Multiple sclerosis (MS) is a chronic autoimmune inflammatory disorder of the central nervous system (CNS). There has been an increase in the prevalence of MS worldwide. There are no epidemiological studies available from India in the last three decades. The prevalence of 3 per 100,000 for India, quoted by the Multiple Sclerosis International Federation (MSIF), is likely to be a gross under estimation. Natural history studies have shown that 10 years after disease onset, approximately 50% of patients are using a cane to walk and 15% require a wheel chair.[1] A significant number of patients with “benign MS” develop cognitive dysfunction into the course of their disease.[2]
Clinical Features
Most of the patients have initial demyelinating events referred to as clinically isolated syndromes (CISs) such as optic neuritis, partial cord syndrome, or an acute brainstem event. If the magnetic resonance imaging (MRI) shows multifocal disease at the time of CIS, nearly half to two-third develop recurrent events and transform into clinically definite MS. Based on the type of presentation, MS is classified into:
Clinically isolated syndromes (CISs)
Relapsing and remitting multiple sclerosis (RRMS)
Secondary progressive multiple sclerosis (SPMS)
Primary progressive multiple sclerosis—progressive from the onset (PPMS)
Primary relapsing multiple sclerosis (PRMS)
An acute relapse refers to an episode of neurological disturbance, of the kind seen in MS that lasts for at least 24 h, and which was not precipitated by intercurrent infection or stress. Typically relapses evolve over days, reach a plateau, and gradually remit over a variable period of time.
Diagnosis
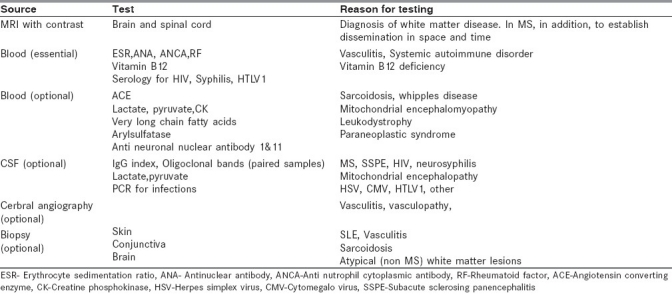
The core requirements for the diagnosis of MS are dissemination of lesions in space and time [Table 1]. The currently accepted classification for MS is the McDonald Diagnostic Criteria[3] which is heavily biased in favor of MRI. Evoked potential studies and cerebrospinal fluid (CSF) evaluation have become less important in the diagnosis of MS and are recommended when evaluating a case of CIS or there is clinical or radiological uncertainty [Table 2].
Table 1.
Diagnostic criteria for multiple sclerosis (Revised Mc Donald criteria)
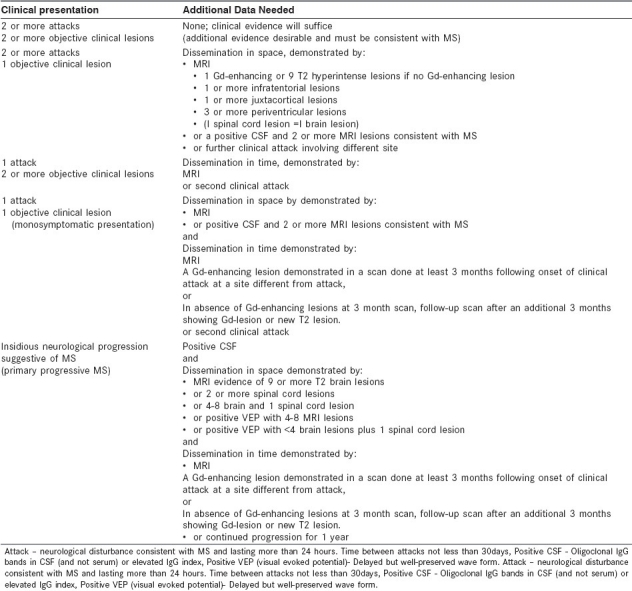
Table 2.
Diagnostic work up for suspected multiple sclerosis
The revised criteria for diagnosis of MS include the following:
At least two attacks with objective clinical evidence of at least two lesions.
