Abstract
Background: The benefit of the Mediterranean-style dietary pattern in mitigating metabolic risk factors for type 2 diabetes and cardiovascular disease has not been well investigated among nondiabetic Americans.
Objective: The aim of this study was to examine the prospective association between the Mediterranean-style dietary pattern and metabolic syndrome.
Design: The Mediterranean-style dietary pattern score (MSDPS) was used to characterize a Mediterranean-style dietary pattern in the Framingham Heart Study Offspring Cohort. We examined the longitudinal association between MSDPS and metabolic syndrome traits (including homeostasis model assessment–insulin resistance, fasting glucose, waist circumference, triglyceride, HDL cholesterol, and systolic and diastolic blood pressure) among 2730 participants of the Framingham Heart Study Offspring Cohort without type 2 diabetes (baseline median age: 54 y; 55% women), who were followed from the fifth (baseline) to the seventh study examinations (mean follow-up time: 7 y), and metabolic syndrome incidence (according to the National Cholesterol Education Program Adult Treatment Panel III definition) in 1918 participants free of the condition at baseline.
Results: A higher MSDPS was associated with lower homeostasis model assessment–insulin resistance (P = 0.02), waist circumference (P < 0.001), fasting plasma glucose (P = 0.03), and triglycerides (P < 0.001) and higher HDL cholesterol (P = 0.02) after adjustment for the corresponding baseline values and for several confounding factors associated with type 2 diabetes risk. Participants in the highest quintile category of the MSDPS had a lower incidence of metabolic syndrome than those in the lowest quintile category (38.5% compared with 30.1%; P = 0.01).
Conclusion: Our study suggests that the consumption of a diet consistent with the principles of the Mediterranean-style diet may protect against metabolic syndrome in Americans.
INTRODUCTION
Insulin resistance is an important part of the etiology of type 2 diabetes mellitus (T2DM) (1), which in turn is an important risk factor for cardiovascular disease (CVD) (2, 3). In addition to hyperinsulinemia, insulin-resistant individuals are often characterized as having multiple metabolic risk factors for CVD, including abdominal obesity, hyperglycemia, hypertension, and dyslipidemia (evidenced by elevated triglycerides or low HDL cholesterol) (4). Metabolic syndrome, which is the term used to refer to the clustering of these metabolic risk factors for CVD (5), confers a 2-fold increase in the risk of developing CVD and a 6-fold increase in the risk of developing T2DM (6).
Dietary patterns rich in whole grains, fruit, vegetables, nuts, and omega-3 fatty acids and low in refined grains and saturated and trans fats have been suggested to offer a significant protection against heart disease (7). One such dietary pattern—the Mediterranean diet—was first shown to benefit heart health in the report of the Seven Countries Study from the 1950s (8). Of 16 different cohorts that participated in this study, the Crete cohort from Greece had the lowest heart disease rate (8), which was attributed in large part to the diet of this region (9). A Mediterranean-style diet has also been favorably associated with reduction in the risk of death, including deaths from CVD in a US population (10).
There remains a lack of information on the relation between a Mediterranean-style diet and both insulin resistance and metabolic risk factors for CVD, both as individual and clustered risk factors, especially in non-Mediterranean populations. Of the few previous cross-sectional studies in different Mediterranean populations, most (11–13), but not all (14), showed that a Mediterranean-style dietary pattern was inversely associated with insulin resistance and metabolic syndrome. Only one study reported a prospective relation between a Mediterranean-style dietary pattern and the incidence of metabolic syndrome (15), but no studies have examined the longitudinal association between a Mediterranean-style dietary pattern and insulin resistance.
The purpose of the current study was to examine the prospective association between a diet consistent with a Mediterranean-style dietary pattern and metabolic syndrome traits and its incidence in nondiabetic US men and women.
