Abstract
B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements, also known as “double-hit” lymphomas (DHL), are rare neoplasms characterized by highly aggressive clinical behavior, complex karyotypes, and a spectrum of pathological features overlapping with Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL) and B-lymphoblastic lymphoma/leukemia (B-LBL). The clinical and pathological spectrum of this rare entity, including comparison to other high-grade B-cell neoplasms, has not been well defined. We conducted a retrospective analysis of clinical and pathologic features of 20 cases of DHL seen at our institution during a 5-year period. In addition, we performed case-control comparisons of DHL with BL and International Prognostic Index (IPI)-matched DLBCL. The 11 men and 9 women had a median age of 63.5 years (range 32-91). Six patients had a history of grade 1-2 follicular lymphoma (FL); review of the prior biopsy specimens in 2 of 5 cases revealed blastoid morphology. Eighteen patients had Ann Arbor stage 3 or 4 disease and all had elevated serum lactate dehydrogenase (LDH) levels at presentation. Extranodal disease was present in 17/20 (85%), bone marrow involvement in 10/17 (59%) and central nervous system (CNS) disease in 5/11 (45%). Nineteen patients were treated with combination chemotherapy, of whom 18 received rituximab and 14 received CNS-directed therapy. Fourteen patients (70%) died within 8 months of diagnosis. Median overall survival in the DHL group (4.5 months) was inferior to both BL (p=0.002) and IPI-matched DLBCL (p=0.04) control patients. Twelve DHL cases (60%) were classified as B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL, 7 cases (35%) as DLBCL, not otherwise specified, and 1 case as B-LBL. Distinguishing features from BL included expression of Bcl2 (p<0.0001), Mum1/IRF4 (p=0.006), Ki-67 <95% (p<0.0001), and absence of EBV-EBER (p=0.006). DHL commonly contained the t(8;22) rather than the t(8;14) seen in most BL controls (p=0.001), and exhibited a higher number of chromosomal aberrations (p=0.0009). DHL is a high-grade B-cell neoplasm with a poor prognosis, resistance to multi-agent chemotherapy, and clinical and pathological features distinct from other high-grade B-cell neoplasms. Familiarity with the morphologic and immunophenotypic spectrum of DHL is important in directing testing to detect concurrent IGH-BCL2 and MYC rearrangements when a karyotype is unavailable. The aggressive clinical behavior and combination of genetic abnormalities seen in these cases may warrant categorization as a separate entity in future classifications and call for novel therapeutic approaches.
Keywords: MYC, BCL2, diffuse large B-cell lymphoma, Burkitt lymphoma, cytogenetics, high-grade B-cell lymphoma
Introduction
B-cell lymphomas commonly exhibit chromosomal translocations involving immunoglobulin genes (IG). The t(14;18)(q32;q21) results in the juxtaposition of BCL2 with enhancer elements of the IG heavy chain (IGH). This translocation is a hallmark of follicular lymphoma (FL) and is also found in 20-30% of de novo diffuse large B-cell lymphoma (DLBCL) (16, 56). Translocations involving MYC at 8q24 and IG partners, including IGH and kappa (IGK) and lambda (IGL) light chains, are characteristic of Burkitt lymphoma (BL), and are also found in 5-15% of unselected cases of DLBCL (2, 17, 24). B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are rare, and are characterized by highly aggressive clinical behavior, complex karyotypes, and a broad morphologic and immunophenotypic spectrum that overlaps with BL and DLBCL, and occasionally B-lymphoblastic lymphoma/leukemia (B-LBL) (30). Because these “double-hit” lymphomas (DHL) share pathologic features with other high-grade B-cell neoplasms, their diagnosis and classification may be difficult, and their rarity and poor response to therapy make selection of appropriate treatment challenging.
Approximately 200 cases of DHL have been reported in the literature, mostly as case reports and small series (3, 4, 6, 8-10, 12, 13, 15, 18-20, 22, 25-27, 29-33, 36, 39, 40, 43, 47, 49-54, 59). Most reported patients have de novo disease, while a minority have a history of grade 1-2 FL and develop DHL secondarily, presumably by acquisition of a MYC translocation (8, 12, 18, 19, 26, 27, 31, 49-51, 54, 59). Few studies have directly compared the pathologic features and clinical outcome of DHL with BL and DLBCL (37, 42), and only a single series has classified DHL using criteria of the 2008 World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues (18, 48).
We conducted a retrospective analysis of the clinical, morphologic, immunophenotypic, cytogenetic, and molecular genetic features of 20 cases of DHL seen at our institution to define further the clinical and pathologic spectrum of this rare entity and to classify cases according to the 2008 WHO Classification. In addition, we performed a case-control comparison with BL cases seen over the same time period, in order to identify distinguishing clinicopathologic features that facilitate early and correct identification of DHL. We also performed a case-control comparison with a group of International Prognostic Index (IPI)-matched DLBCL cases treated at the same institution to elucidate clinical differences between DHL and DLBCL. Based on the distinctive clinicopathologic features of DHL that we identified, we outline circumstances in which additional testing by fluorescence in situ hybridization (FISH) or PCR on diagnostic tissue samples may be helpful to confirm or exclude a diagnosis of DHL if a conventional karyotype is unavailable.