At least two attacks with objective clinical evidence of one lesion plus dissemination in space1 shown on MRI or two or more MRI lesions consistent with MS plus positive CSF finding or second clinical attack.
One attack with objective clinical evidence of at least two lesions plus dissemination in time2 on MRI or second clinical attack.
One attack with objective clinical evidence of one lesion, plus dissemination in space shown on MRI or two or more MRI lesions consistent with MS plus positive CSF findings and dissemination in time shown on MRI or second clinical attack.
Insidious neurologic progression suggestive of MS plus one year of disease progression determined retrospectively or prospectively and two of the following: Positive brain MRI results (nine T2 lesions or at least four T2 lesions with positive visual evoked potential), positive spinal cord MRI results with two focal T2 lesions, and positive CSF findings.
Treatment of multiple sclerosis
Treatment of MS includes treatment of acute relapses, disease-modifying drugs, and treatment of various symptoms and complications associated with MS [Table 3 and Figure 1].
Table 3.
Treatment recommendations in multiple Sclerosis

Figure 1.
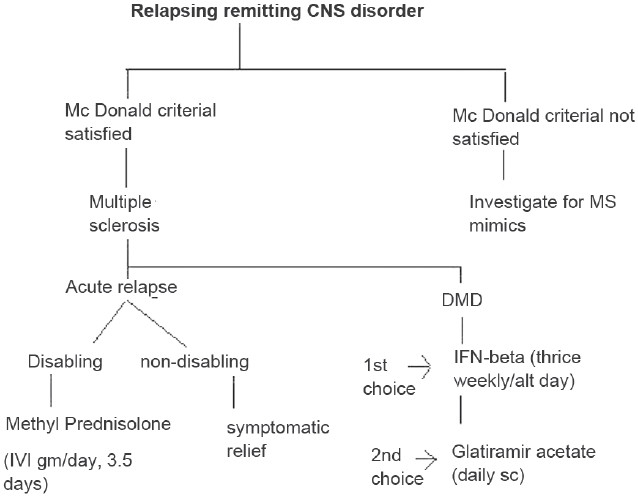
Treatment algorithm for multiple sclerosis
Note
The revised MRI criteria for dissemination in space are three of the following: One or more gadolinium-enhancing lesions or nine T2 hyperintense lesions; one or more infratentorial lesions; one or more juxtacortical lesions; or three or more periventricular lesions.
The revised MRI criteria for dissemination in time are detection of gadolinium enhancement at least 3 months after the onset of the first clinical event or detection of a new T2 lesion appearing at any time compared with a reference scan done at least 30 days after the onset of the initial clinical event.
Treatment of acute relapse
Prevention
Several factors may trigger relapses or may exacerbate existing symptoms and MS patients should be educated about preventive measures. Infections especially of the urinary tract should be anticipated and aggressively controlled. It is safe to vaccinate these patients. Stressful life events and physical trauma may also cause relapse.
Steroids
Not all relapses need to be treated. Steroids are best indicated for the treatment of disabling relapses such as optic neuritis, significant motor disability, acute ataxia, etc. The exact mechanism of action is not clear, but some of the possible mechanisms include: antiedema effects, stabilization of blood–brain barrier, reduction of proinflammatory cytokines, and apoptosis of T cells.[4]
The optic neuritis treatment trial (OPTT) established the standard of care.[5] High dose intravenous (IV) methyl prednisolone in a dose of 1 g/day for 3 days followed by a short course of oral steroids in tapering dose was shown to be significantly better than oral corticosteroids in the hastening rate of recovery following mono ocular optic neuritis. Time to next relapse was also prolonged in those who received parenteral steroids though this effect was not significant after the first 10 years following OPN. Most neurologists use IV methyl prednisolone in doses of 1 g/day for 3–5 days.[6] Oral taper with corticosteroids is a controversial area in MS therapeutics, with many neurologists preferring to avoid it. In some situations when patients do not respond well to initial 3–5 days of parenteral methyl prednisolone, the course may be repeated once again. It is also advisable to give not more than three courses of steroids per year in MS patients.[7] Transient hyperglycemia and hypertension should be looked out for and so also worsening of preexisting acid peptic disease. The specific precaution to be taken before steroid administration is to exclude infections particularly urinary. Plasmapheresis and intravenous immunoglobulin are not standard recommendations in the treatment of MS relapse.