SUBJECTS AND METHODS
Study sample
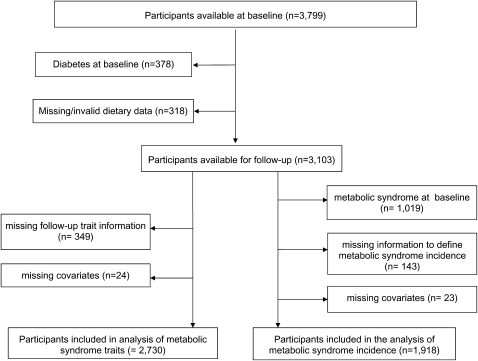
The Framingham Heart Study Offspring Cohort is a longitudinal community-based study of CVD among the offspring of the participants of the Framingham Heart Study Original Cohort (16). Of the 3799 participants in the fifth examination cycle (1991–1995), which we used as the baseline for the current analyses, we excluded participants with diabetes (n = 378) on the basis of previous clinical diagnosis, the use of insulin or oral hypoglycemic medication, or a fasting blood glucose ≥126 mg/dL (Figure 1). Among the remaining eligible participants, 318 participants were excluded because of missing or invalid food-frequency questionnaire (FFQ) data (ie, reported energy intakes of <600 kcal/d for all or >4000 kcal/d for women and >4200 kcal/d for men or >12 blank food items), which left 3103 nondiabetic participants with valid dietary data. After further exclusion of participants who did not attend the follow-up (n = 349) and those who were missing covariates (n = 24), 2730 participants were followed over a mean of 7 y for the longitudinal analyses of metabolic syndrome traits. For the analysis of the incidence of metabolic syndrome, 1019 participants with prevalent metabolic syndrome at baseline (the fifth examination) were excluded from the 3103 nondiabetic participants with valid dietary data. After further exclusion of those with incomplete information to define metabolic syndrome incidence at follow-up (n = 143) or with missing covariates (n = 23), 1918 participants were followed over a mean of 7 y to assess the incidence of metabolic syndrome. The institutional review boards for human research at Boston University and at Tufts Medical Center approved the study protocols and procedures.
FIGURE 1.
Selection of the study sample.
Assessment of individualrsquos diet conformity to a Mediterranean-style dietary pattern
Dietary intake was assessed by using the Harvard semiquantitative FFQ (17). The FFQ was mailed to the participants before the examination, and all participants were asked to bring the completed questionnaire with them to their appointment. The FFQ consisted of 126 items, including a list of foods together with a standard serving size and a selection of 9 frequency categories ranging from “never or less than once per month” to “6+/day.” The questionnaires also allowed participants to add up to 3 additional foods usually consumed that were not listed on the FFQ, as well as types of cold breakfast cereal and cooking oil usually used. Participants were asked to report their frequency of consumption of each food item during the past year. Nutrient intakes were calculated by multiplying the frequency of consumption of each unit of food from the FFQ by the nutrient content of the specified portion. The nutrient values were calculated on the basis of the US Department of Agriculture food composition database and supplemented by other published sources and personal communications from laboratories and manufacturers (17).
The Mediterranean-style dietary pattern score (MSDPS) was developed to assess the conformity of an individual’s diet to a traditional Mediterranean-style dietary pattern as defined by the Mediterranean diet pyramid (18). The development of the MSDPS is discussed in greater detail elsewhere (19) and is summarized briefly below. The MSDPS is based on the recommended intakes of 13 food groups in the Mediterranean diet pyramid, ie, whole-grain cereals, fruit, vegetables, dairy, wine, fish, poultry, olives/legumes/nuts, potatoes, eggs, sweets, meat, and olive oil. With the exception of olive oil, each food group was scored from 0 to 10 depending on the degree of correspondence with the recommendation (eg, consuming 60% of the recommended servings would result in a score of 6). Exceeding the recommendations resulted in a lower score proportional to the degree of overconsumption (eg, exceeding the recommendation by 60% would result in a score of 4). A negative score due to this overconsumption penalty was defaulted to zero. Olive oil’s scoring was categorical in nature on the basis of the exclusive use of olive oil (score 10), the use of olive oil along with other vegetable oils (score 5), or no olive oil (score 0). The sum of the 13 component scores was standardized to a 0–100 scale and weighted proportionally by a continuous factor from 0 to 1, which reflected the proportion of energy intake attributed to the consumption of foods included in the Mediterranean diet pyramid. The total MSDPS ranged from 0 to 100.
To account for long-term dietary exposure and to reduce within-person variability, the MSDPS was calculated as a mean score obtained from the dietary data of the fifth (1991–1995), sixth (1995–1998), and seventh (1998–2001) examinations, and this mean score was applied to the longitudinal analysis of metabolic syndrome traits. For the analysis of metabolic syndrome incidence, the MSDPS was calculated as a mean score calculated from the dietary data of the fifth examination (baseline) up to the examination at which metabolic syndrome incidence was ascertained.