Methods
Identification of Cases and Controls
The Partners HealthCare Institutional Review Board (IRB) granted approval for the study before its initiation. The files of the Massachusetts General Hospital (MGH) Pathology Department were searched for cases of B-cell lymphoma on which cytogenetic and/or FISH analysis had been performed and had revealed concurrent IGH-BCL2 and MYC rearrangements. In some cases, tissue had been sent for cytogenetic analysis at the time of frozen section evaluation at the discretion of the frozen section pathologist, while in other cases lacking cytogenetic analysis, FISH to detect IGH-BCL2 and MYC and gene rearrangements had been performed at the time of diagnosis because BL was a diagnostic consideration based on the morphology and/or immunophenotype. A total of 19 cases from 2004-2009 were identified. One additional case (case 10) identified from this time period contained a MYC rearrangement and 8 copies of an intact BCL2 confirmed by paraffin FISH, and was included in the study because of the functional equivalence of multiple copies BCL2 to t(14;18) (11, 35, 58). Pathology, flow cytometry and cytogenetics reports, and H&E- and immunohistochemical-stained slides of these cases were reviewed by 4 hematopathologists to confirm the diagnosis and to classify each case according to the 2008 WHO Classification. Medical records were reviewed to determine the IPI score (1), prior history of lymphoma, clinical presentation, therapeutic regimen, response to therapy, outcome and overall survival (OS).
BL control cases were identified by review of the MGH pathology files from 2000-2008; a total of 29 consecutive cases in which a diagnosis of “Burkitt lymphoma” or “atypical Burkitt lymphoma” had been rendered were identified. Pathology, flow cytometry and cytogenetics reports, and H&E- and immunohistochemical stained slides of these cases were reviewed by 4 hematopathologists for a consensus diagnosis of BL according to criteria of the 2008 WHO Classification (28). Four cases that did not meet morphologic, immunophenotypic, and/or cytogenetic criteria for BL were excluded, yielding a total of 25 BL control patients whose medical records were reviewed to determine site of involvement by lymphoma, serum lactate dehydrogenase (LDH) level at presentation, outcome and OS. This control group included 7 patients ≤20 years old, none of whom were known to be HIV-positive, 9 HIV-positive adults, and 9 HIV-negative adults.
DLBCL control cases were selected from an IRB-approved clinical database of patients diagnosed with DLBCL and treated at the MGH from 2000-2008. Two IPI-matched controls were randomly selected and matched to each DHL case, yielding a total of 40 DLBCL patients whose pathology reports were reviewed to confirm the diagnosis, and whose medical records were reviewed to determine site of involvement by lymphoma, LDH level at presentation, outcome and OS.
Immunophenotyping and In Situ Hybridization Studies
Formalin- or B+-fixed, paraffin-embedded tissues from diagnostic biopsies of DHL cases and BL control cases were characterized by immunohistochemistry using previously described methods (44) with some or all of the following antibodies, if not already performed at the time of diagnosis: CD20 (L26 epitope, prediluted, Ventana Medical Systems, Tucson, AZ), CD79a (prediluted, Ventana), Pax5 (prediluted, Ventana), Bcl6 (1:10 dilution, Dako, Carpinteria, CA), CD10 (prediluted, Ventana), Bcl2 (clone 124, prediluted, Ventana), Mum1/IRF4 (1:20 dilution, Dako), Ki-67 (clone 30-9, prediluted, Ventana), terminal deoxynucleotidyl transferase (TdT, prediluted, Ventana). One case that lacked Bcl2 protein expression using clone 124 (targeting amino acids 41-54) was subsequently analyzed using clone C-2 (targeting amino acids 1-205) (Santa Cruz Biotechnology, Santa Cruz, CA). In situ hybridization for EBV-encoded RNA (INFORM EBER Probe, Ventana) was performed as previously described (44).
In cases with material available for flow cytometry at the time of diagnosis, 4-color flow cytometry was performed using previously described methods (44) for the following antibodies: CD45-peridinin chlorophyll protein (PerCP), CD19-PerCP (SJ25C1), CD19-phycoerythrin (PE; 4G7), CD20-PerCP (L27), CD10-fluorescein isothiocyanate (FITC; W8E7), kappa light chain-FITC (TB28-2), lambda light chain-phycoerythrin (PE; 1-155-2), TdT-FITC (HT1, HT4, HT8, and HT9) (BD Biosciences, San Jose, CA), and CD10-PE (SS2/36) (Dako).
Cytogenetic and Molecular Genetic Analyses
GTG-banded metaphases were obtained from unstimulated overnight lymph node or bone marrow cultures according to standard cytogenetic protocols in 17 tumors (11 DHL, 6 BL) at the time of diagnosis. Chromosome analysis was performed at a level of 400 bands or greater and the karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN) 2005 (46).
In DHL and BL control cases lacking a karyotype, FISH analyses to identify MYC, BCL6 and either BCL2 or IGH-BCL2 rearrangements were attempted on interphase nuclei of paraffin-embedded tissue at the time of diagnosis. In case 11, MYC and IGH-BCL2 FISH was performed on a cerebrospinal fluid (CSF) air-dried cytospin and in cases 12 and 17, FISH was performed on abnormal metaphases from tumor cells. All FISH probes were purchased from Abbott Molecular (Des Plaines, IL) and included the Vysis LSI MYC Dual Color Break Apart Rearrangement Probe to identify any rearrangement at 8q24, the Vysis LSI BCL6 Dual Color Break Apart Rearrangement Probe to identify any rearrangement at 3q27, the Vysis LSI BCL2 Dual Color Break Apart Rearrangement Probe to identify any rearrangement at 18q21, the Vysis IGH/BCL2 Dual Color Dual Fusion Translocation Probe to identify t(14;18)(q32;q21), and the Vysis CEP 18 (D18Z1) SpectrumOrange Probe. For formalin-fixed, paraffin-embedded tissue samples, interphase FISH was performed using standard cytogenetic protocols either on whole nuclei extracted from 50μm tissue sections or on 4μm tissue sections cut and fixed onto slides (57). Fifty to 100 nuclei were scored for each sample, and a sample was considered positive for the rearrangement if >15% of nuclei exhibited a break-apart and/or fusion signal.
