Malaria is one of the most common treatable and preventable infectious diseases in the world; and 300–500 million malaria cases occur annually, leading to 1.5–2.7 million deaths in tropical countries. About one-third of these cases occur in Asia. In hyperendemic area, most of the children acquire infection by the age of 5 years. Children younger than 6 months enjoy immunity from their mothers. In India the disease occurs in all the agegroups.
Most clinicians would consider any manifestation of cerebral dysfunction in a patient with malaria as cerebral malaria. These manifestations include impairment of consciousness (confusion, delirium, obtundation, stupor, or coma), convulsions, focal neurologic deficit, and psychosis. These abnormalities can as well be due to hypoglycemia or high fever only. Hence a precise definition has been recommended, which has been summarized in Box 1.
Box 1.

Definition of cerebral malaria Practical definition
Clinical Picture
The mean incubation period is 12 days but it may be as short as 6–12 h in children and non immune person. The prodrome comprises of malaise, headache, and myalgia; chill usually precedes the fever. High fever of 104–105°F, shaking chills, rigors, and sweating are typical of malaria but the patient may have irregular pattern or continuous fever as well. Rarely a patient may have hypothermia due to shock and endotoxemia. Fever in P. falciparum malaria occurs every 3rd day but may not be obvious because of multiple exposures and several cycles of parasitemia. Nausea, vomiting, abdominal pain, tachycardia, arthralgia, weakness, muscle tenderness, hepatosplenomegaly, and chest rales may occur in some patients. Seizures may be focal or generalized, occurring more commonly in children (70%) compared to adults (20%), and some patients may have status epilepticus. Rarely meningismus, focal neurologic signs (aphasia, hemiplegia, ataxia, chorea, and tremor), and neuro-ophthalmologic signs (gaze deviation, oculomotor palsy, nystagmus, and ocular bobbing) may be present. Retinal hemorrhage may occur in 15% of patients. The systemic manifestations of severe malaria complicate the clinical picture and include severe anemia, renal failure, jaundice, pulmonary edema, adult respiratory distress syndrome, hypoglycemia, hemoglobinuria, secondary infection, and septicemia. The presence of mult-iorgan dysfunction adversely affect the prognosis.
The cerebral malaria has a mortality of 20%–50%, which is higher in children. Poor prognostic predictors are the level of consciousness and presence of other organ dysfunction. Recurrent seizures, absent corneal reflex, decerebration, retinal hemorrhage, age < 3 years, heavy parasitemia, (>20%), peripheral leukocytosis presence of pigmented polymorphonuclear leucocytes (>5%), lactic acidosis, elevated CSF lactate and serum transaminase, and low antithrombin III level are associated with poor prognosis. Those who survive usually have complete recovery. Transient neurologic sequelae occur in 10%–18% being higher in children and include ataxia, hemiparesis, memory disturbance, visual field defects, cognitive impairment, and behavioral abnormalities, which generally reverse in 4–8 weeks. A postmalaria neurological syndrome characterized by acute onset of confusion, seizure, ataxia, myoclonus, tremor, and aphasia is described in patients successfully treated for cerebral malaria in the absence of parasitemia. This syndrome is attributed to immunologic mechanisms as it responds to corticosteroids.
Diagnosis
Diagnosis of cerebral malaria requires demonstration of asexual form of P. falciparum in peripheral blood smear, in thick and thin blood smear films stained by Giemsa stain. Absence of parasites in some patients may be due to sequestration of parasitized RBCs in cerebral circulation or earlier treatment with antimalarial drugs. In such a situation, atleast 3 smears 6 h apart should be examined. At least 3 smears should be negative before excluding cerebral malaria. The rapid diagnostic test (antigen detection test) and PCR may be helpful.
CSF examination is necessary to exclude other causes of febrile encephalopathy. CSF is generally normal in cerebral malaria, however, mild pleocytosis (10–50 cells/mm3) and protein rise up to 200 mg/dL may be seen. CT and MRI are usually normal or show edema and cortical or subcortical infarcts in watershed zone in 15%–20% patients. EEG shows nonspecific abnormalities, such as diffuse slowing, spike wave discharges, and burst suppression pattern. Cerebral malaria may occur rarely with negative blood smear; therefore a high index of suspension is required.
Treatment
Cerebral malaria is a neurologic emergency requiring urgent intervention. In endemic area, treatment should be started without waiting for confirmation of the diagnosis. The treatment includes specific antimalarial therapy, supportive therapy for multiorgan dysfunction, and management of associated complications. The drug of choice for cerebral malaria is parenteral artemisinin derivatives or quinine because of widespread resistance to chloroquine. Artesunate has been reported to reduce mortality by 34.7% compared to quinine in a randomized controlled trial in Asian adults. It also reduced the occurrence of convulsion, coma and hypoglycemia. The dose schedule of antimalarial drugs is presented in Box 2. In patients with cardiac disease and in older people, QTc interval should be monitored and quinine should be discontinued if QTc interval exceeds 25% of the basal value. Once the patient can swallow orally he can be switched to oral therapy In renal and hepatic dysfunction the dose of antimalarial should be reduced after 48 h. In patients undergoing hemodialysis or hemofiltration, dose adjustment is not necessary.