Outcome measurements
Six biomarkers were used to identify metabolic syndrome traits, including homeostasis model assessment-insulin resistance [HOMA-IR = (fasting glucose × fasting insulin)/22.5] (20), fasting plasma glucose, waist circumference, plasma triglycerides, HDL cholesterol, and systolic and diastolic blood pressures. We used the values of these biomarkers that were measured at the fifth and seventh examinations. Fasting insulin concentration was measured in EDTA plasma as total immunoreactive insulin (Coat-A-Count insulin; Diagnostic Products, Los Angeles, CA) at exam 5 and with a human-specific insulin assay (Linco Inc, St Louis, MO) at exam 7. Fasting plasma glucose was measured in fresh specimens with a hexokinase reagent kit (A-Gene glucose test; Abbot, South Pasadena, CA). Triglyceride was measured enzymatically and the HDL cholesterol fraction was measured after the precipitation of low-density lipoprotein cholesterol and very-low-density lipoprotein cholesterol with dextran sulfate magnesium. The intra-assay coefficients of variation were <3% for glucose, triglyceride, and HDL cholesterol and 5–10% for insulin. Waist circumference was measured at the level of the umbilicus while the participant was standing. Blood pressure was measured to the nearest 2 mm Hg with a mercury-column sphygmomanometer on the left arm after the subject had been seated quietly for 5 min. Two readings obtained by a physician were averaged to calculate the systolic and diastolic blood pressures. The use of medications including insulin, oral hypoglycemic, antihyperlipidemic, or antihypertensive was determined during the physical examination.
The incidence of metabolic syndrome was ascertained at either the sixth or seventh examination among 1918 individuals (1120 women) free of the condition at the baseline fifth examination. Metabolic syndrome was defined according to the modified definition of the National Cholesterol Education Program Adult Treatment Panel III guidelines as ≥3 of any of the following: hyperglycemia (fasting plasma glucose ≥100 mg/dL) or current use of insulin or oral hypoglycemic medication; increased waist circumference (>102 cm in men or >88 cm in women); hypertriglyceridemia (≥150 mg/dL); low HDL cholesterol (<40 mg/dL in men or <50 mg/dL in women) or current use of lipid-lowering treatment; and elevated blood pressure (≥130/85 mm Hg) or current treatment of hypertension (5).
Covariates
Height (to the nearest 0.25 inch) and weight (to the nearest 0.25 lb) were measured with the subject standing, with shoes off, wearing only a hospital gown. Body mass index (BMI; in kg/m2) was calculated as weight/height (squared). A change in BMI was calculated as the difference between BMI values measured at the seventh examination (for the analysis of metabolic syndrome traits) or at the time of ascertainment of metabolic syndrome incidence and at the fifth examination (baseline). Other covariates included age (in y); sex; smoking within the past year (0, 1–15, 16–25, >25 cigarettes/d); multivitamin use (yes/no); estrogen-replacement therapy (ERT) in postmenopausal women only (yes/no); physical activity level (PAL) assessed as a weighted average of the proportion of a typical day spent sleeping and performing sedentary, slight, moderate, or heavy physical activities (expressed in metabolic equivalents) (21); and energy intakes (kcal/d) as an average intake calculated from the dietary data of the fifth, sixth, and seventh examinations (for the analysis of metabolic syndrome traits) or of the fifth examination (baseline) up to the examination at which the metabolic syndrome incidence was ascertained (for the analysis of metabolic syndrome incidence). Because self-reported dietary intake tends to underestimate energy intake relative to energy expenditure measured by the doubly labeled water method (22), we used the Schofield equation to predict a participant’s resting metabolic rate (23) and identified under-reporters (yes/no) as those in whom the ratio of reported energy intake to predicted resting metabolic rate was less than the Goldberg cutoff for a sedentary PAL of 1.35 (24).
Statistical analysis
Statistical analyses were conducted with SAS statistical software (version 9; SAS Institute, Cary, NC). The MSDPS was normally distributed. We used the MSDPS as a continuous measure and also divided this score into approximate quintile categories for analysis. The outcome variables were metabolic syndrome traits including HOMA-IR, fasting glucose, waist circumference, triglyceride, HDL cholesterol, and systolic and diastolic blood pressures. Except for diastolic blood pressure, all other traits of the metabolic syndrome were positively skewed; therefore, a natural logarithmic transformation was applied to normalize their distributions. To express these variables on their original scale, geometric means were calculated by taking the exponent of the adjusted least-squares means.
