Abstract
Context:
Diabetes mellitus is a chronic metabolic disorder of endocrinal origin with multiorgan involement. Today's physician has a lot many options to choose for treating type 2 diabetes, but does not always manages to achieve optimal glycemic control. The newer drug bromocriptine acts by novel hypothalamic circadian rhythm resetting mechanism.
Objective:
To evaluate the efficacy and safety of bromocriptine QR in type 2 diabetes.
Materials and Methods:
105 patients according to inclusion and exclusion criteria were randomized into three groups by simple randomization. Group 1 received bromocriprine 2.4 mg once daily, group 2 received metformin 500 mg twice daily while group 3 received bromocriprine 1.6 mg daily and metformin 500 mg twice daily. Baseline measurement of fasting and postprandial blood sugar, HbA1C and BMI were followed up at 6th and 12th weeks. Safety evaluation was done by questioning the patient and also through routine hematological and biochemical parameters. Z test was used for analysis.
Results:
Group 1 showed significant reduction in fasting and postprandial sugar and HbA1c at 12 weeks. While groups 2 and 3 showed even higher reduction in these parameters albeit with slightly more adverse drug events like nausea, vomiting compared to group 1.
Conclusion:
Bromocriptine QR is an effective and safe antidiabetic drug which can be employed as monotherapy or in conjuction with metformin to achieve and maintain optimal glycemic control.
Keywords: Bromocriptine, pharmacotherapy, type 2 diabetes, circadian rhythm
INTRODUCTION
Diabetes mellitus is a syndrome characterized by chronic hyperglycemia and disturbances of carbohydrate, fat, and protein metabolism occurs due to deficiency of insulin (type 1)[1] or insensitivity of the target tissues to insulin (type 2).[2] When untreated, it leads to multiorgan damage, specially cardiovascular, renal, neurological, and ocular.[3]
Currently, a wide variety of oral antidiabetic agents and insulin are available for the treatment of type 2 diabetes. Several oral antidiabetic agents like metformin, sulfonylureas, meglitinides, thiazolidinediones are currently available. Some of them increase the insulin secretion from pancreas while others increase peripheral utilization of glucose by increasing the sensitivity of peripheral tissues to utilize glucose. Oral therapy for type 2 diabetes,[4] when used appropriately, can safely assist patients to achieve glycemic target in the short term to medium term. However, the progressive nature of type 2 diabetes usually requires a combination of two or more oral agents in long term. Issues of safety and tolerability, notably weight gain, often limit the optimal application on antidiabetic drugs such as sulfonylureas, meglitinides and thiazolidinediones.[5]
Therefore, there is a need for novel oral antidiabetic agents with different mechanism of action from existing oral antidiabetic agents. Bromocriptine mesylate quick release formulation has been approved by FDA in May 2009 for the treatment of type 2 diabetes in adults as an adjunct to diet and exercise to improve glycemic control.[6] The concept of using this D2 receptor agonist for the treatment of type 2 diabetes came while studying the metabolism of migrating birds [Figure 1].[7] The hibernation theory of circadian neuroendocrine rhythm which forms the basis for the use of bromocriptine use proposes that animals hibernating in the winter not only put on a lot of weight before they hibernate but also they become insulin resistant/ glucose intolerant which help them to conserve glucose and lipids for energy production. Extensive animal studies have shown that decreased hypothalamic dopaminergic tone may be involved in the pathogenesis of this insulin resistance. Similar circadian cycle is seen in all vertebrates except humans.
Figure 1.

Migrating birds
The normal circadian cycle that results in leaner body in the summer and heavier body in winter is disturbed in humans because of abundant caloric intake round the year as in modern times human do not worry about food availability during summer resulting in the absence of lean phase. Stimulation of the hypothalamus promotes the release of several hormones that respond to traditional shift in caloric intake and storage. Quick release bromocriptine, given once in the morning, stimulates the hypothalamus to reset circadian rhythm if it was permanently stuck in a winter rhythm (insulin resistant phase),[8] thus improving insulin resistance and peripheral glucose disposal by central mechanism.
