Abstract
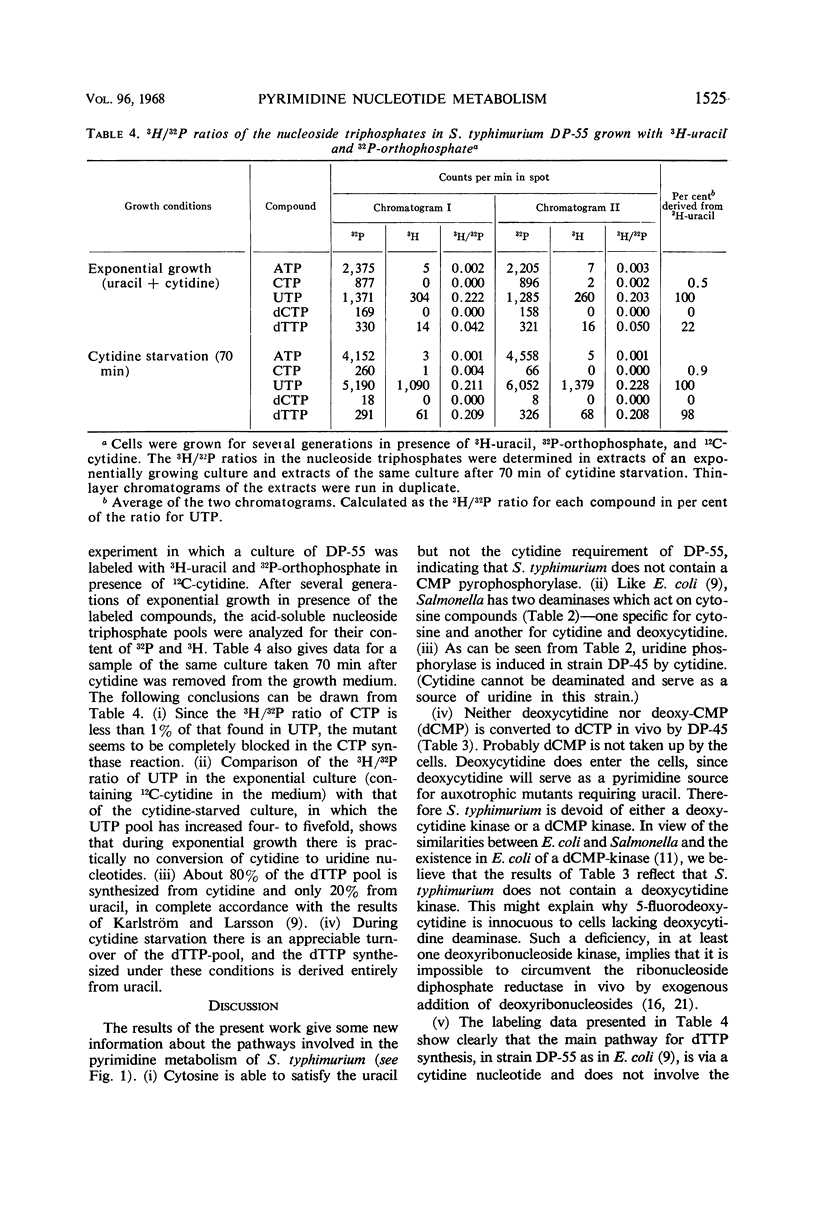
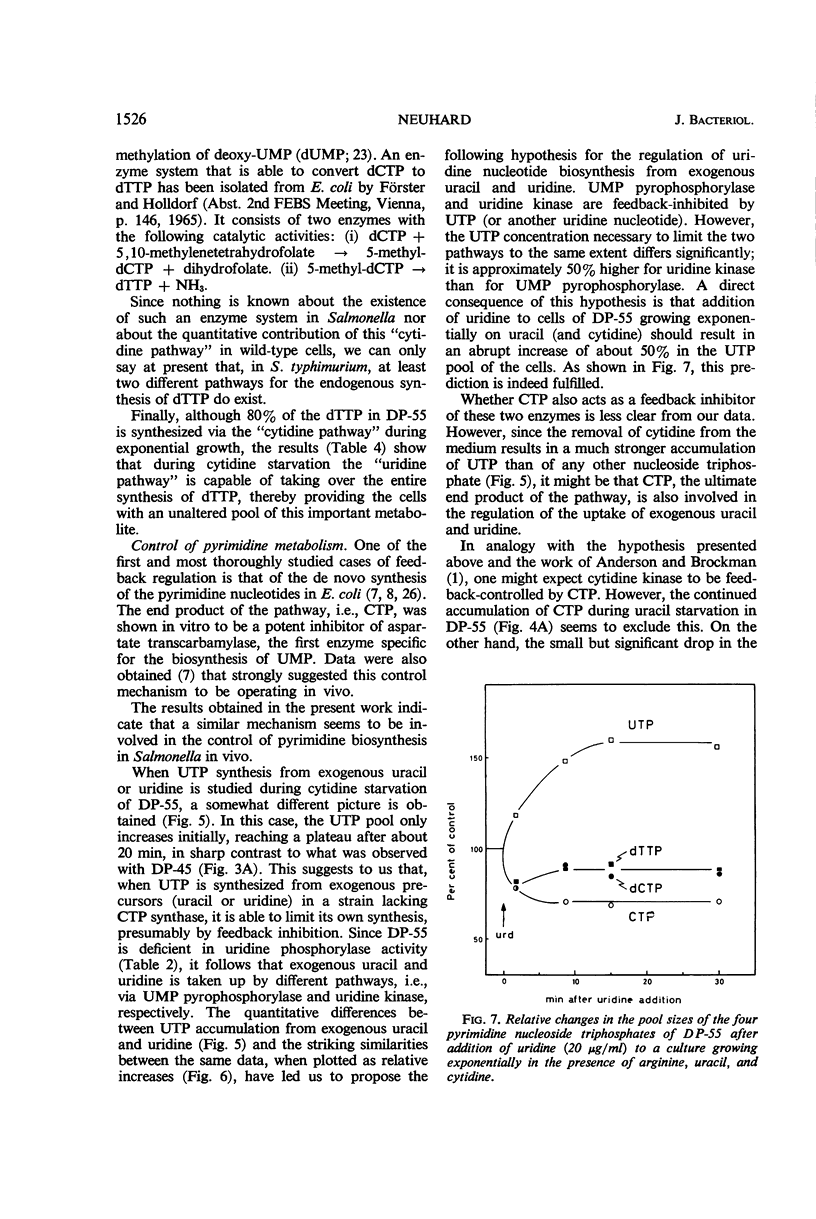
The nucleoside triphosphate pools of two cytidine auxotrophic mutants of Salmonella typhimurium LT-2 were studied under different conditions of pyrimidine starvation. Both mutants, DP-45 and DP-55, are defective in cytidine deaminase and cytidine triphosphate (CTP) synthase. In addition, DP-55 has a requirement for uracil (uridine). Cytidine starvation of the mutants results in accumulation of high concentrations of uridine triphosphate (UTP) in the cells, while the pools of CTP and deoxy-CTP drop to undetectable levels within a few minutes. Addition of deoxycytidine to such cells does not restore the dCTP pool, indicating that S. typhimurium has no deoxycytidine kinase. From the kinetics of UTP accumulation during cytidine starvation, it is concluded that only cytidine nucleotides participate in the feedback regulation of de novo synthesis of UTP; both uridine and cytidine nucleotides participate in the regulation of UTP synthesis from exogenously supplied uracil or uridine. Uracil starvation of DP-55 in presence of cytidine results in extensive accumulation of CTP, suggesting that CTP does not regulate its own synthesis from exogenous cytidine. Analysis of the thymidine triphosphate (dTTP) pool of DP-55 labeled for several generations with 32P-orthophosphate and 3H-uracil in presence of 12C-cytidine shows that only 20% of the dTTP pool is derived from uracil (via the methylation of deoxyuridine monophosphate); 80% is apparently synthesized from a cytidine nucleotide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. P., BROCKMAN R. W. FEEDBACK INHIBITION OF URIDINE KINASE BY CYTIDINE TRIPHOSPHATE AND URIDINE TRIPHOSPHATE. Biochim Biophys Acta. 1964 Nov 15;91:380–386. doi: 10.1016/0926-6550(64)90067-2. [DOI] [PubMed] [Google Scholar]