Age- and sex-adjusted participant characteristics were compared across quintile categories of the MSDPS by using the SAS procedures PROC GLM (for continuous characteristics) and PROC LOGISTIC (for dichotomous characteristics). We performed analysis of covariance (PROC GLM) to examine the relation between MSDPS and the geometric means of metabolic syndrome traits at the follow-up visit, with adjustment for their corresponding baseline values and potential confounders, which included baseline age, sex, BMI, smoking dose, PAL, ERT (in postmenopausal women only), average energy intake, accuracy of reporting energy intake, and change in BMI. We considered the follow-up trait measures with adjustment for the baseline corresponding values rather than change in trait between baseline and follow-up because of the different insulin assays at these 2 time points. To maximize the sample size, covariates that did not affect the association were not included in the final model. A subsidiary analysis was applied to the longitudinal analysis of metabolic syndrome traits by excluding participants with metabolic syndrome at the baseline. We applied PROC GLM to compare the cumulative incidence of metabolic syndrome over 7 y of follow-up across quintile categories of MSDPS, with adjustment for the potential confounders outlined above. Tests for trends across quintile categories of MSDPS were based on linear regression by assigning the median MSDPS for each quintile category to each individual in that category and then by treating it as a continuous variable. We reported previously that the inability of the FFQ used in the present analysis to quantify energy intake accurately could instigate a higher MSDPS (19). Thus, we tested the potential interaction between the accuracy of reporting energy intakes (accurate or underreporting) and the MSDPS on predicting metabolic syndrome. With the use of Bonferroni correction to adjust for multiple comparison, an interaction with an observed P value of <0.007 would be deemed significant. For all other analyses, statistical significance was defined as P value <0.05.
RESULTS
The median quintile MSDPS ranged from 15.0 to 31.9 (Table 1). Compared with participants in the lowest quintile category of MSDPS, those in the highest quintile category were older, more likely to be women, multivitamin users, and ERT users, more likely to have a greater change in BMI over follow-up, and less likely to be current smokers. The MSDPS was positively associated with total energy intakes only among those whom we classified as under-reporter participants, but not among those classified as accurate reporters. There were no significant associations between the MSDPS and BMI, PAL, or the use of cholesterol or blood pressure medications.
TABLE 1.
Participants’ characteristics across quintile (Q) categories of the Mediterranean-style dietary pattern score (MSDPS) in the Framingham Heart Study Offspring Cohort1
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend2 | |
| n | 521 | 538 | 547 | 576 | 548 | |
| MSDPS3 | 15.1 (4.05–17.8) | 19.8 (17.9–21.6) | 23.2 (21.7–25.0) | 26.9 (25.1–29.0) | 31.9 (29.1–49.6) | |
| Age (y)4 | 52.4 (51.6, 53.3) | 53.5 (52.7, 54.3) | 53.7 (52.9, 54.5) | 55.4 (54.7, 56.2) | 54.8 (54.0, 55.6) | <0.001 |
| Women (%)5 | 43 (39, 47) | 53 (49, 57) | 54 (50, 58) | 56 (52, 60) | 70 (65, 74) | <0.001 |
| BMI (kg/m2) | 26.7 (26.3, 27.1) | 27.0 (26.6, 27.4) | 26.8 (26.5, 27.2) | 26.7 (26.4, 27.1) | 26.3 (26.0, 26.7) | 0.08 |
| PAL (MET score/h) | 1.42 (1.40, 1.44) | 1.42 (1.40, 1.44) | 1.44 (1.42, 1.46) | 1.44 (1.42, 1.46) | 1.44 (1.42, 1.46) | 0.06 |
| Smokers (%) | 30 (27, 34) | 21 (18, 24) | 16 (13, 20) | 12 (9, 15) | 13 (10, 16) | <0.001 |
| Multivitamin users (%) | 22 (18, 26) | 25 (21, 29) | 27 (23, 31) | 33 (29, 37) | 35 (31, 39) | <0.001 |
| ERT use in women (%) | 15 (10, 20) | 14 (9, 18) | 16 (12, 20) | 20 (16, 25) | 24 (20, 27) | <0.001 |
| Cholesterol medication use (%) | 6 (3, 8) | 7 (5, 9) | 7 (5, 9) | 7 (5, 9) | 6 (4, 8) | 0.88 |
| Blood pressure medication use (%) | 14 (11, 17) | 17 (14, 20) | 16 (13, 19) | 19 (16, 22) | 14 (11, 17) | 0.49 |
| Energy intakes (kcal/d)6 | ||||||
| Accurate reporters (n = 813) | 2272 (2221, 2329) | 2366 (2313, 2419) | 2332 (2280, 2385) | 2319 (2267, 2371) | 2348 (2296, 2402) | 0.17 |
| Underreporters (n = 1917) | 1409 (1379, 1441) | 1551 (1518, 1586) | 1598 (1564, 1634) | 1629 (1593, 1665) | 1683 (1647, 1721) | <0.001 |
| Change in BMI (kg/m2)7 | 0.46 (0.26, 0.66) | 0.86 (0.67, 1.06) | 0.71 (0.51, 0.90) | 0.92 (0.73, 1.11) | 0.85 (0.66, 1.05) | 0.01 |
PAL, physical activity level; MET, metabolic equivalent task; ERT, estrogen replacement therapy. Values are either proportions or geometric means (and 95% CIs), adjusted for age and sex, unless indicated otherwise.