Hence, there is a rationale for this drug, which attacks the problem of insulin resistance, type 2 diabetes and obesity (which form a conglomere) in a completely new way. Therefore, bromocriptine may prove to be a revolution in the field of treatment of type 2 diabetes. Very few studies have been carried out to clinically evaluate bromocriptine in type 2 diabetes mellitus and no such study was done in Indian population. So, the present study is undertaken to evaluate the efficacy and safety of bromocriptine monotherapy as well as its low dose combination with metformin in type 2 diabetes.
MATERIALS AND METHODS
This was a prospective, randomized, double blind, parallel study carried out at the outpatient department of an endocrinology hospital over 12 weeks after obtaining approval by Institutional Ethics Committee.
Newly diagnosed adult type 2 diabetes patients of either sex with fasting blood sugar in the range of 126 to 200 mg/dl were included after taking informed consent [Figure 2]. The following category of patients were excluded: type 1 diabetes mellitus, diabetic ketoacidosis, known hepersensitivity to ergot alkaloids or bromocriptine, nursing mothers, psychiatric disorder, syncopal migraine or patients taking antihypertensives.
Figure 2.
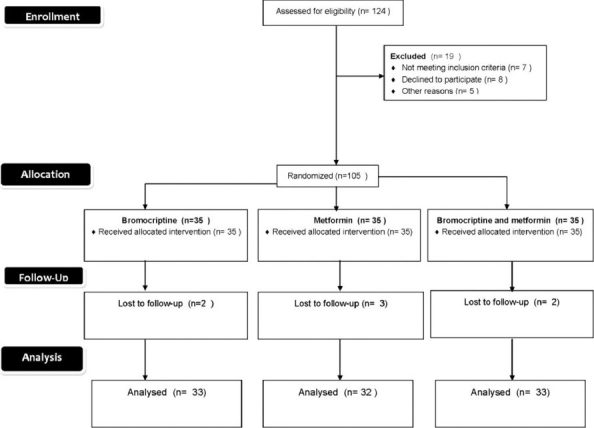
Participant flow
Sample size determined from previous studies was large but for feasibility a smaller sample was chosen. 105 patients were included in the study and randomized into 3 groups of 35 each. Simple randomization was done and allocation was concealed by employing different investigators for each step of random number generation, enrolloment, assignment of patients to treatment groups. Participants and investigators were blinded to achieve double blind. Patients in group 1 received bromocriptine QR 2.4 mg once daily within 2 h of waking in the morning and with food to reduce the risk for gastrointestinal adverse effects such as nausea. Group 2 received oral metformin 500 mg two times daily. Group 3 received bromocriptine QR 1.6 mg as in group 1 and metformin 500 mg two times daily.
As metformin is a standard first line drug for type 2 diabetes, it was chosen as the standard to compare against. Most patients eventually need two or more drugs in real life management of type 2 diabetes. Therefore a third group comprising the combination of both drugs was added with low dose bromocriptine.
After enrolment, detailed medical history and baseline values of fasting blood sugar (FBS), post prandial sugar (PPBS) and HbA1C and BMI were recorded. Follow up was done at 6th and 12th weeks of study period.
Efficacy was assessed by measuring FBS (mg/dl), PPBS (mg/dl) at 6th and 12th weeks while HbA1C (%) and BMI (weight /Height2) at 12th week.
Safety was assessed in terms of systemic adverse effects by questioning patients at each visit and objective signs were assessed by clinical and biochemical examination. Routine hematological (Hb and TLC) and biochemical investigations (serum creatinine, SGPT, SGOT) were done at baseline and at the 12th week of study period.
Data was checked for normality and then analyzed by Z test for difference between two means, P value < 0.05 was taken as significant while P value >0.05 was considered as insignificant.
RESULTS
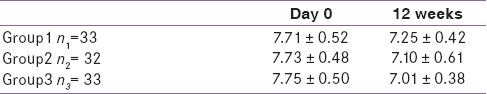
Baseline values of all three groups were comparable with respect to age, sex, habits, FBS, PPBS, HbA1c, and BMI [Table 1].
Table 1.
