—C2B—C3B | −1.2 (2) |
| C13A—C1A—C2A—C3A | 173.59 (13) | C13B—C1B—C2B—C3B | −179.85 (13) |
| O2A—C2A—C3A—C4A | 156.42 (14) | O2B—C2B—C3B—C4B | 155.50 (14) |
| C1A—C2A—C3A—C4A | −25.91 (19) | C1B—C2B—C3B—C4B | −27.87 (19) |
| C2A—C3A—C4A—C23A | 169.58 (12) | C2B—C3B—C4B—C23B | 169.74 (13) |
| C2A—C3A—C4A—C22A | −70.06 (16) | C2B—C3B—C4B—C22B | −69.55 (17) |
| C2A—C3A—C4A—C5A | 50.97 (16) | C2B—C3B—C4B—C5B | 51.22 (17) |
| C23A—C4A—C5A—C6A | −168.10 (13) | C23B—C4B—C5B—C6B | −166.56 (12) |
| C3A—C4A—C5A—C6A | −48.84 (16) | C22B—C4B—C5B—C6B | 73.19 (16) |
| C22A—C4A—C5A—C6A | 72.29 (16) | C3B—C4B—C5B—C6B | −47.44 (16) |
| C2A—C1A—C6A—O1A | −175.93 (13) | C2B—C1B—C6B—O1B | −175.04 (13) |
| C13A—C1A—C6A—O1A | 7.1 (2) | C13B—C1B—C6B—O1B | 3.6 (2) |
| C2A—C1A—C6A—C5A | 4.7 (2) | C2B—C1B—C6B—C5B | 3.8 (2) |
| C13A—C1A—C6A—C5A | −172.24 (13) | C13B—C1B—C6B—C5B | −177.60 (14) |
| C7A—O1A—C6A—C1A | 5.0 (2) | C7B—O1B—C6B—C1B | 13.7 (2) |
| C7A—O1A—C6A—C5A | −175.62 (12) | C7B—O1B—C6B—C5B | −165.29 (12) |
| C4A—C5A—C6A—C1A | 23.1 (2) | C4B—C5B—C6B—C1B | 22.4 (2) |
| C4A—C5A—C6A—O1A | −156.26 (12) | C4B—C5B—C6B—O1B | −158.66 (12) |
| C6A—O1A—C7A—C12A | −6.9 (2) | C6B—O1B—C7B—C12B | −11.9 (2) |
| C6A—O1A—C7A—C8A | 172.65 (12) | C6B—O1B—C7B—C8B | 167.92 (12) |
| C12A—C7A—C8A—C9A | −27.0 (2) | C12B—C7B—C8B—C9B | −17.6 (2) |
| O1A—C7A—C8A—C9A | 153.46 (12) | O1B—C7B—C8B—C9B | 162.62 (12) |
| C7A—C8A—C9A—C10A | 50.47 (16) | C7B—C8B—C9B—C25B | 167.61 (12) |
| C7A—C8A—C9A—C25A | 169.68 (13) | C7B—C8B—C9B—C24B | −72.01 (16) |
| C7A—C8A—C9A—C24A | −70.65 (16) | C7B—C8B—C9B—C10B | 47.59 (16) |
| C25A—C9A—C10A—C11A | −169.89 (13) | C25B—C9B—C10B—C11B | −177.59 (13) |
| C24A—C9A—C10A—C11A | 69.58 (16) | C8B—C9B—C10B—C11B | −58.43 (16) |
| C8A—C9A—C10A—C11A | −51.00 (16) | C24B—C9B—C10B—C11B | 61.91 (17) |
| C9A—C10A—C11A—O3A | −156.99 (14) | C9B—C10B—C11B—O3B | −144.04 (15) |
| C9A—C10A—C11A—C12A | 25.40 (19) | C9B—C10B—C11B—C12B | 38.10 (18) |
| O1A—C7A—C12A—C11A | 178.17 (13) | O1B—C7B—C12B—C11B | 174.40 (12) |
| C8A—C7A—C12A—C11A | −1.3 (2) | C8B—C7B—C12B—C11B | −5.4 (2) |
| O1A—C7A—C12A—C13A | −3.2 (2) | O1B—C7B—C12B—C13B | −7.2 (2) |
| C8A—C7A—C12A—C13A | 177.30 (14) | C8B—C7B—C12B—C13B | 173.05 (13) |
| O3A—C11A—C12A—C7A | −175.04 (14) | O3B—C11B—C12B—C7B | 176.85 (14) |
| C10A—C11A—C12A—C7A | 2.6 (2) | C10B—C11B—C12B—C7B | −5.3 (2) |
| O3A—C11A—C12A—C13A | 6.3 (2) | O3B—C11B—C12B—C13B | −1.6 (2) |
| C10A—C11A—C12A—C13A | −176.08 (13) | C10B—C11B—C12B—C13B | 176.29 (12) |
| C6A—C1A—C13A—C12A | −15.15 (19) | C7B—C12B—C13B—C1B | 21.57 (18) |
| C2A—C1A—C13A—C12A | 167.88 (12) | C11B—C12B—C13B—C1B | −160.03 (12) |
| C6A—C1A—C13A—C14A | 106.62 (15) | C7B—C12B—C13B—C14B | −102.17 (16) |
| C2A—C1A—C13A—C14A | −70.35 (16) | C11B—C12B—C13B—C14B | 76.23 (16) |
| C7A—C12A—C13A—C1A | 13.27 (19) | C6B—C1B—C13B—C12B | −19.82 (19) |
| C11A—C12A—C13A—C1A | −168.10 (12) | C2B—C1B—C13B—C12B | 158.77 (12) |
| C7A—C12A—C13A—C14A | −107.48 (16) | C6B—C1B—C13B—C14B | 102.80 (16) |
| C11A—C12A—C13A—C14A | 71.14 (16) | C2B—C1B—C13B—C14B | −78.61 (16) |
| C1A—C13A—C14A—C19A | 120.01 (14) | C12B—C13B—C14B—C19B | −123.15 (15) |
| C12A—C13A—C14A—C19A | −119.65 (14) | C1B—C13B—C14B—C19B | 115.69 (15) |