Disease-modifying drugs in multiple sclerosis
The disease-modifying drugs (DMDs) [Table 4] are used for the relapsing forms of MS and have multiple benefits:[8,9] (1) decreases in the frequency and severity of relapses or no relapses: (2) less development of disability or sustained improvement; (3) well maintained or improved quality of life; and (4) less MRI lesion burden and less development of CNS atrophy.
Table 4.
Disease modifying drugs in multiple sclerosis
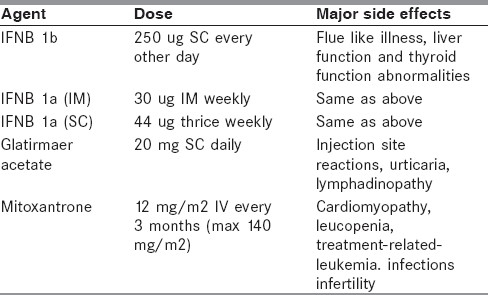
Although a variety of DMDs has become available for the treatment in MS, only interferon-beta (IFN-β), glatiramir acetate, mitoxantrone, and the very recently launched Natalizumab (Tysabri) are currently available in India.
Interferon-β
Interferons are produced naturally in human lymphocytes and modulate the immune system by influencing the production of protein products. Avonex and Rebif are forms of interferon β-1a, but the strength of the individual doses and the injection routes differ. Avonex is prepackaged as a 30-mg dose that is given once a week intramuscularly. Rebif is provided in two dose strengths, 22 mg and 44 mg, and is injected subcutaneously three times a week, beginning therapy with the lower dose. Betaseron, interferon b-1b, is given subcutaneously every other day. In several class I studies, interferon-b has been shown to reduce the attack rate in RRMS patients and also reduce T2 lesion load on MRI.[9,10] However, there is little evidence from long-term follow-up studies that it slows down sustained disability progression.
Glatiramer acetate
Glatiramer acetate (Copaxone) is a synthetic drug that interferes with antigen presentation.[11] It is given as a single, daily, 20-mg subcutaneous injection. In several class I studies, glatiramer acetate has been shown to reduce the attack rate in patients with RRMS, reduce lesion load on T2W MRI and possibly also slows sustained disability progression. It is appropriate to consider glatiramer acetate for treatment in any patient who has RRMS. Although it may be that glatiramer acetate also is helpful in patients with progressive disease, there is no convincing evidence to support this hypothesis.
Mitoxantrone
It is an anthrocenedione chemotherapeutic agent (similar to adriamycin) that has been approved for the treatment of relapsing remitting and secondary progressive MS. Three clinical trials including a large phase III study support the use of mitoxantrone in RRMS.[12–14] However, its use is associated with cardio-toxicity and leukemia in some patients who were in remission five or more years after treatment.[15] At present the best indication for mitroxantrone would be in patients with aggressive RRMS (more than two relapses in a year or worsening lesion load while on a primary disease modifying agent) and probably patients with poor response to IFN-β. However, in view of dosing limitations, at best, mitoxantrone can be used as an inducer of remission in RRMS.[16] Maintenance therapy with other DMDs is required. The best combination perhaps would be short course of mitoxantrone combined with a DMD with a good safety profile such as b-interferon or glatiramir acetate. There is no robust evidence to support the use of mitoxantrone in primary PPMS, or in the later stages of SPMR (EDSS > 6).
Natalizumab
It is a humanized monoclonal antibody directed against β-integren, an adhesion molecule expressed on all leukocytes except neurtrophils.[16] The drug has been shown to be effective in suppression of relapses, disability, and MRI lesion activity, and the drug is very well tolerated. Natalizumab is given once a month as infusion. The major concern with natalizumab is the development of progressive multifocal leukoencephalopathy (PML).[17] By February 2011, at the time of writing this article the reported cases were 95, 20 of whom have died. The risk of PML rises with duration of treatment from, <1/1000 in the first year to a little more than 1/1000 after the second year.