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- BROCKMAN R. W., DAVIS J. M., STUTTS P. Metabolism of uracil and 5-fluorouracil by drug-sensitive and by drug-resistant bacteria. Biochim Biophys Acta. 1960 May 6;40:22–32. doi: 10.1016/0006-3002(60)91311-1. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTY K. P., HURLBERT R. B. Role of glutamine in the biosynthesis of cytidine nucleotides in Escherichia coli. Biochim Biophys Acta. 1961 Mar 4;47:607–609. doi: 10.1016/0006-3002(61)90563-7. [DOI] [PubMed] [Google Scholar]
- CRAWFORD I., KORNBERG A., SIMMS E. S. Conversion of uracil and orotate to uridine 5'-phosphate by enzymes in lactobacilli. J Biol Chem. 1957 Jun;226(2):1093–1101. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Karlström O., Larsson A. Significance of ribonucleotide reduction in the biosynthesis of deoxyribonucleotides in Escherichia coli. Eur J Biochem. 1967 Dec;3(2):164–170. doi: 10.1111/j.1432-1033.1967.tb19512.x. [DOI] [PubMed] [Google Scholar]
- Karlström O. Mutants of Escherichia coli defective in ribonucleoside and deoxyribonucleoside catabolism. J Bacteriol. 1968 Mar;95(3):1069–1077. doi: 10.1128/jb.95.3.1069-1077.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Zimmerman S. B., Kornberg S. R., Josse J. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. INFLUENCE OF BACTERIOPHAGE T2 ON THE SYNTHETIC PATHWAY IN HOST CELLS. Proc Natl Acad Sci U S A. 1959 Jun;45(6):772–785. doi: 10.1073/pnas.45.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., NEUHARD J. STUDIES ON THE ACID-SOLUBLE NUCLEOTIDE POOL IN THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI DURING THYMINE STARVATION. I. ACCUMULATION OF DEOXYADENOSINE TRIPHOSPHATE IN ESCHERICHIA COLI 15 T-A-U-. Biochim Biophys Acta. 1964 Apr 27;80:542–551. doi: 10.1016/0926-6550(64)90298-1. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Randerath E., Randerath K. Ion-exchange thin-layer chromatography. 13. Resolution of complex nucleoside triphosphate mixtures. Anal Biochem. 1965 Nov;13(2):211–222. doi: 10.1016/0003-2697(65)90191-0. [DOI] [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in Escherichia coli. IV. Effects of hydroxyurea. Biochim Biophys Acta. 1967 Aug 22;145(1):1–6. doi: 10.1016/0005-2787(67)90647-8. [DOI] [PubMed] [Google Scholar]