Box 2.

Treatment of severe falciparum malaria (WHO 2010)[1]
Artemether and artesunate are advantageous because of low toxicity, ease of administration, and greater efficacy. Artesunate, which is water soluble has the advantage of i.v. or i.m. administration and does not produce hypotension or hypoglycemia. In cerebral malaria mafloquine is not preferred because of neuropsychiatric complications. In patients with hyperparsitemia more than 20%, exchange transfusion is recommended.
Complications of malaria, such as adult respiratory distress syndrome, renal failure, convulsions , jaundice, severe anemia, hypoglycemia, disseminated intravascular coagulation, and shock, need special attention. Corticosteroids are not beneficial and although phenobarbitone reduces the seizures, it increases mortality specifically in children. Intensive care management using artificial ventilation, hemofiltration or hemodialysis may significantly improve the outcome.
Bacterial Meningitis
Acute bacterial meningitis (ABM) refers to inflammatory response to bacterial infection of pia-arachnoid and CSF of the subarachnoid space. In view of the continuity of subarachnoid space over the brain, spinal cord, nerve roots, and nerves, these structures may also be affected. ABM occurs throughout the world, although its precise figures are not available in various Asian countries, its mortality and morbidity is likely to be higher in the developing countries. Worldwide there are 3 main pathogens responsible for ABM: Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis, which account for 75%–80% patients with ABM after the neonatal period. During the neonatal period, the major pathogens responsible for ABM are group B streptococci, Escherichia coli, and other enteric bacilli and Listeria monocytogenes.
The most common organisms causing bacterial meningitis are S. pneumoniae, S. meningitidis, and H. influenzae, which spread by droplet or exchange of saliva. Microbial adherence to mucosa, invasion of mucosal barrier, bacteremia, tissue tropism, and host defence mechanisms determine the invasive nature of the microbial disease. The pathogens colonizing the nasopharynx when cause bacteremia and breach the blood–brain barrier, it results in bacterial meningitis. Meningitis can also occur due to parameningeal infection, after traumatic or surgical disruption of blood-brain barrier and rupture of cerebral abscess into the subarachnoid space. The leptomeningeal inflammation results in hyperemia of meningeal venules and capillaries and increases permeability of these vessels, resulting in exudation of protein and migration of neutrophils into pia- and subarachnoid spaces.
Clinical features
The classical clinical picture of adult bacterial meningitis includes headache, fever, neck stiffness, and often with cerebral dysfunction in 65% patients. Nausea, vomiting, myalgia, and photophobia are also common. The meningeal sings (neck stiffness, Kernig's or Brudzinski's) are present in 50% adult patients and less commonly in neonates and older people. Absence of meningeal signs does not rule out the diagnosis of bacterial meningitis. Cerebral dysfunction manifests with confusion, delirium, and declining consciousness ranging from lethargy to coma. Seizures occur in 40% patients. Focal neurologic deficit (hemiparesis, monoparesis, or aphasia) may develop due to cortical or subcortical infarction or ischemia, increased intracranial pressure (ICP), or associated subdural empyema. Neurologic deficit and seizures are more common in pneumococcal compared with H. influenzae or meningococcal meningitis. Papilledema is rare and should suggest an alternative diagnosis, such as brain abscess. In neonates, classical features of meningitis may be replaced by listlessness, shrill cry, refusal to feed, irritability or other nonspecific manifestations. In older people, the onset may be gradual with lethargy and obtundation with or without meningeal signs or fever. A high index of suspicion and low threshold for performing lumbar puncture is recommended.
Certain clinical features suggest specific diagnosis, such as prominent rash in extremities in meningococcemia, Staphylococcus aureus, Acinetobacter spp., and rickettesioses. Pneumonia and otitis media or sinusitis is found in 30% patients with pneumococcal or H. influenzae. S. pneumoniae is the causative organism for bacterial meningitis due to CSF rhinorrhea or otorrhea. Listeria, gram-negative bacilli and group B streptococci may result in encephalitis in extremes of age.
Diagnosis
Diagnosis of bacterial meningitis is based on CSF examination. Prompt blood culture and CSF examination are indicated in all the patients with suspected meningitis. Cranial CT scan before lumbar puncture is indicated when focal findings, altered sensorium, or evidence of raised intracranial pressure are present. When CT scan is not available, blood culture should be drawn and empiric antibiotic therapy should be started.