Other drugs used in the treatment of MS include azothiaprine, methotrexate, cyclophosphamide, and mycophenolate moftil. However, none of them have been established for use by phase III studies.
Pregnancy
During pregnancy relapse rates are low but it definitely increases in the immediate 3 months postpartum.[18] Ideally women with MS should plan their pregnancy and stop disease-modifying agents 3 months prior to conception and restart the same after breast feeding is over. IFN-β and natalizumab are Category C pregnancy drugs (where potential benefits may warrant the use of the drug in pregnant women despite potential risks), glatiramer acetate is a Category B (no demonstrable side effects in the fetus during any trimester) and mitoxantrone is a Category D (positive evidence of human fetal risk based on adverse reaction data, but potential benefits may warrant use of the drug in pregnant women despite potential risks) drug. There are no contraindications for breast feeding while on DMD.
Role of disease-modifying agents in clinically isolated syndromes
Several placebo-controlled phase III clinical trials (class I evidence) have studied patients with CIS and evidence of silent lesions on MRI. There was substantial reduction in new MRI lesions and risk of subsequent exacerbations in patients treated with interferon β-1a (Avonex or Rebif),[19] interferon β-1b (Betaseron), and more recently with glatiramer acetate (Copaxone). Patients initially treated with placebo and later converted to active treatment never achieved the magnitude of benefit seen in the patients who received early treatment with disease modifying therapy.
Symptomatic treatment of multiple sclerosis
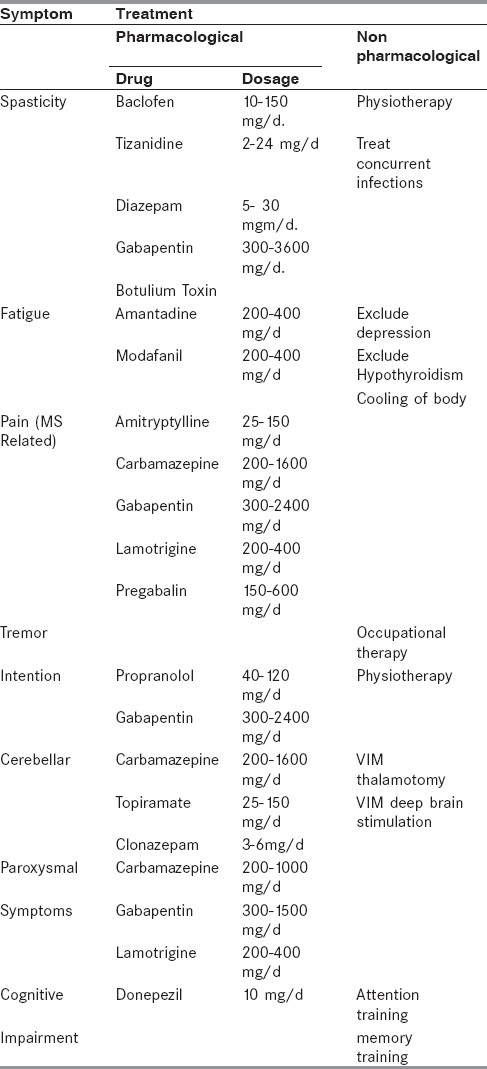
Spasticity, fatigue, tonic spasms, parasthesia, depression, sexual, and voiding dysfunctions are some of the symptoms that require pharmacological interventions[20] [Table 5].
Table 5.
