Introduction
Acute disseminated encephalomyelitis (ADEM) is a monophasic, postinfectious or postvaccineal acute inflammatory demyelinating disorder of central nervous system (CNS).[1,2] The pathophysiology involves transient autoimmune response directed at myelin or other self-antigens, possibly by molecular mimicry or by nonspecific activation of autoreactive T-cell clones.[3] Histologically, ADEM is characterized by perivenous demyelination and infiltration of vessel wall and perivascular spaces by lymphocytes, plasma cells, and monocytes.[4]
The annual incidence of ADEM is reported to be 0.4–0.8 per 100,000 and the disease more commonly affects children and young adults, probably related to the high frequency of exanthematous and other infections and vaccination in this age group.[5–9] There seems to be no gender predominance.[10]
Clinical Features
In ADEM, neurologic deficits develop 3–6 weeks following an antecedent event. The onset can be abrupt or may evolve over a period of several days. Prodromal illness may precede the neurologic symptoms.[10] ADEM can affect any part of the neuraxis and thus the clinical presentation is variable and usually polysymptomatic [Box 1]: altered mental status, pyramidal dysfunction, cerebellar ataxia, brainstem syndromes, optic neuritis, myelitis, and rarely myeloradiculopathy and extrapyramidal syndromes.[11,12] Seizures are not uncommon, can be focal or generalized. Encephalitic illness is more common in children younger than 3 years. [Box 2][12] Rarely ADEM may present with features of intracranial space occupying lesion, with tumefactive demyelinating lesions.[13–17]
Box 1.
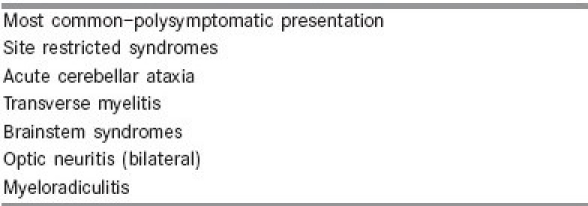
Acute disseminated encephalomyelitis: Clinical syndromes
Box 2.

Common clinical and laboratory features of ADEM
Certain clinical presentations may be specific with certain infections: cerebellar ataxia for varicella infection, myelitis for mumps, myeloradiculopathy for Semple antirabies vaccination, and explosive onset with seizures and mild pyramidal dysfunction for rubella.[18,19] Acute hemorrhagic leukoencephalitis and acute necrotizing hemorrhagic leukoencephalitis of Weston Hurst represent the hyperacute, fulminant form of postinfectious demyelination.[20]
Diagnosis
Cerebrospinal fluid (CSF) is abnormal in about two-thirds of patients and shows a moderate pleocytosis with raised proteins.[21] Oligoclonal band in CSF is usually absent in ADEM whereas it is a common finding in the CSF in patients with multiple sclerosis (MS).[22]
Magnetic resonance imaging (MRI) is the imaging modality of choice to demonstrate white matter lesion in ADEM and MS. A recent study in children suggested the presence of any 2 of the MRI features: (1) absence of bilateral diffuse pattern; (2) presence of black holes; and (3) presence of 2 or more periventricular lesions help to differentiate MS from ADEM. The sensitivity and specificity of these criteria was 81% and 95%. respectively. In this study the total number of lesions did not differentiate ADEM from MS but periventricular lesions were more frequent in MS.[23] A study done to compare the MRI pattern of lesions, which could help to differentiate ADEM from MS found the following characteristics: solitary lesion, unilateral large lesion, cortical lesions, and subcortical grey matter (basal ganglia and thalamus) involvement.[24] Other studies suggested that bilateral thalamic lesion may be diagnostic of ADEM.[15,16,25–28]
Differential Diagnosis
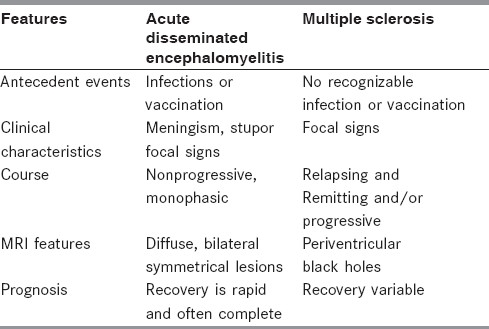
Monophasic ADEM has to be differentiated from the first attack of MS. In the absence of a biological marker, the distinction between ADEM and MS cannot be made with certainty at the time of first presentation.[15] However, certain clinical features are more indicative of ADEM [Box 1 and Table 1].[15,16] In addition, MRI features may be diagnostic of MS or ADEM. Differentiating ADEM from the first attack of MS is of therapeutic importance as early institution of disease modifying drugs will modify the course of MS.