- Neuhard J. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. 3. On the regulation of the deoxyadenosine triphosphate and deoxycytidine triphosphate pools of Escherichia coli. Biochim Biophys Acta. 1966 Oct 24;129(1):104–115. doi: 10.1016/0005-2787(66)90012-8. [DOI] [PubMed] [Google Scholar]
- PAEGE L. M., SCHLENK F. Bacterial uracil riboside phosphorylase. Arch Biochem Biophys. 1952 Sep;40(1):42–49. doi: 10.1016/0003-9861(52)90071-4. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem. 1956 Aug;221(2):757–770. [PubMed] [Google Scholar]
- Peterson R. N., Boniface J., Koch A. L. Energy requirements, interactions and distinctions in the mechanisms for transport of various nucleosides in Escherichia coli. Biochim Biophys Acta. 1967 Sep 9;135(4):771–783. doi: 10.1016/0005-2736(67)90108-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S., Carr H. S., Pollak R. D. Studies with hydroxyurea. VI. Effects of hydroxyurea on the metabolism of sensitive and resistant strains of Escherichia coli. Biochim Biophys Acta. 1967 Nov 21;149(1):228–245. doi: 10.1016/0005-2787(67)90704-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. THE CHROMOSOMAL LOCATION OF THE STRUCTURAL GENE FOR OROTIDYLIC ACID PYROPHOSPHORYLASE IN ESCHERICHIA COLI. J Mol Biol. 1964 May;8:771–771. doi: 10.1016/s0022-2836(64)80124-8. [DOI] [PubMed] [Google Scholar]
- WAHBA A. J., FRIEDKIN M. The enzymatic synthesis of thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J Biol Chem. 1962 Dec;237:3794–3801. [PubMed] [Google Scholar]
- WANG T. P., SABLE H. Z., LAMPEN J. O. Enzymatic deamination of cytosine nucleosides. J Biol Chem. 1950 May;184(1):17–28. [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]
- Yan Y., Demerec M. Genetic analysis of pyrimidine mutants of Salmonella typhimurium. Genetics. 1965 Sep;52(3):643–651. doi: 10.1093/genetics/52.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]