Symptomatic treatment of MS
Conclusions
The key to successful management of MS is early diagnosis and early initiation of treatment with disease-modifying agents. Creating awareness among primary care doctors especially ophthalmologists, pediatricians, and physicians with interest in neurology, go a long way in achieving an early diagnosis. The exorbitant cost of DMD makes it unaffordable to vast majority of patients with MS in India. The necessity is now to have well-designed clinical trials which could re-examine the role of cheaper drugs such as azathioprine, in the Indian setting.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Weinshenker BG, Bass B, Rice PA, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of Multiple Sclerosis: A geographically based study . Clinical course and disability. Brain. 1989;112:133–46. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Amato MP, De Stefano N. Longitudinal follow-up of benign Multiple Sclerosis at 20 years. Neurology. 2007;69:938–9. doi: 10.1212/01.wnl.0000281903.32474.15. [DOI] [PubMed] [Google Scholar]
- 3.McDonald WI, Compston A, Edan G. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 4.Gold R, Buttgereit F, Toyka KV. Mechanism of action of glucocorticosteroid hormones: Possible implications for therapy of neuroimmunological disorders. J Neuroimmunol. 2001;117:1–8. doi: 10.1016/s0165-5728(01)00330-7. [DOI] [PubMed] [Google Scholar]
- 5.Beck RW. The optic neuritis treatment trial: Three-year follow-up results. Arch Ophthalmol. 1995;113:136–7. doi: 10.1001/archopht.1995.01100020014004. [DOI] [PubMed] [Google Scholar]
- 6.Tremlett HL, Luscombe DK, Wiles CM. Use of corticosteroids in multiple sclerosis by consultant neurologists in the United Kingdom. J Neurol Neurosurg Psychiatry. 1998;65:362–5. doi: 10.1136/jnnp.65.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam SM, Kyriakides T, Lawden M, Newman PK. Methylprednisolone in multiple sclerosis: A comparison of oral with intravenous therapy at equivalent high dose. J Neurol Neurosurg Psychiatry. 1993;56:1219–20. doi: 10.1136/jnnp.56.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodin DS, Frohman EM, Garmany GP, Jr, Halper J, Likosky WH, Lublin FD, et al. Disease modifying therapies in multiple sclerosis.Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58:169–78. doi: 10.1212/wnl.58.2.169. [DOI] [PubMed] [Google Scholar]
- 9.Goodin DS. Disease-modifying therapy in multiple sclerosis: Update and clinical implications. Neurology. 2008;71:S8–S13. doi: 10.1212/WNL.0b013e31818f3d8b. [DOI] [PubMed] [Google Scholar]
- 10.Kappos L, Traboulsee A, Constantinescu C, Erälinna JP, Forrestal F, Jongen P, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67:944–53. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 11.Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56:702–8. doi: 10.1212/wnl.56.6.702. [DOI] [PubMed] [Google Scholar]
- 12.Edan G, Miller D, Clanet M, Confavreux C, Lyon-Caen O, Lubetzki C, et al. Therapeutic effect of mitoxantrone combined with methylprednisolone in multiple sclerosis: A randomised multicentre study of active disease using MRI and clinical criteria. J Neurol Neurosurg Psychiatry. 1997;62:112–8. doi: 10.1136/jnnp.62.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edan G, Morrissey S, Le Page E. Rationale for the use of mitoxantrone in multiple sclerosis. J Neurol Science. 2004;223:35–9. doi: 10.1016/j.jns.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Millefiorini E, Gasperini C, Pozzilli C, D’Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. J Neurol. 1997;244:153–9. doi: 10.1007/s004150050066. [DOI] [PubMed] [Google Scholar]
- 15.Ellis R, Boggild M. Therapy related acute leukemia with Mitoxantrone: What is the risk and can we minimize it? Mult Scler. 2009;15:505–8. doi: 10.1177/1352458508100967. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi PA, Giovannoni G, Confavreux C, Galetta SL, Havrdova E, Hutchinson M, et al. The incidence and significance of anti-natalizumab antibodies: Results from AFFIRM and SENTINEL. Neurology. 2007;69:1391–403. doi: 10.1212/01.wnl.0000277457.17420.b5. [DOI] [PubMed] [Google Scholar]
- 17.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 18.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis.Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 19.Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–9. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 20.Henze T, Riekmann P, Toyka KV. Symptomatic Treatment of Multiple Sclerosis-Multiple Sclerosis Therapy Consensus Group (MSTCG) of the German Multiple Sclerosis Society. Eur Neurol. 2006;56:78–105. doi: 10.1159/000095699. [DOI] [PubMed] [Google Scholar]