Statistical Analysis
All statistical analyses were performed using SAS version 9. Fisher’s exact test was used for comparing proportions and Student’s t-test was used for comparing means between 2 groups. Matching between the DHL and DLBCL groups was taken into account by using conditional logistic regression. OS was calculated from date of diagnosis to date of death or last follow-up. Distributions of OS were estimated using the method of Kaplan and Meier and differences in OS were assessed by the stratified log-rank test. Cox regression was used to analyze the effect of continuous variables on OS (5). Significance was tested at the α=0.05 level.
Results
Clinical Features
Clinical characteristics of DHL patients are summarized in Tables 1 and 2. The 20 patients included 11 men and 9 women with a median age of 63.5 years (range: 32-91). None were known to be HIV-positive. Six patients (30%) had a reported history of FL, all histologic grade 1-2 of 3, diagnosed 6 months to 12 years before their DHL diagnosis. Patients were treated with a variety of multiagent chemotherapy regimens that could be broadly divided into 2 categories: moderate intensity regimens (CHOP, R-CHOP and R-ICE) in 13 patients (70%) and high intensity regimens (CODOX-M/R-IVAC and R-EPOCH) in 6 patients (30%) (Table 1). All were treated with curative intent, except for patient 20 who received a single cycle of R-CHOP chemotherapy for palliation. Rituximab was included in 18 cases (90%) and 14 patients (70%) received CNS-directed therapy with intrathecal or high-dose systemic methotrexate. Treatment was not known in case 16. Eight patients (42%) achieved a CR, of whom 3 eventually died: 1 relapsed and died of disease, 1 had persistent neutropenia and died of infection, and 1 died of an unknown cause. After a median follow-up of 7.3 months (range: 1.5-26.6), 6 patients (30%) were alive (4 with no evidence of disease, 1 alive with disease who relapsed after 1 year in CR, and 1 diagnosed recently, undergoing chemotherapy) and 14 patients (70%) had died. Four patients remain alive in CR (median follow-up: 18.2 months, range: 8.9-26.6), all of whom were treated with R-CHOP. However, treatment regimen was not significantly correlated with response or OS (data not shown). The sole clinical variable that was significantly correlated with outcome was ECOG performance status (PS) of ≥2 (p=0.04). In addition, age at diagnosis was marginally significant in the univariate Cox regression analysis (p=0.06). The hazard ratio associated with older age (>60 years) was 2.7 (95% CI: 0.84-8.8).
Table 1.
Clinical Characteristics of Double-Hit Lymphoma Patients
| Case | Age/ Sex |
Stage | LDH (U/L)* |
IPI score |
Disease site(s) at presentation |
Prior history of lymphoma |
Therapy# | BM disease |
CNS disease |
Response | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52/M | 4 | 2142 | 3 | Stomach | Yes | R-ICE, M, Auto-SCT |
+ | + | NR | DOD |
| 2 | 68/M | 3 | 1646 | 3 | Axillary LN | No | CHOP | ND | ND | NR | DOD |
| 3 | 66/F | 4 | 419 | 5 | Cervical LN | No | CODOX-M/ R-IVAC |
− | − | NR | DOD |
| 4 | 75/F | 4 | 6413 | 5 | BM, PB | No | R-CHOP, M | + | + | PR | DOD |
| 5 | 51/M | 4 | 2995 | 3 | Retroperitoneum, BM, PB |
No | R-EPOCH, M | + | − | CR | Relapsed, AWD |
| 6 | 58/M | 4 | 270 | 4 | Retroperitoneum | No | CODOX-M/ R-IVAC |
+ | − | CR | Relapsed, DOD |
| 7 | 76/F | 3 | 390 | 4 | Perirenal mass | No | R-CHOP, M | + | − | NR | DOD |
| 8 | 68/F | 4 | 335 | 5 | Retroperitoneum | Yes | R-CHOP | ND | ND | CR | Died, infection |
| 9 | 64/M | 4 | 380 | 5 | Retroperitoneum, abdomen |
No | R-CHOP | − | − | NR | DOD |
| 10 | 32/M | 4 | 1382 | 4 | Testicle | Yes | R-CHOP, M | + | ND | CR | Died, cause unknown |
| 11 | 63/M | 4 | 218 | 4 | Retroperitoneum, CNS |
Yes | R-EPOCH, M | − | + | NR | DOD |
| 12 | 66/F | 4 | 255 | 3 | Cervical LN, BM | Yes | R-CHOP, M | + | ND | CR | AND |
| 13 | 41/F | 2 | 1564 | 2 | Retroperitoneum | No | CODOX-M/ R-IVAC |
− | − | PR | DOD |
| 14 | 52/M | 3 | 339 | 2 | Abdomen | No | R-CHOP | − | ND | CR | AND |
| 15 | 42/F | 4 | 656 | 4 | Inguinal LN | No | R-CHOP, M | − | ND | CR | AND |
| 16 | 91/F | NK | NK | 2^ | Right neck soft tissue |
No | NK | ND | ND | NR | DOD |
| 17 | 62/M | 4 | 798 | 3 | Retroperitoneum | No | R-CHOP, M | + | + | NR | DOD |
| 18 | 63/F | 4 | NK | 3^ | Left leg soft tissue |
Yes | R-CHOP, M, Auto-SCT |
+ | ND | CR | AND |
| 19 | 75/M | 4 | 3640 | 3 | BM, PB | Yes | R-EPOCH, M | + | − | Therapy ongoing |
AWD |
| 20 | 69/M | 4 | 10,840 | 4 | BM, PB | No | R-CHOP (palliative) | + | ND | NR | DOD |
Serum lactate dehydrogenase (LDH) normal range: 110-210 U/L.