Baseline data of group 1, group 2 and group3
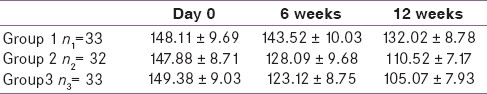
Mean FBS reduction in group 1 compared with day 0, at 6 weeks was not significant (P<0.05) while that at 12 weeks was statistically significant (P<0.05) [Table 2]. In groups 2 and 3, reduction at 6 and 12 weeks was significant (P<0.05) when compared to baseline. 12 weeks reduction of group 3 was significantly greater than that of group1 (P<0.05).
Table 2.
Effect on mean fasting blood sugar (mg/dl) at 6 and 12 weeks
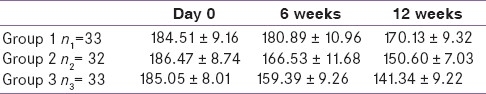
Group 1 mean PPBS reduction when compared with day 0, at 6 weeks was not significant (P<0.05) while that at 12 weeks was statistically significant (P<0.05) [Table 3]. In groups 2 and 3, reduction at 6 and 12 weeks was significant (P<0.05) when compared to baseline. 12 weeks reduction of group 3 was significantly greater than that of group1 (P<0.05).
Table 3.
Effect on mean postprandial blood sugar(mg/dl) at 6 and 12 weeks
Mean reduction in glycosylated hemoglobin at 12 weeks when compared with respective baseline values was statistically significant (P<0.05) in all three treatment groups [Table 4]. Reduction in group 3 was significantly more (P<0.05) compared to group 1.
Table 4.
Effect on mean HbA1c level (%) at 12 weeks
All three treatment groups showed reduction in mean BMI which when compared with baseline was found to be statistically not significant (P<0.05) [Table 5].
Table 5.
Effect on mean body mass index at 12 weeks

In the present study, significant common adverse drug events observed were nausea, vomiting, and headache in all the three groups with comparable incidence. These events were slightly more common in group 3. None of the groups had adverse impact on Hb level, total leucocyte count, serum creatinine, SGPT and SGOT.
DISCUSSION
Diabetes mellitus is a chronic metabolic syndrome of endocrine origin with multiorgan involvement and complications. Currently wide variety of oral hypoglycemic agents and insulin are available for the treatment of type 2 diabetes.
There is a need for novel antidiabetic agents with different mechanism of action from existing drugs.The traditional oral agents are often associated with an increased risk of adverse events (hypoglycemia, weight gain) and typically may become less effective over time as patients undergo progressive beta cell failure and often fail to achieve target glycemic control in patients, even when used as combination therapy. Bromocriptine, a dopamine receptor (D2) agonist used for hyperprolactinemia and Parkinsonism, has recently been approved for the treatment of type 2 diabetes in adjunct to diet and exercise to improve glycemic control. Studies carried out have shown that it can reduce FBS, PPBS and HbA1c level significantly. No study has been carried out to evaluate the effectiveness of bromocriptine in type 2 diabetes mellitus in Indian population until now. So, the present study was planned to evaluate the efficacy and safety of bromocriptine in type 2 diabetes as monotherapy and in combination with metformin.
As depicted in Graph 1, mean FBS in group1 ( bromocriptine) was reduced by 4.79 mg/dl at 6 weeks and by 16.09 mg/dl at 12 weeks. When compared to baseline reduction at 6 weeks, it was found to be statistically insignificant (P>0.05), whereas at 12 weeks it was significant (P<0.05). In group 2 (metformin), it was decreased by 19.79 mg/dl at 6 weeks and by 37.36 mg/dl at 12 weeks. In group 3 (combination), there was a reduction in mean FBS by 26.26 mg/dl at 6 weeks and by 44.31 mg/dl at 12 weeks. In groups 2 and 3, reduction in mean FBS at 6 and 12 weeks was found to be statistically significant when compared to baseline values (P<0.05). Previous study on bromocriptine by Pijl et al., found FBS reduction by 18 mg/ml after 16 weeks.[9] In group 1, reduction was significant at 12 weeks of treatment and this lag period could be due to its complex mechanism acting through neural circuits and providing a fine tuning of hypothalamic circadian rhythm. In the present study, combination of metformin with low dose bromocriptine significantly reduced FBS as compared to bromocriptine or metformin alone.
