Abstract
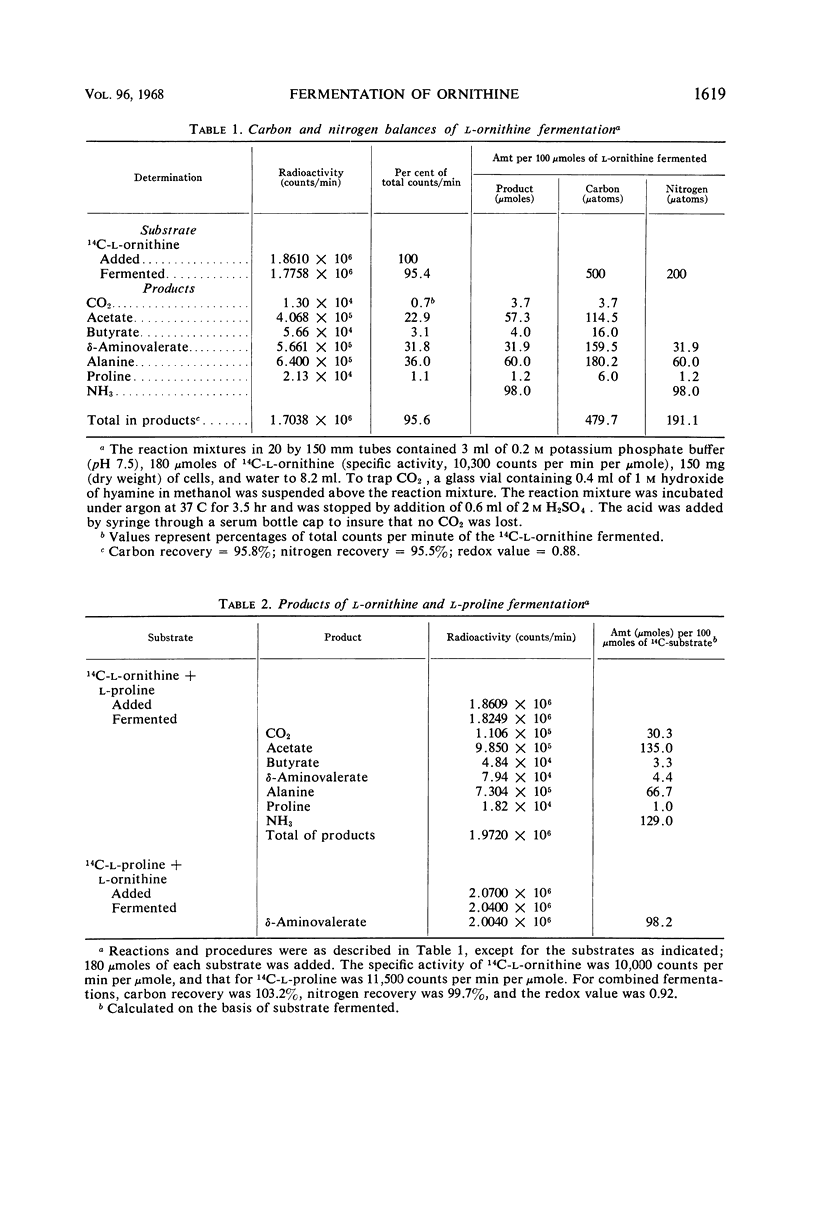
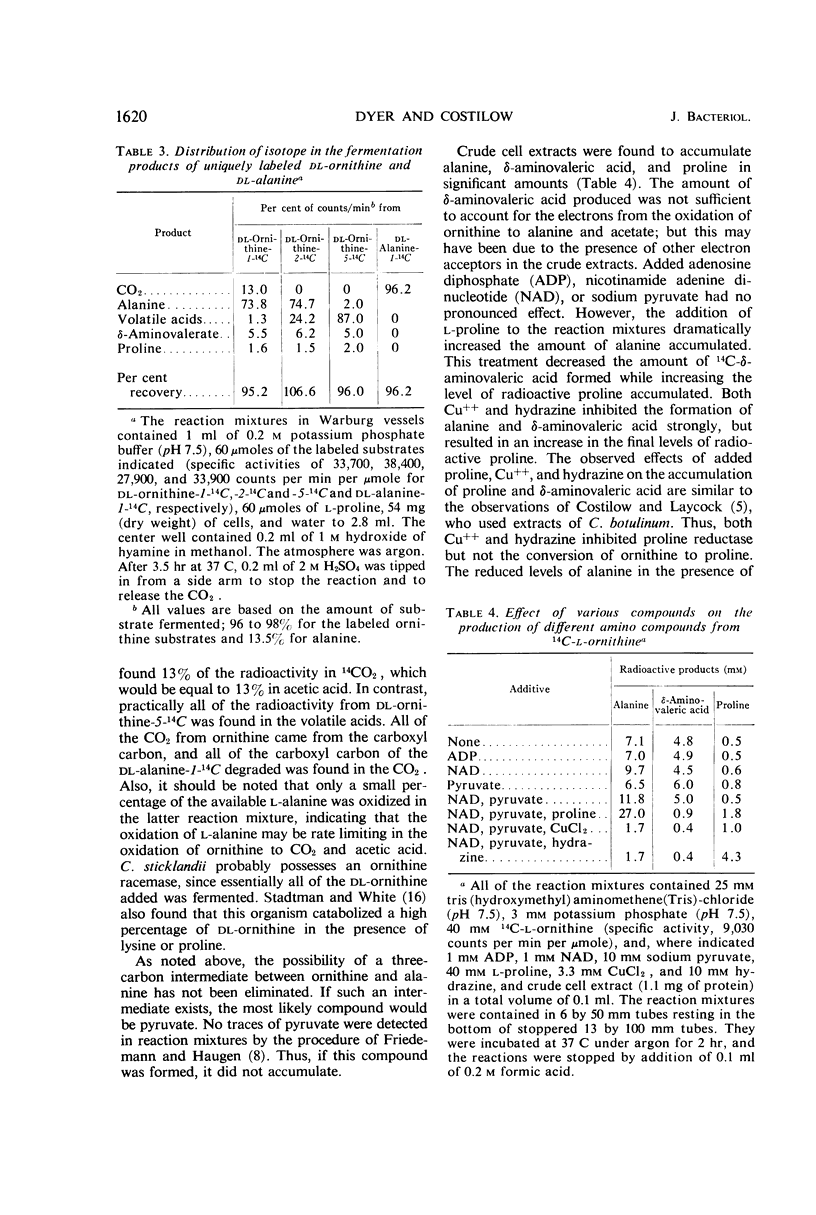
Resting cells of Clostridium sticklandii fermented l-ornithine as a single substrate by a coupled oxidation-reduction with proline as the electron acceptor. The products of the fermentation of ornithine alone were ammonia, alanine, acetate, and δ-aminovalerate, in order of concentration. Traces of CO2, butyrate, and proline were also found. When an equimolar amount of proline was added along with ornithine, very little δ-aminovalerate was produced from the ornithine, but essentially all of the proline was reduced to this compound. The ratios of the other primary products were changed by the addition of proline. The primary products from ornithine fermented in the presence of proline were acetate, ammonia, alanine, and CO2, in order of concentration. Studies with dl-ornithine-1-14C, dl-ornithine-2-14C, and dl-ornithine-5-14C demonstrated that the primary cleavage of this amino acid occurred between carbons 3 and 4. A high percentage of the isotope from carbons 1 and 2 was found in alanine, and most of that from carbon 5 was found in volatile acid. The CO2 formed was derived from the carboxyl carbon. All of the radioactivity from the fermentation of dl-alanine-1-14C was found in 14CO2. The alanine from ornithine was oxidized by d-amino acid oxidase to the same extent as dl-alanine, indicating that it was dl-α-alanine. Preliminary experiments with cell extracts indicated proline is an intermediate in the reduction of ornithine to δ-aminovaleric acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R. N., Laycock L. Proline as an intermediate in the reductive deamination of ornithine to delta-aminovaleric acid. J Bacteriol. 1968 Oct;96(4):1011–1020. doi: 10.1128/jb.96.4.1011-1020.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R. N., Rochovansky O. M., Barker H. A. Isolation and identification of beta-lysine as an intermediate in lysine fermentation. J Biol Chem. 1966 Apr 10;241(7):1573–1580. [PubMed] [Google Scholar]