Moderate intensity regimens included R-CHOP (rituximab, cyclophosphamide, adriamycin, vincristine, prednisone), CHOP and R-ICE (rituximab, ifosfamide, carboplatin, etoposide); high intensity regimens included CODOX-M/R-IVAC (cyclophosphamide, vincristine, adriamycin, methotrexate, intrathecal cytarabine, intrathecal methotrexate/rituximab, ifosfamide, etoposide, cytarabine, intrathecal methotrexate) and R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, adriamycin). M indicates CNS-directed therapy with intrathecal or high-dose systemic methotrexate. Auto-SCT indicates autologous stem cell transplantation, which was undertaken in first remission or after the completion of chemotherapy.
Minimum IPI score calculated based on available data.
AND: alive, no evidence of disease, AWD: alive with disease BM: bone marrow, CNS: central nervous system, CR: complete response, DOD: died of disease, F: female, IPI: International Prognostic Index, LDH: serum lactate dehydrogenase, LN: lymph node, M: male, ND: not done, NK: not known, NR: no response, PB: peripheral blood, PR: partial response.
Table 2.
Comparison of Clinical Characteristics of Double-Hit Lymphoma, Burkitt Lymphoma and Diffuse Large B-cell Lymphoma Patients
| DHL (n=20) |
BL (n=25) |
DLBCL (n=40) |
p-value (DHL vs BL) |
p-value (DHL vs DLBCL) |
|
|---|---|---|---|---|---|
| Median age (range) |
63.5 (32-91) |
41 (6-73) |
71 (47-86) |
<0.0001 | 0.03 |
| Males (%) | 11 (55) | 19 (76) | 21 (52.5) | NS | NS |
| Pre-existing FL (%) |
3 confirmed (15%) |
NA | 6 (15%) | NA | NS |
| HIV-positive (%) | 0/6 (0) | 9/18 (50) | 1/15 (7) | 0.05 | NS |
| Median LDH at presentation* (range) |
727 U/L (218 - 10,840) |
365 U/L (112-4862) |
366 U/L (162- 5871) |
0.07 | 0.02 |
| Extranodal disease (%) |
17 (85) | 13 (52) | 37 (90) | 0.03 | NS |
| BM involvement (%) |
10/17 (59) | 7/21 (33) | 6/26 (23) | NS | 0.04 |
| CNS involvement (%) |
5/11 (45) | 7/25 (28) | 6/17 (35) | NS | NS |
| Alive at last follow-up (%) |
6 (30) | 17 (68) | 16 (40) | 0.02 | NS |
| Median OS in months (95% CI) |
4.5 (4.0-7.9) | Not reached |
38.9 (13.2- 83.7) |
0.002 | 0.04 |
Serum lactate dehydrogenase (LDH) normal range: 110-210 U/L.
BL: Burkitt lymphoma, BM: bone marrow, CHL: classical Hodgkin lymphoma, CI: confidence interval, CNS: central nervous system, DHL: double-hit lymphoma, DLBCL: diffuse large B-cell lymphoma, FL: grade 1-2 follicular lymphoma, HIV: human immunodeficiency virus, LDH: lactate dehydrogenase, NA: not applicable, NS: not significant, OS: overall survival.
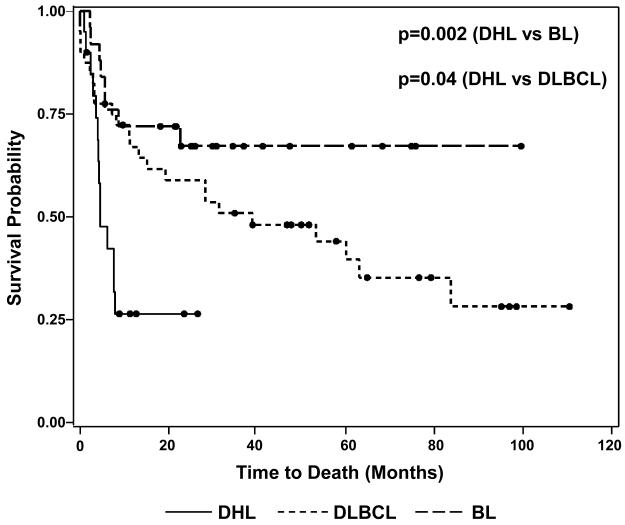
Clinical comparison between the DHL patients and the 2 groups of control patients is summarized in Table 2. Patients in the DHL group had a median serum LDH level at presentation of 727 U/L, nearly twice that of either control group. An LDH cutoff of >630 U/L (>3 times the upper limit of normal in our laboratory) was found to be relatively specific for distinguishing DHL patients from those with BL (88% specificity, p=0.006) and DLBCL (82.5% specificity, p=0.005). DHL patients had a higher prevalence of bone marrow and CNS disease than either BL or DLBCL patients and a higher prevalence of extranodal disease compared with BL patients. OS for DHL patients was markedly worse than for BL (p=0.002) and DLBCL (p=0.04) patients (Fig. 1). The median survival time in the DHL group was only 4.5 months (95% CI: 4.0-7.9), and all observed deaths occurred within 8 months of diagnosis. In contrast, the BL group did not reach its median survival (median follow-up: 31.7 months) and the median survival time of the DLBCL group was 38.9 months (95% CI: 13.2-83.7).
Figure 1.
Kaplan-Meier Overall Survival Distributions for Double-Hit Lymphoma, Burkitt
Lymphoma and IPI-Matched Diffuse Large B-cell Lymphoma Patients
Black circles denote patients who were alive at the time of last follow-up.
DHL: double-hit lymphoma, BL: Burkitt lymphoma, DLBCL: diffuse large B-cell lymphoma.