CSF examinations
Bacterial meningitis reveals raised CSF pressure (20–50 cm of water); protein (100-1000 mg/dl), marked pleocytosis (1000-10,000/mm3, >60% neutrophils) and reduced glucose (<40% of serum glucose). CSF Gram stain is positive in at least 60% of patients and CSF culture in 75%, which may be reduced to 5%–40% if antibiotics were administered before lumbar puncture. For rapid specific diagnosis, latex particle agglutination or counter immunoelectrophoresis may be used if available. A careful Gram stain of CSF is usually sufficient and should be encouraged in developing countries where PCR and other tests may not be available.
CT or MRI may be normal or reveal meningeal enhancement, cerebral edema, communicating or obstructive hydrocephalus, infarction or venous sinus thrombosis.
The patients with bacterial meningitis should be differentiated from viral, tubercular, fungal or parasitic meningitis, subarachnoid hemorrhage and carcinomatous meningitis. If the meningeal signs are less prominent cerebral or epidural abscess, subdural empyema and viral encephalitis should be considered.
Treatment
Antibiotic therapy should be started promptly because delay results in poor outcome of ABM. The selection of antibiotics depends on local resistance pattern, clinical setting in conjunction with allergies, and CSF results. When lumbar puncture is delayed or Gram stain results are inconclusive, empiric therapy is indicated, which is presented in Table 1. Ampicillin, penicillin G or third-generation cephalosporin are typical first-line agents. Ampicillin covers most pneumococcus, meningococcus, and Listeria and third-generation cephalosporins cover gram-negative organisms and ampicillin-resistant H. influenzae. In patients with head injury or after neurosurgical procedures, vancomycin should replace ampicillin. Ceftazidime should be reserved for pseudomonas meningitis. When the CSF Gram stain or culture reports are available, the specific antibiotic should be started. If S. pneumoniae is of unknown antibiotic sensitivity, vancomycin should be added to third-generation cephalosporin to overcome the emerging penicillin-resistant pneumococcus. Gram-positive cocci are treated with vancomycin and third-generation cephalosporins, gram-negative cocci with penicillin G, gram-positive bacilli with ampicillin and aminoglycoside, and gram-negative bacilli with third-generation cephalosporins and aminoglycoside. The duration of therapy is 7–10 days for meningococcal and H. influenzae, 10–14 days for pneumococcal, 14–21 days for L. monocytogenes and group B streptococci, and 21 days for gram-negative bacilli other than H. influenzae.
Table 1.
Treatment of bacterial meningitis
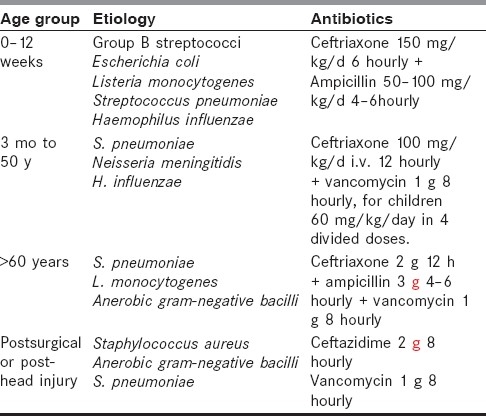
Treatment of bacterial meningitis sometimes may extend to include family members, medical personnel, and other contacts who may require chemoprophylaxis and notification to authorities.
Despite the modern antibacterial therapy, the morbidity and mortality of bacterial meningitis is unacceptably high. Corticosteroid has a beneficial effect in bacterial meningitis as it inhibits the synthesis of proinflammatory cytokines at mRNA level, decrease CSF outflow resistance, and stabilize blood-brain barrier. If the organism is known, corticosteroid co-administration results in greater survival and lower morbidity, especially in H. influenzae and pneumococcal meningitis in both children and adults. In a recent meta-analysis, adjunctive dexamethasone in the treatment of ABM, however, did not seem to significantly reduce death or neurological disability. There were no significant treatment effects in any of the prespecified subgroups. The benefit of adjunctive dexamethasone for all or any subgroup of patients with bacterial meningitis thus remains unproven. Seizure and status epilepticus should be promptly treated with phenytoin, lorazepam, or diazepam.
Tuberculous Meningitis
Tuberculous meningitis (TBM) is the most severe form of tuberculosis and is an important cause of death and disability in adults and children, especially in HIV-infected patients. The diagnosis of TBM is based on clinical, CSF, and radiological (CT/MRI) findings, evidence of extra-CNS TB, and additional laboratory findings.
The clinical findings of TBM are nonspecific in the early stage and include fever, headache, irritability, neck stiffness, vomiting, and focal neurologic signs. Altered sensorium, cranial nerve palsies, and limb paralysis are more commonly found in advanced disease. The symptom onset to the time of presentation ranges from 7 to 30 days. History of close contact with TB or extrapulmonary TB should be considered suggestive of TBM.