| C1A—C13A—C14A—C15A | −58.45 (16) | C12B—C13B—C14B—C15B | 54.28 (18) |
| C12A—C13A—C14A—C15A | 61.90 (16) | C1B—C13B—C14B—C15B | −66.88 (18) |
| C19A—C14A—C15A—C16A | −0.4 (2) | C19B—C14B—C15B—C16B | 0.4 (2) |
| C13A—C14A—C15A—C16A | 178.06 (12) | C13B—C14B—C15B—C16B | −177.13 (14) |
| C21A—O5A—C16A—C15A | 4.6 (2) | C14B—C15B—C16B—O5B | −178.82 (14) |
| C21A—O5A—C16A—C17A | −175.41 (14) | C14B—C15B—C16B—C17B | −0.2 (2) |
| C14A—C15A—C16A—O5A | −179.48 (13) | C21B—O5B—C16B—C15B | −103.40 (16) |
| C14A—C15A—C16A—C17A | 0.5 (2) | C21B—O5B—C16B—C17B | 78.01 (17) |
| C20A—O4A—C17A—C18A | −1.9 (2) | C20B—O4B—C17B—C18B | −6.1 (2) |
| C20A—O4A—C17A—C16A | 177.96 (12) | C20B—O4B—C17B—C16B | 173.68 (13) |
| O5A—C16A—C17A—O4A | −0.01 (18) | C15B—C16B—C17B—O4B | −179.75 (14) |
| C15A—C16A—C17A—O4A | 179.99 (12) | O5B—C16B—C17B—O4B | −1.2 (2) |
| O5A—C16A—C17A—C18A | 179.82 (13) | C15B—C16B—C17B—C18B | 0.1 (2) |
| C15A—C16A—C17A—C18A | −0.2 (2) | O5B—C16B—C17B—C18B | 178.66 (13) |
| O4A—C17A—C18A—C19A | 179.58 (13) | O4B—C17B—C18B—C19B | 179.72 (14) |
| C16A—C17A—C18A—C19A | −0.2 (2) | C16B—C17B—C18B—C19B | −0.1 (2) |
| C15A—C14A—C19A—C18A | 0.0 (2) | C15B—C14B—C19B—C18B | −0.4 (2) |
| C13A—C14A—C19A—C18A | −178.43 (12) | C13B—C14B—C19B—C18B | 177.09 (14) |
| C17A—C18A—C19A—C14A | 0.3 (2) | C17B—C18B—C19B—C14B | 0.3 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10A—H10A···O4Ai | 0.99 | 2.38 | 3.2817 (19) | 152 |
| C18A—H18A···O2Aii | 0.95 | 2.35 | 3.2943 (19) | 176 |
| C18B—H18B···O2Biii | 0.95 | 2.45 | 3.4000 (19) | 175 |
| C20A—H20A···O3Aiv | 0.98 | 2.59 | 3.4144 (19) | 142 |
| C24B—H24D···O4Av | 0.98 | 2.48 | 3.453 (2) | 171 |
Symmetry codes: (i) x, y−1, z; (ii) −x, −y+1, −z; (iii) −x+1, −y, −z+1; (iv) x, y+1, z; (v) −x, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5885).
References
- Banerjee, A. & Mukherjee, A. K. (1981). Stain Technol. 56, 83–85. [DOI] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, 105–107.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Fan, X.-S., Li, Y.-Z., Zhang, X.-Y., Hu, X.-Y. & Wang, J.-J. (2005). Chin. Chem. Lett. 16, 897.
- Hideo, T. (1981). Jpn Tokkyo Koho JP 56 005 480.
- Jin, T. S., Zang, J. S., Wang, A. Q. & Li, T. S. (2005). Synth. Commun. 35, 2339–2345.
- Lambert, R. W., Martin, J. A., Merrett, J. H., Parkes, K. E. B. & Thomas, G. J. (1997). PCT Int. Appl. WO 9 706 178.
- Mehdi, S. H., Hashim, R., Ghalib, R. M., Yeap, C. S. & Fun, H.-K. (2011). Acta Cryst. E67, o1449. [DOI] [PMC free article] [PubMed]
- Menchen, S. M., Benson, S. C., Lam, J. Y. L., Zhen, W., Sun, D., Rosenblum, B. B., Khan, S. H. & Taing, M. (2003). US Patent 6 583 168.
- Poupelin, J. P., Saint-Ruft, G., Foussard-Blanpin, O., Narcisse, G., Uchida-Ernouf, G. & Lacroix, R. (1978). Eur. J. Med. Chem. 13, 67–71.
- Ravindranath, B. & Seshadri, T. R. (1973). Phytochemistry, 12, 2781–2788.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Srihari, P., Mandal, S. S. & Reddy, J. S. S. (2008). Chin. Chem. Lett. 19, 771–774.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811023014/hb5885sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811023014/hb5885Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811023014/hb5885Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report