- Mitruka B. M., Costilow R. N. Arginine and ornithine catabolism by Clostridium botulinum. J Bacteriol. 1967 Jan;93(1):295–301. doi: 10.1128/jb.93.1.295-301.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C. ANAEROBIC DEGRADATION OF LYSINE. II. COFACTOR REQUIREMENTS AND PROPERTIES OF THE SOLUBLE ENZYME SYSTEM. J Biol Chem. 1963 Aug;238:2766–2773. [PubMed] [Google Scholar]
- STADTMAN T. C. Lysine fermentation to fatty acids and ammonia: a cobamide coenzyme-dependent process. J Biol Chem. 1962 Jul;237:2409–2411. [PubMed] [Google Scholar]
- STADTMAN T. C. On the metabolism of an amino acid fermenting Clostridium. J Bacteriol. 1954 Mar;67(3):314–320. doi: 10.1128/jb.67.3.314-320.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN T. C., WHITE F. H., Jr Tracer studies on ornithine, lysine, and formate metabolism in an amino acid fermenting Clostridium. J Bacteriol. 1954 Jun;67(6):651–657. doi: 10.1128/jb.67.6.651-657.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Tsai L. A cobamide coenzyme dependent migration of the epsilon-amino group of D-lysine. Biochem Biophys Res Commun. 1967 Sep 27;28(6):920–926. doi: 10.1016/0006-291x(67)90067-8. [DOI] [PubMed] [Google Scholar]
- Tsai L., Stadtman T. C. Anaerobic degradation of lysine. IV. Cobamide coenzyme-dependent migration of an amino group from carbon 6 of beta-lysine (3,6-diaminohexanoate) to carbon 5 forming a new naturally occurring amino acid, 3,5-diaminohexanoate. Arch Biochem Biophys. 1968 Apr;125(1):210–225. doi: 10.1016/0003-9861(68)90656-5. [DOI] [PubMed] [Google Scholar]



