To the Editor:
The risk of thromboembolic events is 3 to 4-fold higher in patients with inflammatory bowel disease (IBD) than in the general population.1 Venous thromboembolism in IBD occurs usually during exacerbation mainly in patients with more advanced complicated forms of the disease.1 The mechanisms underlying the link of IBD and thromboembolism involve endothelial injury, procoagulant effects of enhanced inflammation, hyperhomocysteinemia, and genetic predisposition.2 Typical prothrombotic abnormalities reported in IBD include elevated fibrinogen, factors (F)V, FVIII, FIX, FXIII, impaired fibrinolysis as well as reduced anticoagulant proteins such as protein S.2,3 Recently, it has been suggested that arterial thrombosis is more prevalent in IBD than in the general population.4
Tissue factor (TF) is the main initiator of blood coagulation in vivo. Although it has been suggested that circulating TF-bearing microparticles are elevated in IBD like in other inflammatory disorders,5 to our knowledge there have been no reports regarding TF levels in Crohn’s disease (CD) or ulcerative colitis (UC) patients. Moreover, increased active FXI (FXIa) levels that are known to confer a higher risk of venous and arterial thrombosis,6 have been observed in patients with active UC.5 No data on FXIa in CD have been published yet.
To evaluate a potential role of procoagulant activity of TF and FXIa, we studied 80 consecutive IBD patients (39M, 41F) aged 34.5±12.1 years (±SD) (range 18 to 69), including 34 subjects with CD and 46 with UC. Active disease was observed at the time of enrollment in 19 CD patients (mean activity 169.2±93.5) and 26 UC assessed using a variation of the Truelove-Witts criteria (mean activity 5.45±3.35). Exclusion criteria were as follows: any acute illness, cancer, hepatic or renal dysfunction, previous venous thromboembolism, MI or stroke, oral anticoagulation or heparin administration. The Jagiellonian University Ethical Committee approved the study, and patients provided written, informed consent.
To determine FXIa and TF in fasting citrated plasma, we used inhibitory monoclonal anti-FXI (αFXI-2) and anti-TF (αTF-5) antibodies at a final 0.1 mg/ml concentration and measured clotting time as previously described.7 The quantitation limit of our TF activity assay is 0.4 pM and that of FXIa assay is 10 pM. Age- and sex-matched healthy individuals (n=12) recruited from the hospital staff showed no detectable TF or FXIa. To assess thrombin generation in plasma, we determined thrombin-antithrombin complexes (TAT) using an ELISA (Dade Behring, Marburg, Germany).
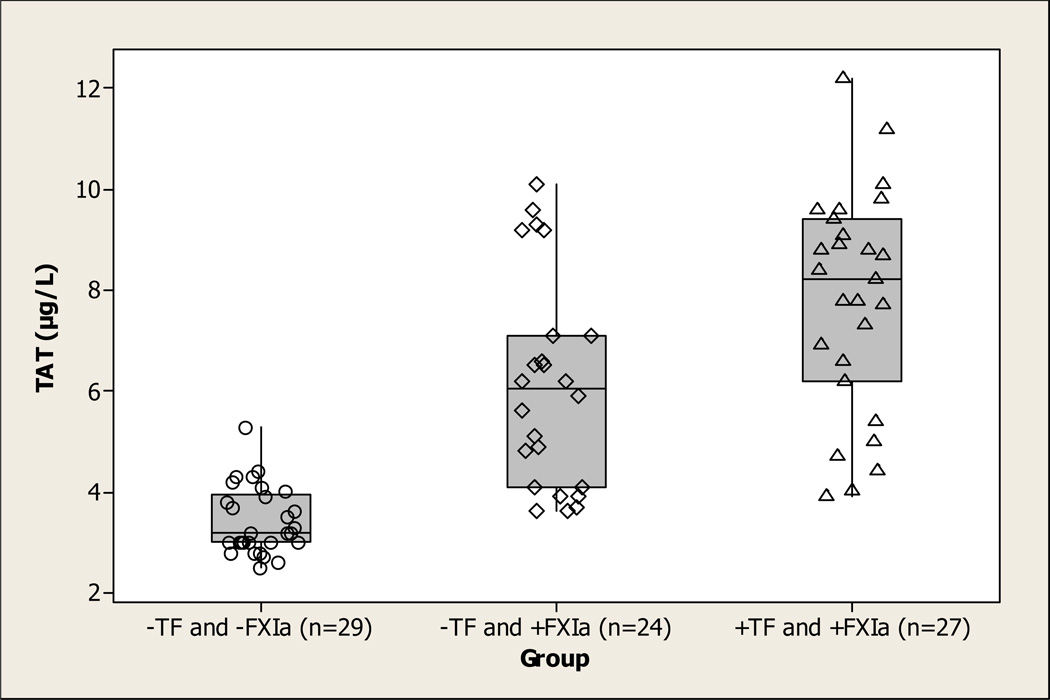
Quantifiable amounts (>10 pM) of plasma FXIa were detected in 53 of 80 (66%) patients with IBD. The concentration of FXIa varied from 17 to 156 pM with an average value of 57.2±33.6 pM. Detectable plasma TF activity was displayed in 27 of 80 (34%) IBD patients, all of whom also had circulating FXIa in plasma. Only 10 IBD patients had TF activity above 0.5 pM in the range from 0.6 to 5.1 pM. The concentration of TF in the remaining samples was below 0.4 pM. TF-positive patients did not differ from those not displaying detectable TF activity in plasma with regard to demographics, fibrinogen, C-reactive protein, creatinine, and disease activity (data not shown). This also held true for FXIa-positive and -negative patients with IBD except for age: the patients with detectable FXIa were older (36.6±12.7 years) than those with no FXIa (30.7±10.0 years, p=0.028). Importantly, plasma TAT correlated with FXIa in IBD (r2=0.49, p<0.001). The highest TAT levels were observed in patients with both detectable active TF and FXIa (7.8±2.2 µg/L); patients with FXIa but no detectable TF had a significantly lower amount (6.1±2.1 µg/L); and the patients with neither FXIa nor TF had the lowest levels (3.4±0.7 µg/L, p<0.05 for all comparisons) of TAT.