Tests for trends were based on linear regression with the use of the MSDPS median quintile category as a continuous variable.
Values are medians; ranges in parentheses.
Values are sex-adjusted means; 95% CIs in parentheses.
Values are age-adjusted proportions; 95% CIs in parentheses.
Calculated by using data at the fifth, sixth, and seventh examinations.
Values are age- and sex-adjusted means (95% CIs in parentheses) and calculated as the mean difference between BMI measured at the fifth (baseline) and seventh (follow-up) examinations. Analyses were controlled for baseline BMI.
Metabolic syndrome traits
Participants with a higher MSDPS had significantly lower waist circumference, HOMA-IR, fasting plasma glucose, or triglyceride and higher HDL cholesterol at the follow-up examination than those with lower scores, after adjustment for their corresponding baseline values and the other risk factors for T2DM, including age, sex, energy intake, smoking dose, BMI, and change in BMI (Table 2). No significant association was shown between the MSDPS and blood pressure. Additional adjustment for ERT (in postmenopausal women only), multivitamin use, PAL, and the accuracy of reporting energy intake did not meaningfully change the associations; therefore, these covariates were not included in the reported models. We did not find significant effect modification (P < 0.007 after Bonferroni correction) that resulted from underreporting of energy intake on the association between the MSDPS and waist circumference (P = 0.54), HOMA-IR (P = 0.82), fasting glucose (P = 0.26), plasma triglyceride (P = 0.38), HDL cholesterol (P = 0.89), or systolic and diastolic blood pressures (P = 0.04 and 0.24, respectively). In subsidiary analyses, these findings were unaffected by excluding participants who had metabolic syndrome at the baseline examination (see Table S1 under “Supplemental data” in the online issue).
TABLE 2.
Metabolic syndrome traits at the seventh follow-up examination across quintile (Q) categories of the Mediterranean-style dietary pattern score (MSDPS) in the Framingham Heart Study Offspring Cohort1
| Q1 (4.05–17.8) | Q2 (17.9–21.6) | Q3 (21.7–25.0) | Q4 (25.1–29.0) | Q5 (29.1–49.6) | P for trend2 | |
| n | 521 | 538 | 547 | 576 | 548 | |
| HOMA-IR3 | 3.38 (3.23, 3.53) | 3.37 (3.23, 3.51) | 3.30 (3.17, 3.44) | 3.27 (3.14, 3.40) | 3.16 (3.03, 3.30) | 0.02 |
| Fasting glucose (mg/dL)4 | 98.5 (97.6, 99.4) | 98.5 (97.6, 99.4) | 98.7 (97.8, 99.6) | 98.2 (97.3, 99.0) | 97.1 (96.3, 98.0) | 0.03 |
| Waist circumference (cm)5 | 98.9 (98.4, 99.4) | 98.2 (97.7, 98.6) | 98.6 (98.1, 99.0) | 98.2 (97.8, 98.6) | 97.1 (96.7, 97.6) | <0.001 |
| Triglyceride (mg/dL)6 | 114 (110, 119) | 112 (108, 117) | 111 (107, 116) | 108 (104, 112) | 103 (99, 107) | <0.001 |
| HDL cholesterol (mg/dL)7 | 53.3 (52.3, 54.2) | 51.9 (51.0, 52.8) | 54.0 (53.1, 54.9) | 53.9 (53.0, 54.8) | 54.0 (53.1, 55.0) | 0.02 |
| Blood pressure (mm Hg)8 | ||||||
| Systolic | 122 (121, 123) | 122 (120, 123) | 121 (120, 122) | 122 (120, 123) | 121 (120, 123) | 0.70 |
| Diastolic9 | 74 (73, 74) | 74 (73, 74) | 73 (73, 74) | 74 (73, 75) | 73 (73, 74) | 0.96 |
Values are least-squares geometric means; 95% CIs in parentheses. HOMA-IR, homeostasis model assessment–insulin resistance. Estimates are from multivariate models with adjustment for the following trait values at the fifth baseline examination: age (y), sex, smoking dose (cigarettes/d), BMI (kg/m2), energy intake (kcal/d), and change in BMI (kg/m2).