Graph 1.
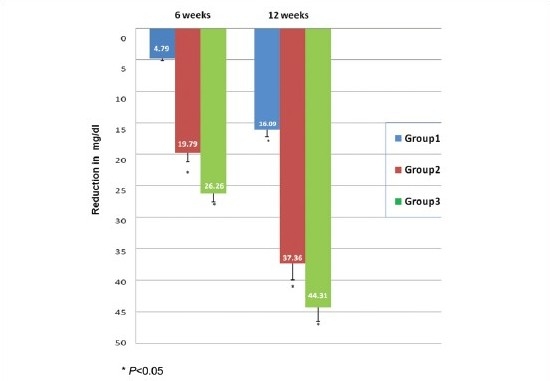
Comparison of reduction in fasting blood sugar level (mg/dl)
As shown in Graph 2, in group 1 reduction in mean PPBS at 6 weeks was 3.92 mg/dl and at 12 weeks it was 14.38 mg/dl. When compared to baseline reduction at 6 weeks it was found to be statistically insignificant (p>0.05), whereas at 12 weeks it was significant (P<0.05). Similar findings were observed by Kamath et al., where the reduction in postprandial sugar was 16 mg/dl.[10] In group 2 mean PPBS decreased by 19.94 mg/dl at 6 weeks and by 35.87 mg/dl at 12 weeks. In group 3 it decreased by 25.66 mg/dl at 6 weeks and by 43.71 mg/dl at 12 weeks. In groups 2 and 3, the reduction in mean fasting blood sugar level at 6 and 12 weeks was found to be statistically significant when compared to baseline values (P<0.05). In the present study, combination of metformin with low dose bromocriptine significantly reduced PPBS as compared to bromocriptine or metformin alone.
Graph 2.
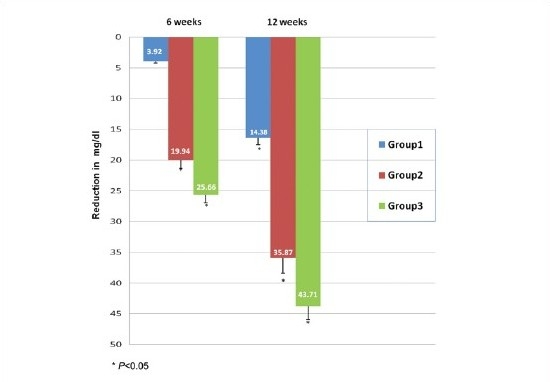
Comparison of reduction in postprandial blood sugar level (mg/dl)
Graph 3 shows the mean reduction in glycosylated hemoglobin at 12 weeks which was 0.46% in group 1, 0.63% in group 2 and 0.74% in group 3. This reduction was statistically significant (P<0.05) when compared with respective baseline values in all the groups. Previous study carried out by Pijl et al., on bromocriptine found a reduction of 0.6% at 16 weeks.[9]
Graph 3.
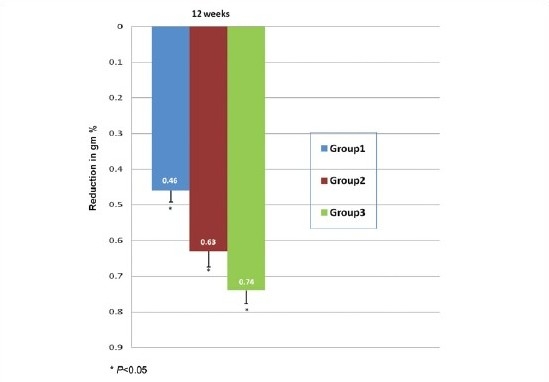
Comparison of reduction in glycosylated haemoglobin levels (gm %)
Thus it can be concluded from the findings of the present study that bromocriptine as monotharapy and in combination is effective in significantly reducing FBS, PPBS and HbA1c level.