Morphologic and Immunophenotypic Characteristics and WHO Classification
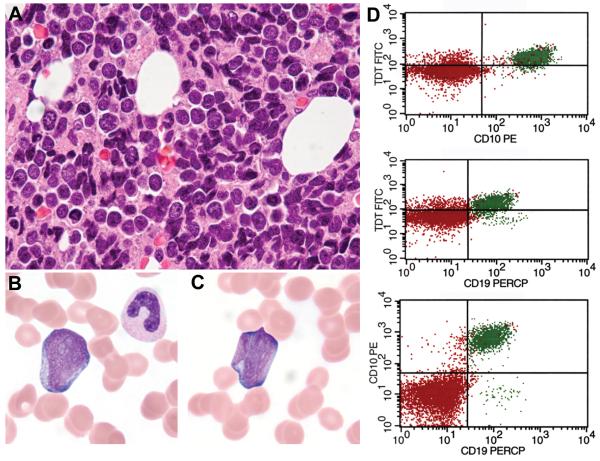
Morphologic and immunophenotypic features are summarized in Tables 3 and 4. Diagnostic tissue samples included lymph nodes (13 cases), extranodal sites (8 cases), bone marrow (5 cases), and peripheral blood (3 cases). Cases were classified according to the 2008 WHO Classification as follows: 12 cases of B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL (BCLU) (60%) and 7 cases of DLBCL, not otherwise specified (DLBCL-NOS) (35%). Case 20 with a leukemic presentation and lymphoblastic morphology and immunophenotype was classified as B-LBL (Fig. 2).
Table 3.
Morphologic, Immunophenotypic and In Situ Hybridization Characteristics of Double-Hit Lymphoma Cases
| Case | WHO Classification |
Morphology | Features of pre-existing lymphoma (if applicable) |
Surface light chain* |
CD20 | Pax5/ CD79a |
CD10 | Bcl6 | Bcl2^ | Mum1 | TdT | Ki-67 (%) |
EBER |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BCLU | Burkitt-like | FL, grade 1/3 (confirmed by morphology and FISH) |
Absent | ND | +/+ | + | − | + | + | − | 100 | − |
| 2 | BCLU | Burkitt-like | − | Lambda | + | +/+ | − | + (wk) | + | + | − | 80 | − |
| 3 | BCLU | Burkitt-like | − | Absent | + | +/+ | + | + | + ^ | − | − | 100 | − |
| 4 | BCLU | Burkitt-like | − | Lambda | + | +/+ | + | + | + | + (wk) | − | 65 | − |
| 5 | BCLU | Burkitt-like | − | Kappa | + | ND/+ | + | + (wk) | + | − | ND | 60 | ND |
| 6 | BCLU | Intermediate | − | Lambda | + | +/+ | + | + | + | − | − | 90 | − |
| 7 | BCLU | Intermediate | − | Lambda | + | +/+ | + | + (wk) | ND | + | − | 100 | − |
| 8 | BCLU | Intermediate | FL, grade 1-2 (confirmed by morphology and FISH) |
Lambda | + | ND/ND | + | + | ND | ND | ND | 25 | − |
| 9 | BCLU | Intermediate | − | Lambda | + | ND/+ | + | + | + | − | − | 95 | − |
| 10 | BCLU | Blastoid | GC-BCL, MYC+, BCL2x8, with high Ki-67 PI and blastoid morphology |
Kappa | + | ND/ND | + | ND | + | − | − | 80 | − |
| 11 | BCLU | Blastoid | GC-BCL with diffuse growth and blastoid morphology (FISH ND) |
Kappa | + | ND/+ | + | + | + | − | ND | 80 | − |
| 12 | BCLU | Blastoid | CHL (by report) | Lambda | + | +/ND | + | + | + | − | − | 70 | − |
| 13 | DLBCL | DLBCL | − | Lambda | + | +/+ | + | + | + | + (wk) | − | 90 | − |
| 14 | DLBCL | DLBCL | − | Lambda | + | +/+ | + | + | + | − | − | 80 | − |
| 15 | DLBCL | DLBCL | − | ND | + | +/ND | − | + | + | + (wk) | − | 80 | − |
| 16 | DLBCL | DLBCL | − | Lambda | + | +/+ | + | + | + | − | ND | 85 | − |
| 17 | DLBCL | DLBCL | − | Absent | + | ND/ND | + | + (wk) | + | + (wk) | − | 90 | − |
| 18 | DLBCL | DLBCL | FL, grade 1-2 (by report) | ND | + | +/ND | ND | + | + | − | − | 95 | − |
| 19 | DLBCL | DLBCL | FL, grade 1-2 (confirmed by morphology and FISH) |
Absent | + | +/ND | + | + (wk) | + | − | − | 80 | − |
| 20 | B-LBL | B-LBL | − | Absent | − | +/+ | + | − | + | + | + | 50 | − |
Surface light chain expression was determined by flow cytometry in each case.
All cases tested were Bcl2+ with clone 124 (targeting amino acids 41-54) except for case 3, which was subsequently tested using clone C-2 (targeting amino acids 1-205) and found to be positive.
BCLU: B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma, B -LBL: B-lymphoblastic lymphoma/leukemia, CHL: classical Hodgkin lymphoma, DLBCL: diffuse large B-cell lymphoma, not otherwise specified, EBER: Epstein-Barr virus encoded RNA, FISH: fluorescence in situ hybridization, FL: follicular lymphoma, GC-BCL: germinal center-phenotype B-cell lymphoma, ND: not done, PI: proliferation index, TdT: terminal deoxynucleotidyl-transferase, WHO: World Health Organization, wk: weak, +: positive, −: negative.
Table 4.