Tests for trends were based on linear regression with the use of the MSDPS median quintile category as a continuous variable.
On the basis of 2394 participants: 44 participants treated with insulin or an oral hypoglycemic at follow-up and 292 participants missing data on HOMA-IR at either baseline or follow-up were excluded.
On the basis of 2529 participants: 44 participants treated with insulin or oral hypoglycemic at follow-up and 157 participants missing data on fasting glucose at either baseline or follow-up were excluded.
On the basis of 2621 participants: 109 participants with missing data on waist circumference at either baseline or follow-up were excluded.
On the basis of 2056 participants: 553 participants treated with cholesterol-lowering medication and 121 participants missing data on triglycerides at either baseline or follow-up were excluded.
On the basis of 2050 participants: 553 participants treated with cholesterol-lowering medication and 127 participants missing data on HDL cholesterol at either baseline or follow-up were excluded.
On the basis of 1815 participants: 913 participants treated with hypertension-lowering medication and 2 participants missing data on blood pressure at either baseline or follow-up were excluded.
Values are least-squares means; 95% CIs in parentheses.
Metabolic syndrome incidence
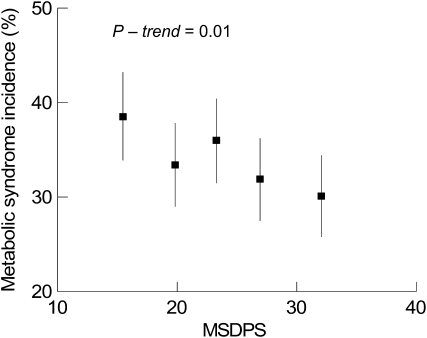
In a model adjusted for age, sex, energy intake, smoking dose, BMI, and change in BMI among participants without metabolic syndrome at the baseline (Figure 2), those in the highest quintile category of MSDPS had the lowest cumulative incidence of metabolic syndrome over 7 y of follow-up [cumulative incidences (95% confidence limits) from the lowest to the highest quintile categories were 38.5% (33.9, 43.2%), 33.4% (29.0, 37.8%), 36.0% (31.5, 40.4%), 31.9% (27.5, 36.2%), and 30.1% (25.8, 34.4%), respectively]. Adjustment for additional covariates, as described above for the analysis of metabolic syndrome traits, did not affect the observed associations. Also, no significant interaction was shown between the MSDPS and the accuracy of reporting energy intake for the metabolic syndrome incidence (P = 0.49).
FIGURE 2.
Cumulative incidence (with 95% CIs) of metabolic syndrome over 7 y of follow-up across quintile categories of the Mediterranean-style dietary pattern score (MSDPS) among 1918 participants at risk at baseline in the Framingham Heart Study Offspring Cohort. Symbols represent proportions for quintile categories, which were estimated from multivariate models adjusted for age (in y), sex, smoking dose (cigarettes/d), average energy intake (kcal/d), BMI (kg/m2), and change in BMI.
DISCUSSION
In this nondiabetic sample, we observed that consuming a diet consistent with the principles of the Mediterranean-style dietary pattern was favorably associated with avoiding metabolic syndrome traits that were assessed longitudinally—specifically, with less abdominal obesity, less insulin resistance, and less atherogenic dyslipidemia. We also showed that a diet consistent with a Mediterranean-style dietary pattern was associated with a lower incidence of metabolic syndrome. Our findings extend the limited data in this field in several distinctive aspects.
First, there are many previous cross-sectional studies that reported inconsistent findings between the Mediterranean-style dietary pattern and both insulin resistance (11, 14) and metabolic syndrome (12–14). However, the prospective design of our study, by which dietary information was measured before the outcome occurred, strengthens the causal inference on the association between the Mediterranean-style dietary pattern and the study’s outcomes.