Though there was a slight decrease in BMI at 12 weeks in all the groups, when compared to baseline values, reduction was statistically insignificant. In present study there was no significant effect on BMI possibly due to short duration of study.
The cerebral mechanisms underlying the behaviours that lead to pathological overeating and obesity are postulated to involve dopamine. Dopamine modulates motivation and reward circuits and hence dopamine deficiency in obese individuals may perpetuate pathological eating as a means to compensate for decreased activation of these circuits.[11] Strategies aimed at improving dopamine function may be beneficial in the treatment of obese individuals. Further extensive studies of long duration need to be carried out to evaluate the effectiveness of dopamine agonists including bromocriptine in obesity and in obese diabetic patients.
Frequency of patients experiencing at least one adverse reaction in bromocriptine treated group 1 was 30.3 %, in metformin treated group 2 was 33.7% while in group 3 treated with bromocriptine and metformin it was 35.3%. Significant common adverse drug events observed were nausea, vomiting, headache in all three groups with incidence not significantly different from each other. Other adverse effects seen in bromocriptine treated groups are fatigue and dizziness. In previous studies, bromocriptine used in diabetes patients has demonstrated good safety and tolerability, most of the side effects were gastrointestinal and tend to be present on initiation or titration of dose and decreased on continuation of therapy. Thus from the results of present study it can be concluded that bromocriptine is a safe antidiabetic agent alone and in combination with metformin.
No drug had any adverse impact on the values of hemoglobin, total leukocyte count (TLC), serum creatinine, SGPT, SGOT.
Apart from the efficacy parameters studied in the present study, various other favorable effects of bromocriptine in animal and human studies have been observed. Multiple animal studies have demonstrated metabolic improvements including significant weight loss, decreased levels of blood sugar and triglycerides, decreased insulin resistance and increased glucose tolerance. Human studies show similar effects, though the results are not as profound. From the safety point of view, bromocriptine meets all the USFDA cardiovascular safety guidelines and demonstrated 40% reduction in cardiovascular end points.[8] Bromocriptine had also shown beneficial effects in patients of type 2 diabetes with dyslipidemia.[9] Bromocriptines’ clinical utility should be maximal in obese, depressed patients with limited mobility and features of insulin resistance.[12]
Despite well-established guidelines for glycemic control, many diabetes patients do not achieve targeted goals or have difficulty in maintaining it. Adherence to prescribed medicine, dietary and lifestyle regimens are all crucial factors in the management of type 2 diabetes. Bromocriptine has the advantage of acting through a completely different mechanism of action from currently available antidiabetic drugs, easy to use single daily dose which may boost compliance, can be combined with other antidiabetic drugs for synergistic effect and may help reduce their dose and consequent adverse reactions. Other desired effects are weight reduction and reduced cardiovascular mortality end points. The dose approved for treatment of type 2 diabetes is 2 to 3 fold lower than usual doses in hyperprolactinemia and 10 to 20 fold lower than treatment for Parkinson's disease.[13,14] These relatively low doses may reduce the risk of adverse effects. Overall, bromocriptine demonstrates good safety and tolerability that most individuals are adherent to therapy.
Why bromocriptine for diabetes concerns India? India has the largest number of diabetic subjects in the world and therefore called as the “diabetes capital of the world”. According to the Diabetes Atlas 2006 published by the International Diabetes Federation, the number of diabetics in India is around 40.9 million which is expected to rise to 69.9 million by 2025 unless urgent preventive steps are taken. “Asian Indian Phenotype” with certain unique clinical and biochemical abnormalities in Indians which include increased insulin resistance, greater abdominal adiposity i.e., higher waist circumference despite lower body mass index, lower adiponectin and higher high sensitive C-reactive protein levels makes Asian Indians more prone to diabetes.[15,16] So any modality of treatment including therapeutic lifestyle change (TLC), pharmacotherapy can gradually but significantly improve the situation and can prevent, retard progression and complications of diabetes and reduce the financial burden on the individual patient and government for the awareness and treatment of the diabetes. A recent survey conducted on Indian physicians about the knowledge and attitude about the use of bromopcriptine in diabetes management demonstrated the interest and high potential associated with bromocriptine use.[17]
The present study is a small study both as regards to the number of patients included, the duration, also dose used was fixed. In India more extensive studies including large number of patients with differing severity and comorbidities, more efficacy parameters and a flexible dosing pattern are required to determine the exact utility of this drug with novel mechanism.