Comparison of Pathologic Characteristics between Double-Hit Lymphoma Cases and Burkitt Lymphoma Controls
| DHL (n=20) |
BL (n=25) |
p-value | |
|---|---|---|---|
| Light chain expression* Absent (%) Kappa (%) Lambda (%) |
5/18 (28) 3/18 (17) 10/18 (56) |
2/13 (15) 6/13 (46) 5/13 (38) |
NS |
| CD10 expression (%) | 17/19 (89) | 21/23 (91) | NS |
| Bcl6 expression (%) | 17/19 (89) | 22/24 (92) | NS |
| CD10 or Bcl6 expression (%) | 20/20 (100) | 24/24 (100) | NS |
| Bcl2 expression (%) | 18/18# (100) | 1/24^ (4) | <0.0001 |
| Mum1 expression (%) | 8/19 (42) | 1/24 (4) | 0.006 |
| Ki-67 proliferation index <95% (%) | 15/20 (75) | 0/24 (0) | <0.0001 |
| EBER expression (%) | 0/19 (0) | 8/24** (33) | 0.006 |
| Complex karyotype (3 or more abnormalities) (%) | 11/11 (100) | 3/6 (50) | 0.03 |
| Median number of cytogenetic abnormalities (range) |
9 (5-20) | 2.5 (2-6) | 0.0009 |
|
MYC partner IGH (%) IGL (%) Unknown (%) |
0/11 (0) 9/11 (82) 2/11 (18) |
5/6 (83) 1/6 (17) 0/6 (0) |
0.001 |
Surface light chain expression was determined by flow cytometry in all DHL and BL cases.
The single DHL case that was negative for Bcl2 expression with clone 124 but positive with clone C-2 (case 3) had a t(14;18) by karyotype.
The single Bcl2+ BL case showed no BCL2 rearrangement by FISH.
Among 8 EBER+ BL cases, 4 arose in HIV+ adults.
BL: Burkitt lymphoma, DHL: double-hit lymphoma, EBER: Epstein-Barr virus encoded RNA, IGH: immunoglobulin heavy chain gene, IGL: immunoglobulin lambda light chain gene.
Figure 2.
B-Lymphoblastic Leukemia with IGH-BCL2 and MYC Rearrangements
The bone marrow core biopsy from case 20 showed medium-sized cells with round nuclei and finely dispersed chromatin in a background of extensive cellular necrosis (A). The peripheral blood contained a significant population of blasts with round to irregular nuclei, prominent nucleoli, dispersed chromatin and deeply basophilic cytoplasm (B-C). Immunophenotyping of the circulating leukemic cells by flow cytometry showed a population of CD19+, CD10+, terminal deoxynucleotidyl transferase (TdT)+ B lymphoblasts (D) that were CD45dim+ and negative for CD20 and surface light chain (not shown).
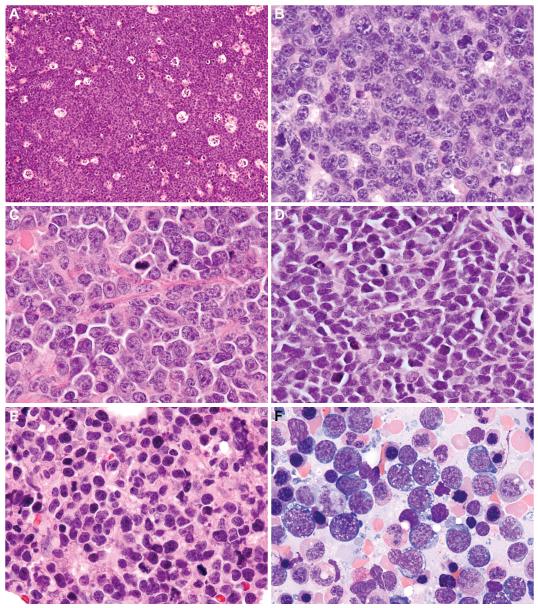
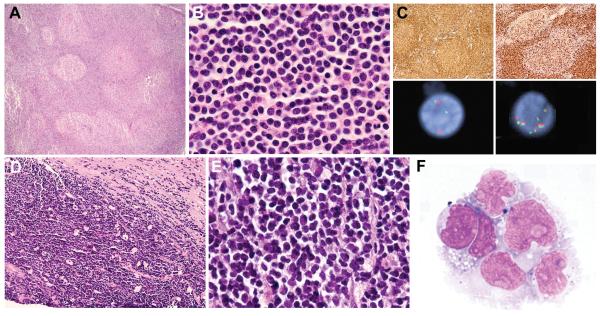
All 12 cases classified as BCLU exhibited a diffuse growth pattern with abundant mitoses and frequent apoptosis (Table 3). Five of 12 (41.7%) contained a conspicuous starry-sky pattern and monomorphous cell population closely resembling BL (“Burkitt-like” morphology, Fig. 3A-B). In 4 of 12 cases (33.3%), there was some morphologic overlap with BL but cell size was variable and a proportion of the cells contained more irregular nuclei and more prominent nucleoli than classic BL; these cases had morphological features intermediate between BL and DLBCL, and therefore were classified as BCLU rather than DLBCL-NOS (“intermediate” morphology, Fig. 3C) (21, 28). Three of 12 BCLU cases (25%) contained small cells with finely dispersed chromatin resembling lymphoblasts (“blastoid” morphology, Fig. 3D); all 3 expressed strong CD20 and surface light chain and 2 cases tested for TdT were negative. Despite the high-grade morphology seen in cases classified as BCLU, the Ki-67 proliferation index (PI) ranged from 25-100% (median: 80%) (Fig. 4A).
Figure 3.