Second, there is limited prospective investigation of the role of the Mediterranean-style dietary pattern in the change in metabolic syndrome traits. In one intervention conducted with patients with metabolic syndrome, HOMA-IR reduced after a 2-y intervention with a Mediterranean-style diet (25). The current study showed that the Mediterranean-style diet was associated with improved insulin resistance. This association remained significant after excluding individuals with metabolic syndrome. Thus, the present study is the first to report the prospective association between a Mediterranean-style dietary pattern and insulin resistance in a healthy sample of general population.
Third, the findings of this study also expand on the existing body of knowledge concerning the inverse association between a Mediterranean-style dietary pattern and metabolic syndrome incidence, from the Mediterranean populations (15) to a non-Mediterranean one, specifically to a US population. In a Spanish population, individuals with higher Mediterranean diet scores had a 1.8% lower incidence of metabolic syndrome than those with lower scores after 6 y of follow-up (15). However, this study applied a diet score that was based on the actual intakes of the study population rather than on the recommended intakes in the Mediterranean diet pyramid. In our study, we showed a greater difference in metabolic syndrome incidence (≈ 7.7%) between participants in the highest and lowest quintiles of MSDPS after 7 y of follow-up. Given the high prevalence of metabolic syndrome in the United States over the past few years (26), the finding of this research, which is the first to report a protective association of a Mediterranean-style diet to metabolic syndrome incidence in a sample of the US population, is particularly important. If a Mediterranean-style diet delays or prevents metabolic syndrome, then individuals following this diet will be less likely to develop T2DM and CVD.
Our longitudinal observation showed that of the 5 components that compose the metabolic syndrome incidence, 4 components (waist circumference, fasting plasma glucose, triglyceride, and HDL cholesterol) were individually associated with the MSDPS. The association between a Mediterranean-style diet and waist circumference in our study was stronger (mean difference between the highest and lowest quintiles of MSDPS: 1.7 cm; P for trend: <0.001) than that of an earlier prospective study among adults in Spain (mean difference: 0.5 cm; P for trend: 0.04) (15). Although this latter study did not find a significant association between the diet score and plasma glucose, triglyceride, or HDL cholesterol—which could be due to the difference in the diet scores—our study’s findings imply that a long-term intake of the Mediterranean-style diet may reduce the development or progression of atherogenic dyslipidemia. It was not unanticipated that our study did not observe a significant association between the MSDPS and blood pressure, given that a large number of participants being treated for hypertension had been excluded from this particular analysis.
Interpretation of the findings from the present study is subject to some limitations. Because the MSDPS assigned an equal weight to each of its food components, the associations in this study were also based on the assumption that each food group composing the Mediterranean-style dietary pattern contributed equally to the outcomes. Nevertheless, the nutritional benefits of Mediterranean-style diet, including those of reducing the risk of metabolic syndrome or the individual metabolic risk factors, are most likely due to the joint effects of the entire diet comprised of all the nutrients rather than the effects of individual food components (9, 27). Another potential limitation of this work concerns the nature of dietary pattern analysis on the basis of a diet score. Of the possible maximum MSDPS of 100, the highest score achieved in this study population was 49.5. Although a higher value of the MSDPS reflects a greater conformity to a Mediterranean-style dietary pattern, an individual’s diet will not conform entirely to the Mediterranean-style dietary pattern unless the person achieves the maximum score of 100. Similarly, there are several different ways in which combinations of food groups composing the MSDPS may produce moderate scores observed in our population sample. Consequently, specific subpatterns within the Mediterranean diet may be associated in a different way with various outcomes in this study, which limits our ability to detect the benefits associated with higher scores. Despite this limitation, the MSDPS was associated with the metabolic syndrome incidence and a majority of the traits. The use of dietary data that derived from an FFQ to calculate the MSDPS is another potential limitation of this research. However, earlier validation studies of the Harvard FFQ showed that many of the foods included in the MSDPS were adequately captured on the FFQ on the basis of correlations with diet records (28). Also, the FFQ is helpful in characterizing individuals’ usual intakes over a long period of time and in ranking individuals according to their usual intake with respect to its association with health outcomes (29). Finally, although the apparent protective association of a Mediterranean-style diet with metabolic syndrome traits and incidence persisted after adjustment for lifestyle and T2DM risk factors, we cannot rule out residual confounding. For example, adjustment for BMI cannot entirely remove the confounding effect of adiposity on the diet-disease associations observed in our study, which is in part due to the limitation of BMI in accurately measuring adiposity. In our study, BMI attenuated the associations between a Mediterranean-style diet and the disease outcomes. Thus, these observed associations might be further weakened if they were adjusted for more accurate adiposity measures.