The diagnosis of TBM depends on CSF examination but CT scan should be done before lumbar puncture if a patient has papilledema, focal signs, altered sensorium, or history of seizure to exclude a pressure cone and avoid possible herniation. CSF in TBM is typically clear, has lymphocytic pleocytosis (10–1000 cells/mm3 with lymphocytic predominance), elevated protein (0.5–3 g/L), and low glucose (CSF to plasma glucose ratio < 50%).
CT or MRI findings are commonly seen in stage 2 and 3 of TBM. Basal meningeal enhancement, hydrocephalus, granuloma, and infarction are common findings and are found in various combinations.
Evidence of extra-CNS TB is obtained by a radiograph of the chest, or a cervical or abdominal lymphadenopathy (on abdominal ultrasonography). Diagnostic sampling of lung, lymph node, liver, bone marrow, ascitic fluid, and gastric aspirate add confidence to the diagnosis of TBM. HIV serology should be undertaken in all patients with TBM.
Other diagnoses, such as cryptococcal meningitis (CSF India Ink and cryptococcal antigen), treponema pallidum (treponema pallidum hemagglutination assays), cytologic tests for malignancies, malaria, and toxoplasma should be excluded in appropriate clinical settings.
Treatment
Four-drug antitubercular treatment with RHZE (rifampicin, isoniazide, pyrazinamide, ethambutol) should be initiated as soon as the diagnosis of TBM is suspected. One should start the therapy without waiting for confirmation [Table 2].
Table 2.
Chemotherapeutic options of tuberculous meningitis in adults
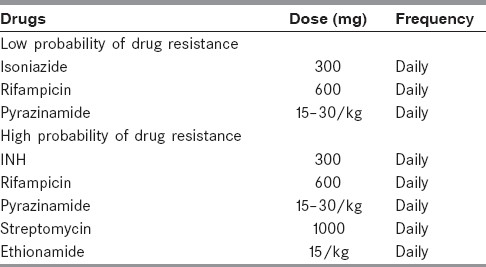
In adults ethambutol and in children streptomycin may be preferred. Ethambutol is preferred to streptomycin because of ototoxicity in adults. Four drugs are recommended for initial 2 months; and INH and rifampicin for a total of 9–12 months. In patients who come late or have had incomplete treatment may be treated for 12–18 months. Some patients with tuberculoma may need longer treatment. DOTS (Directly observed therpay short course ahs not been sufficiently evaluate in TB meningitis .
Corticosteroids are recommended on the basis of randomized controlled trial (RCT) and meta-analysis. Corticosteroids are recommended in patients without HIV infection. A large RCT-recommended dexamethasone treatment for 4 weeks (0.4 mg/kg/day for 1 week, 0.3 mg/kg/day in week 2, 0.2 mg/kg/day in week 3, and 0.1 mg/kg/day in week 4) followed by oral taper (4, 3, 2, and 1 mg/day for 1 week each). Thus patients received 2 months of corticosteroids. Lower doses of prednisolone 40 mg/day, tapered after 1 month may also be considered. But before administering corticosteroids one must be sure of the diagnosis (exclude cryptococcal, syphilis, toxoplasma, and partially treated septic meningitis) and exclude the patients with HIV, diabetes, bedsore, septicemia, and so on.
Baseline investigations
All the patients after the diagnosis of TBM should be tested for HIV and if positive, CD4 count should be done. High-risk individuals for hepatitis should undergo serology for hepatitis B and C. Baseline serum bilirubin, liver enzymes, platelet, and creatinine should be done. Patients on ethambutol should undergo vision testing and red–green color discrimination test. On subsequent follow-up, routine liver and renal function tests are not needed unless there are baseline abnormalities or symptoms related to liver dysfunctions. On monthly follow-up, the patient should be enquired about visual disturbances (blurring or scotoma) and red–green discrimination if patient is on ethambutol therapy for more than 2 months.
Surgery
Surgery has limited but definite role in the management of CNS tuberculosis for secondary complications and when diagnosis is not clear.
Hydrocephalus
Mild to moderate hydrocephalus responds well to medical treatment but surgery is indicated when there is raised intracranial pressure, leading to alteration in sensorium. In a patient with hydrocephalus, ventriculoperitoneal shunt is the treatment of choice. Results of shunt surgery in deeply comatose patients who are decerebrating are poor. In such patients, a preliminary trial of external ventricular drainage may help to distinguish the patients who are likely to benefit from surgery.
At times intracranial tuberculoma may require biopsy and surgery when response to ATT is not adequate or diagnosis is in doubt.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.World Health Organization. Guidelines for the treatment of malaria. (Second edition) 2010 [Google Scholar]