Morphologic Spectrum of Double-Hit Lymphoma
Five double-hit lymphoma cases classified as B-cell lymphoma, unclassifiable (BCLU), with features intermediate between diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL) showed Burkitt-like morphological features, with a prominent starry-sky growth pattern at low magnification (A: cervical lymph node, case 3) and a population of monomorphous medium-sized cells with round to slightly irregular nuclei, finely clumped chromatin and multiple small nucleoli at high magnification (B: axillary lymph node, case 2). Four BCLU cases were morphologically intermediate between BL and DLBCL with greater cytomorphologic variation, including medium-sized to slightly larger cells containing conspicuous nuclear irregularity and single prominent central nucleoli (C: abdominal wall subcutaneous soft tissue, case 9). Three BCLU cases exhibited blastoid cytologic features, with small cells containing finely dispersed chromatin and inconspicuous nucleoli (D: testicle, case 10). Cases classified as diffuse large B-cell lymphoma, not otherwise specified (DLBCL-NOS) contained predominantly large atypical cells with oval to irregular nuclei, including some with prominent central nucleoli (E: bone marrow biopsy, case 19). The corresponding aspirate smear contained a population of large cells with irregular nuclei, prominent nucleoli, and deeply basophilic cytoplasm with conspicuous vacuolation (F).
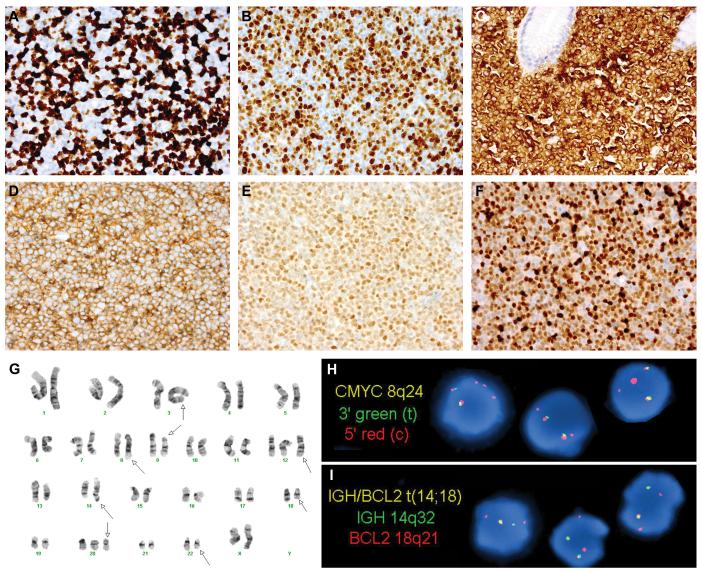
Figure 4.
Immunophenotypic, Cytogenetic and Molecular Genetic Features of Double-Hit Lymphoma
Two examples of double-hit lymphoma (DHL) demonstrating a Ki-67 proliferation index (PI) of <95%: a retroperitoneal lymph node biopsy from case 5, classified as B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma (BCLU), with an overall Ki-67 PI of 60% (A); and an abdominal lymph node biopsy from case 14, classified as diffuse large B-cell lymphoma, not otherwise specified (DBLCL-NOS), with an overall Ki-67 PI of 80% (B). The vast majority of DHL cases expressed Bcl2 by immunohistochemistry for the commonly used antibody, clone 124 (C: stomach polyp, case 1). All DHL cases were of germinal center origin, expressing CD10, Bcl6, or both, as in this cervical lymph node biopsy from case 12 (D: CD10, E: Bcl6). Mum1 was expressed in 8/19 DHL cases, including this axillary lymph node biopsy from case 2 (F). Bone marrow cytogenetic analysis from case 4 showed a complex karyotype (G, arrows), as did all DHL cases. In addition to the t(8;22) and t(14;18), this case contained additional unknown material in the 3q2?7 region (G, arrow indicating chromosome 3). Subsequent fluorescence in situ hybridization (FISH) analysis confirmed a rearrangement involving BCL6 at 3q27 (not shown). Interphase FISH analysis of paraffin-embedded stomach polyp tissue from case 1 confirmed both a MYC rearrangement (H: dual-color, split-apart probe) and an IGH-BCL2 fusion (I: dual-color, dual fusion probe). The FISH patterns with both probes were consistent with unbalanced rearrangements (H-I). This patient’s prior low-grade follicular lymphoma lacked both MYC and BCL2 rearrangements (not shown), suggesting that both translocations were acquired at the time of DHL transformation.
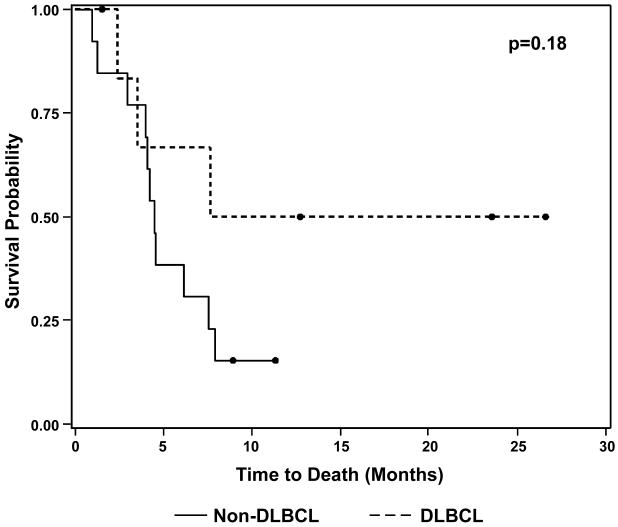
All 7 cases classified as DLBCL-NOS exhibited morphological features outside of the spectrum of BL with predominantly large cells with oval or irregular nuclei; most had a conspicuous population of immunoblasts with prominent central nucleoli, while some contained mainly centroblasts with vesicular chromatin or cells with finely dispersed chromatin (Table 3, Fig. 3E-F). Some cases had frequent mitoses and single-cell necrosis, but all lacked a starry-sky pattern or areas of geographic necrosis. All had a Ki-67 PI of ≥80% (median: 85%, range: 80-95%) (Fig. 4B). Patients with tumors classified as DLBCL-NOS showed a trend toward improved OS compared to other DHL patients (p=0.18) (Fig. 5).