Table 1.
Differential diagnosis: Acute disseminated encephalomyelitis vs multiple sclerosis
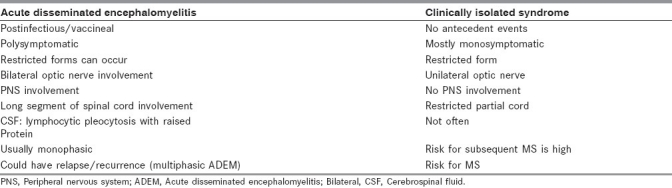
Site restricted syndromes of ADEM may have to be differentiated from Clinical Isolated Syndrome (CIS) [Table 2]. CIS is characterized by the occurrence of a single, clinical (monofocal presentation), demyelinating event with no clinical evidence of MS lesion in space and time. The most common presentation includes optic neuritis, partial myelitis, brainstem syndromes, or multifocal abnormalities.[29]
Table 2.
Site restricted syndromes of acute disseminated encephalomyelitis and clinically isolated syndrome
The patient with a CIS would have sustained a first ever clinical demyelinating event, and has 2 clinically silent lesions on T2-weighted brain MRI, with a size of at least 3 mm, at least one of which is ovoid or periventricular or infratentorial in the first imaging. The revised MS diagnostic criteria are of great value as it enables one to make an earlier diagnosis of MS, based on the development of new lesions on MRI brain, despite the absence of a new clinical event. The MRI brain can predict the conversion to clinical definite MS (CDMS) one cohort study had shown that 88% of patients with an abnormal scan experienced a second event, whereas only 19% of patients with a normal initial scan converted to MS.[30] Most patients with CIS develop either CDMS or new brain MRI-confirmed lesions within a very short period of 18 months, and the presence of gadolinium-enhancing lesions or meeting MS MRI diagnostic criteria further increases this likelihood.[31] The diagnostic criteria of CIS are heavily weighted on imaging features.
In the Optic Neuritis Treatment Trial, the risk of conversion to MS at 10 years is 22% in patients who have a normal imaging of the brain, and 56% in patients with an abnormal brain imaging. O’ Riordan et al found that in patients presenting with optic neuritis, transverse myelitis, and brain stem syndromes, along with an abnormal MRI of brain, had a risk of 83% going on to develop MS at 10 years, while those with a normal brain MR had only a 11% risk.[32]
CSF oligoclonal bands (OCBs) in CIS showed a sensitivity of 91.4% and specificity of 94.1%.[33] Masjuan et al found that 32/33 patients with OCBs went on to develop MS in 6 years, whereas only 3/19 patients without OCBs went on to develop MS.[31]
Treatment
Spontaneous improvement has been documented in patients with ADEM.[34] However, the recovery is incomplete in patients with ADEM not receiving any form of immune modulation treatment. No therapy has been established by controlled trials in ADEM. Use of high-dose steroids, plasma exchange, and intravenous immunoglobulin are based on the analogy of pathogenesis of ADEM with that of MS.[35,36]
Treatment of ADEM includes: (1) supportive, and (2) specific—high-dose intravenous methyl prednisolone, intravenous immunoglobulin (IVIg), and plasma paresis, and (3) physical and rehabilitation therapy [Figure 1].
Figure 1.
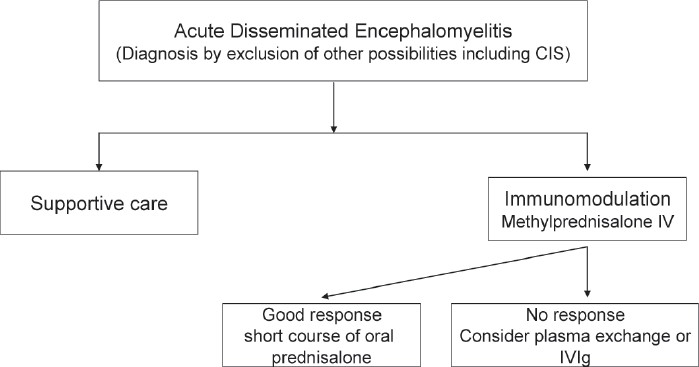
Treatment algorithm—acute disseminated encephalomyelitis
Supportive Care
Supportive care includes airway protection in patients with altered mental status and mechanical ventilation if required. Patients with cervical myelitis may require mechanical ventilation. Other supportive includes: antiseizure medication in patients with seizures, correction of fluid and electrolyte disturbances, and prophylactic anticoagulation for prevention of deep vein thrombosis in patients with high risk.