Results of the present study show that bromocriptine is effective in improving the markers of diabetes like fasting blood sugar, postprandial blood sugar, HbA1c, even though the results are not as profound as that of metformin. Combination with metformin in a low dose was found to be more effective in improving glycemic control than individual drugs alone.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bennet PH. Definition, diagnosis and classification of diabetes mellitus and impaired glucose tolerance. In: Kahn CR, Weir GC, editors. Joslins Diabetes Mellitus. 13th ed. Philadelphia: Lea Febiger Publisher; 1994. pp. 93–200. [Google Scholar]
- 2.Genuth S. Classification and diagnosis of diabetes mellitus. Med Clin North Am. 1982;66:1191–207. doi: 10.1016/s0025-7125(16)31358-x. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane IA. Non Insulin- Dependent diabetes mellitus. In: John P, Williams G, editors. Textbook of Diabetes. London: Blackwell Scientific Publication; 1991. pp. 24–9. [Google Scholar]
- 4.Masters SB, Trevor AJ. Pancreatic hormones and antidiabetic drugs. In: Katzung BG, editor. Basic and Clinical Pharmacology. 11th ed. New Delhi: McGraw Publication; 2010. p. 741. [Google Scholar]
- 5.Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus. Drugs. 2008;68:2131–62. doi: 10.2165/00003495-200868150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Scranton RE, Gaziano JM, Rutty D, Ezrokhi M, Cincotta A. A randomized, double-blind, placebo-controlled trial to assess safety and tolerability during treatment of type 2 diabetes with usual diabetes therapy and either Cycloset or placebo. BMC Endocr Disord. 2007;7:3. doi: 10.1186/1472-6823-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hennesy T. Old Parkinson's drug tweaked into diabetes treatment. The Associated Press. 2009 [Google Scholar]
- 8.Gaziano JM, Cincotta AH, O‘Connor CM, Ezrokhi M, Rutty D, Ma ZJ, et al. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care. 2010;33:1503–8. doi: 10.2337/dc09-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. A novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000;23:1154–61. doi: 10.2337/diacare.23.8.1154. [DOI] [PubMed] [Google Scholar]
- 10.Kamath V, Jones CN, Yip JC, Varasteh BB, Cincotta AH, Reaven GM, et al. Effects of quick-release form of bromocriptine (Ergoset) on fasting and postprandial plasma glucose, insulin, lipid and lipoprotein concentrations in obese nondiabetic hyperinsulinenic women. Diabetes Care. 1997;20:1697–701. doi: 10.2337/diacare.20.11.1697. [DOI] [PubMed] [Google Scholar]
- 11.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 12.Kalra S. Bromocriptine: A revolution in diabetes care. Int J Clin Cases Invest. 2011;1:2. [Google Scholar]
- 13.Calne DB, Teychenne PF, Leigh PN, Bamji AN, Greenacre JK. Treatment of parkinsonism with bromocriptine. Lancet. 1974;2:1355–6. doi: 10.1016/s0140-6736(74)92219-3. [DOI] [PubMed] [Google Scholar]
- 14.Biller BM, Luciano A, Crosignani PG, Molitch M, Olive D, Rebar R, et al. Guidelines for the diagnosis and treatment of hyperprolactinemia. J Reprod Med. 1999;44:1075–84. [PubMed] [Google Scholar]
- 15.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 16.Abate N, Chandalia M. Ethnicity, type 2 diabetes and migrant Asian Indians. Indian J Med Res. 2007;125:251–8. [PubMed] [Google Scholar]
- 17.Das AK, Kalra S. Bromocriptine in diabetes management: Knowledge and attitudes of Indian physician. Int J Clin Cases Invest. 2011;2:5. [Google Scholar]