Like most epidemiologic studies, the results of this study alone cannot be directly translated into clinical practice, yet they provide fundamental information on the relation of a Mediterranean style diet pattern to intermediate risk factors for T2DM and CVD at the population level. For example, by using the regression coefficient derived from the multivariate linear regression model of the MSDPS–metabolic syndrome association (0.0045), we estimated that the cumulative incidence of metabolic syndrome in this study population could be reduced by ≈4.5% by increasing the MSDPS by 10 points, which assumes a linear association across the full range of this relation.
This research suggests that a long-term consumption of a Mediterranean-style diet may be one effective dietary strategy for protecting against metabolic syndrome, a risk factor for T2DM and CVD. However, further studies are warranted to confirm the potential benefit of a Mediterranean-style diet in mitigating metabolic syndrome.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MER: designed the score, created and designed the project, conducted the analysis, and drafted the manuscript; JBM, JTD, NMM, and PFJ: assisted in the creation and design of the project. MER, JBM, JTD, NMM, and PJF: participated in the revision and approval of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Olefsky JM, Nolan JJ. Insulin resistance and non-insulin-dependent diabetes mellitus: cellular and molecular mechanisms. Am J Clin Nutr 1995;61:980S–6S [DOI] [PubMed] [Google Scholar]
- 2.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544–50 [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007;115:e69–171 [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Kiechl S, Willeit J, et al. Metabolic syndrome: epidemiology and more extensive phenotypic description. Cross-sectional data from the Bruneck Study. Int J Obes Relat Metab Disord 2003;27:1283–9 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–72 [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. JAMA 2002;288:2569–78 [DOI] [PubMed] [Google Scholar]
- 8.Keys AB. Seven countries: a multivariate analysis of death and coronary heart disease. Cambridge, MA: Harvard University Press, 1980 [Google Scholar]
- 9.Simopoulos AP. The Mediterranean diets: what is so special about the diet of Greece? The scientific evidence. J Nutr 2001;131:3065S–73S [DOI] [PubMed] [Google Scholar]
- 10.Mitrou PN, Kipnis V, Thiebaut AC, et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461–8 [DOI] [PubMed] [Google Scholar]
- 11.Panagiotakos DB, Tzima N, Pitsavos C, et al. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: the ATTICA Study. J Am Coll Nutr 2007;26:32–8 [DOI] [PubMed] [Google Scholar]
- 12.Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J 2004;147:106–12 [DOI] [PubMed] [Google Scholar]
- 13.Babio N, Bullo M, Basora J, et al. Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr Metab Cardiovasc Dis 2009;19:563–70 [DOI] [PubMed] [Google Scholar]
- 14.Alvarez Leon EE, Henriquez P, Serra-Majem L. Mediterranean diet and metabolic syndrome: a cross-sectional study in the Canary Islands. Public Health Nutr 2006;9:1089–98 [DOI] [PubMed] [Google Scholar]
- 15.Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, Basterra-Gortari FJ, Nunez-Cordoba JM, Martinez-Gonzalez MA. Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care 2007;30:2957–9 [DOI] [PubMed] [Google Scholar]
- 16.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4:518–25 [DOI] [PubMed] [Google Scholar]
- 17.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26; discussion 1127–36 [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health and Welfare Supreme Scientific Health Council of Greece Dietary guidelines for adults in Greece. Arch Hellenic Med 1999;16:516–24 [Google Scholar]
- 19.Rumawas ME, Dwyer JT, McKeown NM, Meigs JB, Rogers G, Jacques PF. The development of the Mediterranean-style dietary pattern score and its application to the American diet in the Framingham Offspring Cohort. J Nutr 2009;139:1150–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med 1979;139:857–61 [PubMed] [Google Scholar]
- 22.Schoeller DA, Schoeller DA. Validation of habitual energy intake. Public Health Nutr 2002;5:883–8 [DOI] [PubMed] [Google Scholar]
- 23.Schofield WN. Predicting basal metabolic rate: new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(suppl 1):5–41 [PubMed] [Google Scholar]
- 24.Goldberg GR, Black AE, Jebb SA, et al. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569–81 [PubMed] [Google Scholar]
- 25.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292:1440–6 [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 27.Minich DM, Bland JS. Dietary management of the metabolic syndrome beyond macronutrients. Nutr Rev 2008;66:429–44 [DOI] [PubMed] [Google Scholar]
- 28.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 29.Willett W. Nutritional epidemiology. 2nd ed. New York, NY: Oxford University Press, 1998 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.