Immunomodulation
Intravenous methyl prednisolone is the first-line drug (10–30 mg/kg/day, up to a maximum of 1 g/day) for 3–5 days are being used (Class IV).[3,4,12,37,38] With this modality of treatment, full recovery has been reported in 50%–80% of patients. Methylprednisolone-treated patients had significantly better outcome with respect to disability status when compared with those treated with dexamethasone.[39] Oral corticosteroid treatment is continued with gradual tapering over 6 weeks to reduce the risk of relapses. However, these regimens are not based on controlled randomized trials. The role of corticosteroids in patients presenting late in the course of the disease is questionable. Any type of vaccination should be avoided during the first 6 months following recovery.
If high-dose corticosteroids fail, the next step will be plasma exchange (PE) and there is Class Ib evidence for PE.[40–42] A course of 4–6 PEs have been shown to be associated with moderate to marked and sustained improvement. One could remove a large volume of plasma per exchange if there are no problems of autonomic dysfunction. Predictors associated with improvement include male sex, preserved reflexes, and early initiation of treatment.[39,40] In centers that do not have this facility for conventional PE, one could modify and improvise to do a small volume manual plasma exchange—by doing a phlebotomy, centrifuging the blood, remove 250–300 mL of plasma and return the cells. One could do this twice a day for 7–10 days.[43]
Intravenous immunoglobulin (IVIg) (0.4 gm/kg/day for 5 days) is another option, but there is a constraint of high cost and the evidence for this modality of treatment in ADEM is Class IV.[44] The improvement is seen within 2–3 days.[45–47] In the absence of randomized controlled trials and with the available evidence, either plasma exchange or IVIg, could be the second-line treatment, when corticosteroids fail. The choice of second-line treatment should be individualized, depending on the severity of the disease, complications, and comorbidities. For example, autonomic dysfunction and hypotension would preclude the use of PE.[42] Anecdotal reports suggest that IVIg may be more effective in patients with peripheral nervous system involvement and PE in patients with tumefactive demyelination. Methyl prednisolone along with IVIg has been successfully used in patients with atypical features and could be tried for fulminant, aggressive, and atypical disease.[48]
Cyclophosphamide[10] and hypothermia[49] have been used with success in patients with fulminant ADEM. Decompressive hemi-craniectomy has been reported to be life saving in patients with massive life-threatening cerebral edema refractory to conventional medical management.[50,51]
Relapsing/Recurrence/Multiphasic Acute Disseminated Encephalomyelitis
Although ADEM is typically a monophasic illness, occasionally it can have biphasic or multiphasic course. Different terminologies have been used for an event that occurs within 4 weeks of treatment or within the first 3 months, relapsing ADEM. However, some researchers view new event temporally related to the initial disease process, and thought to be related to early steroid withdrawal and hence terminology steroid dependent or pseudo-relapsing ADEM.
If there is another event 4 weeks after steroid withdrawal or 3 months after the first episode, and if both clinically and radiologically, the same site is involved, this entity is called recurrent ADEM.[52] But there needs to be a prerequisite that this patient was in complete remission or as in a stable plateau phase of incomplete remission. If one or more ADEM relapses occur, including encephalopathy and multifocal deficits occur, involving new areas of the neuraxis on MRI and neurologic examination, this entity is multiphasic ADEM.[53] If only the imaging criteria are used these entities would fulfill the criteria for MS and hence these patients need to be referred to a tertiary center with expertise in management of these problems. The present evidence suggests that these episodes are probably related to the ongoing active disease and the autoimmune disease is probably due to persistent antigen/epitope progression and these episodes occur in relation to the initial episode.