This study is the first to demonstrate that as many as ⅔ of IBD patients had circulating FXIa and ⅓ had active TF in their plasmas, with no differences between CD and UC. Previously, we have shown that 96% of patients with acute myocardial infarction (AMI) and 76% of subjects with a history of MI have circulating FXIa.7 38% of AMI patients and only 6% of stable CAD patients showed detectable TF activity.7 In the current study, circulating levels of TF in the IBD patients were similar to those in acute coronary ischemia, whereas FXIa coagulant activity was only slightly lower compared to that observed in stable angina subjects who survived AMI.7 A correlation between thrombin generation (TAT) and FXIa activity confirms that, most likely, thrombin, not the contact pathway, accounts for FXI activation in this disease. These abnormalities may predispose to venous thrombosis and arterial thromboembolic events observed in CD and UC patients. Of note is that the presence of measurable TF and FXIa did not correlate with clinical activity of IBD or with inflammatory markers, suggesting a novel mechanism of thrombosis. This might in part explain clinical observations indicating that in UC an increased risk of thrombosis is observed in the mild form of disease or even during remission.1
Figure 1.
Boxplot of TAT levels in IBD patients. Patients are separated into three groups: those that do not contain detectable TF or FXIa (n=29, ○), those that contain detectable FXIa but not TF (n=24, ◇), and those that contain both detectable FXIa and TF (n=27, △).
REFERENCES
- 1.Miehsler W, Reinisch W, Valic E, et al. Is inflammatory bowel disease an independent and disease specific risk factor for thromboembolism? Gut. 2004;53:542–548. doi: 10.1136/gut.2003.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danese S, Papa A, Saibeni S, et al. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. 2007;101:174–186. doi: 10.1111/j.1572-0241.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 3.Kume K, Yamasaki M, Tashiro M, et al. Activations of coagulation and fibrinolysis secondary to bowel inflammation in patients with ulcerative colitis. Intern Med. 2007;17:1323–1329. doi: 10.2169/internalmedicine.46.0237. [DOI] [PubMed] [Google Scholar]
- 4.Ha C, Magowan S, Accortt NA, et al. Risk of arterial thrombotic events in inflammatory bowel disease. Am J Gastroenterol. 2009;104:1445–1451. doi: 10.1038/ajg.2009.81. [DOI] [PubMed] [Google Scholar]
- 5.SriRajaskanthan R, Winter M, Muller AF. Venous thrombosis in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2005;17:697–700. doi: 10.1097/00042737-200507000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Seligsohn U. Factor XI in haemostasis and thrombosis: Past, present and future. Thromb Haemost. 2007;98:84–89. [PubMed] [Google Scholar]
- 7.Butenas S, Undas A, Gissel MT, et al. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]