Abstract
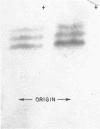
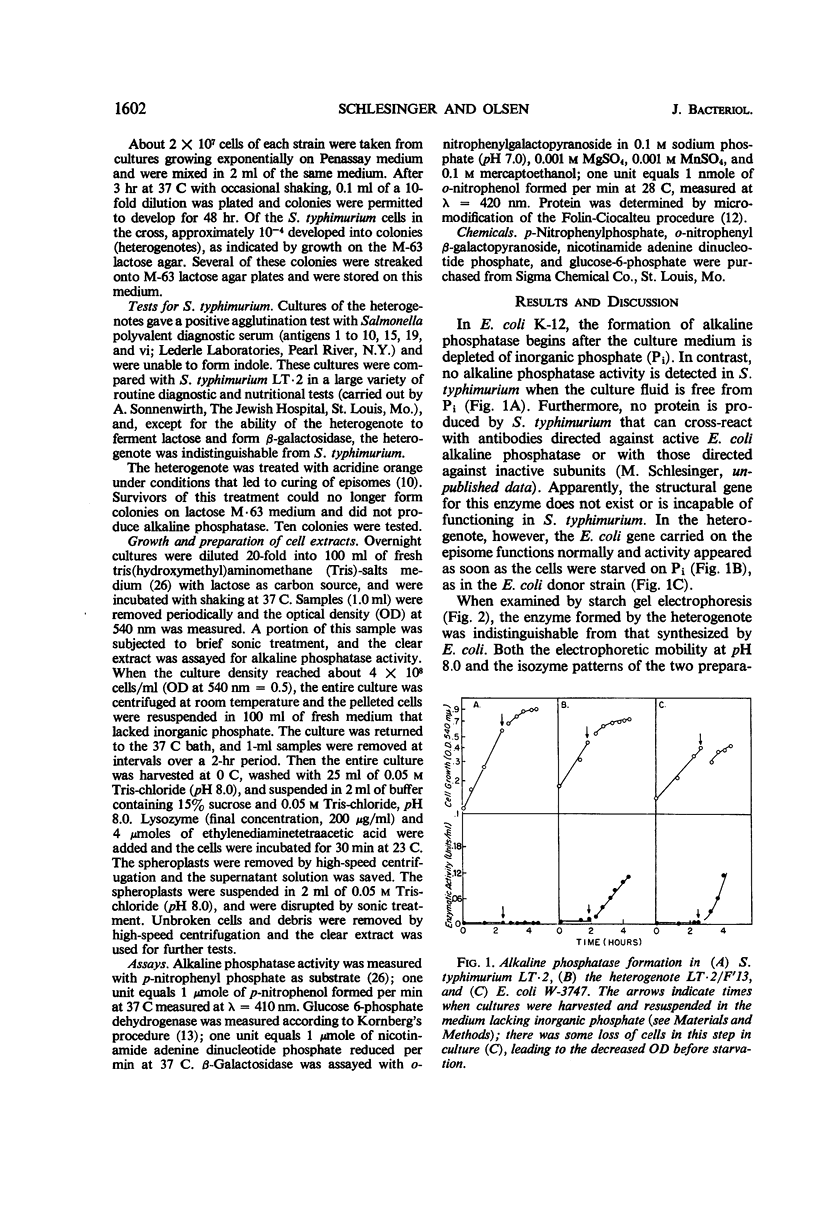
The Escherichia coli structural gene for alkaline phosphatase was inserted into Salmonella typhimurium by episomal transfer in order to determine whether this enzyme would continue to be localized to the periplasmic space of the bacterium even though it was formed in a cell that does not synthesize alkaline phosphatase. The S. typhimurium heterogenote synthesized alkaline phosphatase under conditions identical to that observed with E. coli. This enzyme appeared to be identical to that synthesized by E. coli, and was quantitatively released from the bacterial cell by spheroplast formation with lysozyme. These results showed that localization is not a property unique to the E. coli cell and suggested that, in E. coli, enzyme location is related to the structure of the protein. Formation of alkaline phosphatase in the S. typhimurium heterogenote was repressed in cells growing in a medium with excess inorganic phosphate, even though only one of the three regulatory genes for this enzyme is on the episome. Thus, S. typhimurium can supply the products of the other two regulatory genes essential for repression even though this bacterium seems to lack the structural gene for alkaline phosphatase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON L. S., SPILMAN W. M., CAREY W. F. Hybridization of Salmonella species by mating with Escherichia coli. Science. 1959 Sep 4;130(3375):566–567. doi: 10.1126/science.130.3375.566. [DOI] [PubMed] [Google Scholar]
- CRICK F. H. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–163. [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Complementation in vivo between structural mutants of alkaline phosphatase from E. coli. J Mol Biol. 1963 Jul;7:13–22. doi: 10.1016/s0022-2836(63)80015-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- GOMORI G. Alkaline phosphatase of cell nuclei. J Lab Clin Med. 1951 Apr;37(4):526–531. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. M., FALKOW S., BARON L. S. RECIPIENT ABILITY OF SALMONELLA TYPHOSA IN GENETIC CROSSES WITH ESCHERICHIA COLI. J Bacteriol. 1964 Jan;87:54–60. doi: 10.1128/jb.87.1.54-60.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A., Bykov A. S. Elektronnomikroskopicheskoe izuchenie sekretsii shchelochnoi fosfatazy kletkami Escherichia coli. Dokl Akad Nauk SSSR. 1967 Jul 21;175(3):718–720. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Miyake T. Exchange of Genetic Material between Salmonella Typhimurium and Escherichia Coli K-12. Genetics. 1962 Aug;47(8):1043–1052. doi: 10.1093/genetics/47.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- ROTHMAN F., BYRNE R. Fingerprint analysis of alkaline phosphatase of Escherichia coli K12. J Mol Biol. 1963 Apr;6:330–340. doi: 10.1016/s0022-2836(63)80092-3. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Conformational states of the subunit of Escherichia coli alkaline phosphatase. Biochemistry. 1967 Nov;6(11):3552–3559. doi: 10.1021/bi00863a029. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Hydrogen ion equilibria of conformational states of Escherichia coli alkaline phosphatase. Biochemistry. 1968 Jun;7(6):2080–2085. doi: 10.1021/bi00846a009. [DOI] [PubMed] [Google Scholar]
- SIGNER E. R., TORRIANI A., LEVINTHAL C. Gene expression in intergeneric merozygotes. Cold Spring Harb Symp Quant Biol. 1961;26:31–34. doi: 10.1101/sqb.1961.026.01.008. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Revised linkage map of Salmonella typhimurium. Bacteriol Rev. 1967 Dec;31(4):354–372. doi: 10.1128/br.31.4.354-372.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Andersen L. Multiple molecular forms of the alkaline phosphatase of Escherichia coli. Ann N Y Acad Sci. 1968 Jun 14;151(1):159–170. doi: 10.1111/j.1749-6632.1968.tb11886.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of a defective alkaline phosphatase subunit by a mutant of Escherichia coli. J Biol Chem. 1967 Apr 10;242(7):1604–1611. [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. The reversible dissociation of the alkaline phosphatase of Escherichia coli. II. Properties of the subunit. J Biol Chem. 1965 Nov;240(11):4293–4298. [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. I. Substitution of triazolealanine for histidine. J Biol Chem. 1967 Jul 25;242(14):3369–3372. [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Hybrids of Escherichia and Salmonella. Science. 1960 Mar 18;131(3403):813–815. doi: 10.1126/science.131.3403.813. [DOI] [PubMed] [Google Scholar]