Prognosis
Earlier studies reported a mortality rate of 20% with a high incidence of neurologic sequelae in those who survived probably it was related to high incidence of postmeasles ADEM.[8] However, recent studies suggest a favorable prognosis.[2,24,54] Prolonged altered mental state was associated with both mortality and morbidity. Multiple or single extensive lesions on MRI lesions may be associated with disability.[18]
The long-term prognosis of this entity depends on the etiology, with postmeasles patients having a higher mortality rate and significant morbidity in survivors. The prognosis of nonmeasles cases is favorable and most studies report a full recovery in 50%–75% of patients, in a period of 1–6 months after the illness.[3,4] The most common sequelae are focal motor deficits, could range from mild ataxia to hemiparesis. The principal determinants for the extent of neuronal and axonal damage are duration and severity of inflammation in brain and spinal cord. A hyperacute onset, severe neurologic deficits as a result of aggressive disease, and unresponsiveness to steroids are poor prognostic indicators.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tenembaum S, Chitnis T, Ness J, Hahn JS. International Pediatric MS Study Group. Acute Disseminated Encephalomyelitis. Neurology. 2007;68:S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 2.Krupp LB, Banwell B, Tenembaum S. The International Pediatric MS Study Group.Consensus definitions proposed for pediatric multiple Sclerosis and related childhood disorders. Neurology. 2007;68:S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8. [DOI] [PubMed] [Google Scholar]
- 3.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–12. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 4.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123:2407–22. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Munger K. Epidemiology of Multiple Sclerosis.From Risk factors to Prevention. Semin Neurol. 2008;28:17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- 6.Panicker JN. Bangalore: National Institute of Mental Health and Neurosciences, NIMHANS (Deemed University); 2004. Acute Disseminated Encephalomyelitis: Clinical Profile and Predictors of Outcome (dissertation) [Google Scholar]
- 7.Menge T, Hemmer B, Nessler S, Wiendl H, Neuhaus O, Hartung HP, et al. Acute disseminated encephalomyelitis: An update. Arch Neurol. 2005;62:1673–80. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 8.Anlar B, Basaran C, Kose G, Guven A, Haspolat S, Yakut A, et al. Acute disseminated Encephalomyelitis in Children: Outcome and Prognosis. Neuropediatrics. 2003;34:194–9. doi: 10.1055/s-2003-42208. [DOI] [PubMed] [Google Scholar]
- 9.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: A long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–31. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute Disseminated Encephalomyelitis: A followup study of 40 adult patients. Neurology. 2001;56:1313–8. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 11.Wingerchuk DM, Weinshenker BG. Multiple Sclerosis: Epidemiology, genetics, classification, natural history and clinical outcome measures. Neuroimaging Clin North Am. 2000;10:611–24. [PubMed] [Google Scholar]
- 12.Singh S, Alexander M, Korah IP. Acute Disseminated Encephalomyelitis: Pictorial Essay. Am J Roentgenol. 1999;173:1101–7. doi: 10.2214/ajr.173.4.10511187. [DOI] [PubMed] [Google Scholar]
- 13.Idrissova ZR, Boldyreva MN, Dekonenko EP, Malishev NA, Leontyeva IY, Martinenko IN, et al. Acute disseminated encephalomyelitis in children: Clinical features and HLA-DR linkage. Eur J Neurol. 2003;10:537–46. doi: 10.1046/j.1468-1331.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Alexander M, Sase N, Korah IP. Solitary Hemispheric Demyelination in Acute Disseminated Encephalomyelitis. Australas Radiol. 2003;47:29–36. doi: 10.1046/j.1440-1673.2003.t01-2-01126.x. [DOI] [PubMed] [Google Scholar]
- 15.Murthy JM. Acute disseminated encephalomyelitis. Neurol India. 2002;50:238–43. [PubMed] [Google Scholar]
- 16.Murthy JM, Yangala R, Meena AK, Jaganmohan Reddy J. Acute disseminated encephalomyelitis: Clinical and MRI study from South India. J Neurol Sci. 1999;132:133–8. doi: 10.1016/s0022-510x(99)00094-5. [DOI] [PubMed] [Google Scholar]
- 17.Krivickas LS, Hochberg FH, Freeman S. Chronic inflammatory demyelinating polyradiculoneuropathy with tumefactive central demyelination. Muscle Nerve. 2006;33:283–8. doi: 10.1002/mus.20412. [DOI] [PubMed] [Google Scholar]
- 18.Murthy SK, Faden HS, Cohen ME, Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110:e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 19.Noorbaksh F, Johnson RT, Emery D, Power C. Acute Disseminated Encephalomyelitis: Clinical and Pathogenesis features. Neurol Clin. 2008;26:759–80. doi: 10.1016/j.ncl.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuperan S, Ostrow P, Landi MK, Bakshi R. Acute Hemorrhagic Leukoencephalitis vs ADEM: FLAIR MRI and neuropathology findings. Neurology. 2003;2:721–2. doi: 10.1212/01.wnl.0000048493.82053.4c. [DOI] [PubMed] [Google Scholar]
- 21.Francis GS, Deguette P, Antel JP. Infl ammatory demyelinating disease of the central nervous system. In: Bradely WG, Daroff RB, Fenichel GM, editors. Neurol Clin Prac. 3nd ed. Vol. 2. Boston: Butterworth Heinemann; 1995. pp. 1307–43. [Google Scholar]
- 22.Rust RS, Dodson W, Prensky A. Classification and outcome of acute disseminated encephalomyelitis. Ann Neurol. 1997;42:491. [Google Scholar]
- 23.Callen DJ, Shroff MM, Branson HM, Li DK, Lotze T, Stephens D, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009;72:961–7. doi: 10.1212/01.wnl.0000338630.20412.45. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Prabhakar S, Korah IP, Warade SS, Alexander M. Acute Disseminated Encephalomyelitis and Multiple Sclerosis.Magnetic Resonance Imaging Differentiation. Australas Radiol. 2000;44:404–11. doi: 10.1046/j.1440-1673.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Kesselring J, Miller DH, Robb SA, Kendall BE, Moseley IF, Kingsley D, et al. Acute disseminated encephalomyelitis. Brain. 1990;113:291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]
- 26.Andreula CF, Recchia Luciana NM, Milella D. Magnetic resonance imaging in the diagnosis of acute disseminated encephalomyelitis. Int J Neuroradiol. 1997;3:21–34. [Google Scholar]
- 27.Baum PA, Barkovich AJ, Koch TK, Berg BO. Deep gray matter involvement in children with acute disseminated encephalomyelitis. AJNR Am J Neuroradiol. 1994;15:1275–83. [PMC free article] [PubMed] [Google Scholar]
- 28.Okumura A, Hayakawa M, Watanabe K, Kito M, Negoro T, Kawamura M. Two cases of acute disseminated encephalomyelitis with lesion in the thalamus or basal ganglia. No to Hattatsu. 1992;24:278–82. [PubMed] [Google Scholar]
- 29.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and Progression of disability in multiple sclerosis. N Engl J Med. 2000;343:1430–8. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 30.Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–64. doi: 10.1056/NEJMoa011341. [DOI] [PubMed] [Google Scholar]
- 31.CHAMPS Study Group. MRI predictors of early conversion to clinically defi nite MS in the CHAMPS placebo group. Neurology. 2002;59:998–1005. doi: 10.1212/wnl.59.7.998. [DOI] [PubMed] [Google Scholar]
- 32.O’Riordan JI, Thompson AJ, Kingsley DP, MacManus DG, Kendall BE, Rudge P, et al. The prognostic value of Brain MRI in Clinically Isolated Syndromes of the CNS : 10 year follow up. Brain. 1998;121:495–503. doi: 10.1093/brain/121.3.495. [DOI] [PubMed] [Google Scholar]
- 33.Masjuan J, Alvarez-Cermeño JC, García-Barragán N, Díaz-Sánchez M, Espiño M, Sádaba MC, et al. Clinically Isolated Syndromes: A new oligoclonal band test accurately predicts conversion to MS. Neurology. 2006;66:576–8. doi: 10.1212/01.wnl.0000198253.35119.83. [DOI] [PubMed] [Google Scholar]
- 34.Rust RS, Dodson W, Prensky A. Classification and outcome of acute disseminated encephalomyelitis. Ann Neurol. 1997;42:491. [Google Scholar]
- 35.Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, et al. Comparison of MRI Criteria at first presentation to predict conversion to clinically definite Multiple Sclerosis. Brain. 1997;120:2059–69. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 36.Noseworthy JH, Hartung HP. Multiple Sclerosis and Related Conditions.Neurological Therapeutics. Principles and Practice. (2nd Ed) 2006;1:1225–50. [Google Scholar]
- 37.Wingerchuk DM. Current Evidence and Therapeutic Strategies in Multiple Sclerosis. Semin Neurol. 2008;28:56–68. doi: 10.1055/s-2007-1019128. [DOI] [PubMed] [Google Scholar]
- 38.Straub J, Chofflon M, Delavelle J. Early high-dose intravenous methylprednisolone in acute disseminated encephalomyelitis: A successful recovery. Neurology. 1997;49:1145–7. doi: 10.1212/wnl.49.4.1145. [DOI] [PubMed] [Google Scholar]
- 39.Sakakibara R, Hattori T, Yasuda K, Yamanishi T. Micturitional disturbance in acute disseminated encephalomyelitis (ADEM) J Auton Nerv Syst. 1996;60:200–5. doi: 10.1016/0165-1838(96)00054-9. [DOI] [PubMed] [Google Scholar]
- 40.Weinshenker BG, O’Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46:878–86. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: Predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 42.Miyazawa R, Hikima A, Takano Y, Arakawa H, Tomomasa T, Morikawa A. Plasmapheresis in fulminant acute disseminated encephalomyelitis. Brain Dev. 2001;23:424–6. doi: 10.1016/s0387-7604(01)00256-x. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S. Manual (small) volume Plasmapheresis: An effective and safe therapeutic procedure in Acute Neurological Illness. Ann Indian Acad Neurol. 2004;7:439–40. [Google Scholar]
- 44.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Disc. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 45.Sahlas DJ, Miller SP, Guerin M, Veilleux M, Francis G. Treatment of acute disseminated encephalomyelitis with intravenous immunoglobulin. Neurology. 2000;54:1370–2. doi: 10.1212/wnl.54.6.1370. [DOI] [PubMed] [Google Scholar]
- 46.Kleiman M, Brunquell P. Acute disseminated encephalomyelitis: Response to intravenous immunoglobulin? J Child Neurol. 1995;10:481–3. doi: 10.1177/088307389501000612. [DOI] [PubMed] [Google Scholar]
- 47.Pittock SJ, Keir G, Alexander M, Brennan P, Hardiman O. Rapid clinical and CSF response to intravenous gamma globulin in acute disseminated encephalomyelitis. Eur J Neurol. 2001;8:725. doi: 10.1046/j.1468-1331.2001.00195.x. [DOI] [PubMed] [Google Scholar]
- 48.Straussberg R, Schonfeld T, Weitz R, Karmazyn B, Harel L. Improvement of atypical acute disseminated encephalomyelitis with steroids and intravenous immunoglobulins. Pediatr Neurol. 2001;24:139–43. doi: 10.1016/s0887-8994(00)00229-0. [DOI] [PubMed] [Google Scholar]
- 49.Takata T, Hirakawa M, Sakurai M, Kanazawa I. Fulminant form of acute disseminated encephalomyelitis: Successful treatment with hypothermia. J Neurol Sci. 1999;165:94–7. doi: 10.1016/s0022-510x(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 50.Von Stuckrad-Barre S, Klippel E, Foerch C, Lang JM, du Mesnil de Rochemont R, Sitzer M. Hemicraniectomy as a successful treatment of mass effect in acute disseminated encephalomyelitis. Neurology. 2003;61:420–1. doi: 10.1212/01.wnl.0000073540.35919.ae. [DOI] [PubMed] [Google Scholar]
- 51.Refai D, Lee MC, Goldenberg FD, Frank JI. Decompressive hemicraniectomy for acute disseminated encephalomyelitis: Case report. Neurosurgery. 2005;56:E872. doi: 10.1227/01.neu.0000156201.46473.a8. [DOI] [PubMed] [Google Scholar]
- 52.Cohen O, Steiner-Birmanns B, Biran I, Abramsky O, Honigman S, Steiner I. Recurrence of ADEM at previously affected brain site. Arch Neurol. 2001;58:797–801. doi: 10.1001/archneur.58.5.797. [DOI] [PubMed] [Google Scholar]
- 53.Singhi PD, Ray M, Singhi S, Khandelwal NK. Acute disseminated encephalomyelitis in North Indian children: Clinical profile and follow-up. J Child Neurol. 2006;21:851–73. doi: 10.1177/08830738060210100201. [DOI] [PubMed] [Google Scholar]
- 54.Kesselring J, Miller DH, Robb SA, Kendall BE, Moseley IF, Kingsley D, et al. Acute disseminated encephalomyelitis. Brain. 1990;113:291–302. doi: 10.1093/brain/113.2.291. [DOI] [PubMed] [Google Scholar]