Figure 5.
Differences in Kaplan-Meier Overall Survival Distributions for Double-Hit Lymphoma Patients by 2008 WHO Classification
Black circles denote patients who were alive at the time of last follow-up.
DLBCL: diffuse large B-cell lymphoma, not otherwise specified.
Among the 6 patients with a prior history of grade 1-2 FL, tissue from the initial FL was available for review in 5 (Table 3). The prior diagnosis of grade 1-2 FL was confirmed by morphologic examination and immunohistochemistry in 3 cases (case 1 with Burkitt-like BCLU, case 8 with BCLU with intermediate morphology, and case 19 with DLBCL-NOS). None of them showed evidence of a MYC rearrangement and a BCL2 rearrangement was present in 2 of 3 by FISH analysis of the initial lymphoma samples. In 2 cases (cases 10 and 11 with blastoid BCLU), review of the prior biopsies revealed morphological findings consistent with the blastoid variant of FL (38, 41, 55). In case 10 (Fig. 6A-C), a diagnosis of grade 1-2 FL had been made on a supraclavicular lymph node; 2 years later, DHL, with a MYC rearrangement and 8 copies of BCL2, developed in the testis. Subsequent FISH analysis of the supraclavicular lymph node revealed the same genetic abnormalities. In case 11 (Fig. 6D-F), the diagnosis of grade 1-2 FL had been made on 2 small retroperitoneal core needle biopsies; 6 months after the diagnosis, DHL developed in the central nervous system (CNS). Tissue from the prior biopsies was not available for FISH analysis to determine initial MYC status.
Figure 6.
Pre-Existing Low-Grade Follicular Lymphomas with Unusual Features
Examination of prior low-grade follicular lymphomas in 2 patients revealed findings unusual for this diagnosis. In case 10 with testicular double-hit lymphoma (DHL; illustrated in Fig. 3D), review of a supraclavicular lymph node biopsy from 2 years earlier showed architectural effacement by a follicular proliferation of B cells (A). Cells within and outside of follicles were small with blastoid features, including round nuclei, evenly dispersed chromatin and absent nucleoli (B); typical centrocytes were not identified. Both follicular and extrafollicular B cells were strongly CD20+, CD10+ and Bcl2+ (C, upper left). The follicular component weakly co-expressed Bcl6, but showed absent follicular dendritic cell staining (not shown). Ki-67 stain showed an unusually high proliferation index (PI) of approximately 40% within follicles and 90% outside of follicles (C, upper right). Fluorescence in situ hybridization (FISH) analysis with dual color break apart probes revealed a MYC rearrangement (C, lower left) and 8 copies of BCL2 (C, lower right), the same genetic abnormalities seen in the subsequent DHL. In case 11, core needle biopsies of a retroperitoneal mass showed a CD10+, Bcl6+, Bcl2+ B-cell neoplasm with a diffuse growth pattern (absent staining for follicular dendritic cell antigens), a focal starry-sky pattern (D) and patchy necrosis. The cells were small to medium-sized; some were centrocyte-like, but the majority had oval nuclei with finely dispersed chromatin and absent nucleoli (E), prompting a diagnosis of diffuse grade 1-2 FL with blastoid features. Tissue from the initial biopsies was not available for cytogenetics or FISH analysis. Six months later, the patient relapsed with a CD10+ B-cell lymphoma involving the central nervous system (F), confirmed by FISH to have an IGH-BCL2 fusion as well as a MYC rearrangement (FISH analysis not shown).
All 18 DHL cases tested for Bcl2 by immunohistochemistry were positive (Fig. 4C). Case 3 was Bcl2-negative with the commonly used antibody (clone 124), and positive with a different Bcl2 antibody (clone C-4) recognizing a longer amino acid segment of the protein (34). The majority of cases (18/20, 90%) had a germinal center B-cell like (GCB) immunophenotype (either CD10+, or Bcl6+, Mum1− if results of CD10 staining were not available), while only 2 (cases 2 and 15) had a non-GCB immunophenotype (CD10−, Bcl6+, Mum1+) (Fig. 4D-F) (14). Significant differences distinguishing DHL cases from BL controls included Bcl2 (p<0.0001) and Mum1/IRF4 (p=0.006) protein expression, Ki-67 PI <95% (p<0.0001), and EBER-negativity (p=0.006) (Table 4, Fig. 4A-F). Within the DHL group, Mum1/IRF4 expression or Ki-67 PI had no significant correlation with response to therapy, outcome or OS (data not shown).
Cytogenetic and Molecular Genetic Characteristics
Cytogenetic and molecular genetic features of DHL are summarized in Tables 4 and 5. Cytogenetic analysis, available in 11 cases, showed a complex karyotype in all (≥3 numerical or structural aberrations), with a higher median number of aberrations than BL controls (p= 0.0009) (Fig. 4G). Among the DHL cases, the MYC partner was frequently IGL (82%) or unknown (18%), while in BL cases the MYC partner was usually IGH (83%) or rarely IGL (17%), a difference that was statistically significant (p=0.001). There was no significant correlation between number of karyotypic abnormalities and response to therapy, outcome or OS within the DHL group (data not shown). Besides the t(14;18) and t(8q24), there was no cytogenetic abnormality common to all DHL cases. However, certain numerical and structural aberrations were seen frequently, including trisomy 12 (6 cases) and trisomy 7 (5 cases). Two cases had i(17)(q10), resulting in loss of heterozygosity for TP53 at 17p13.
Table 5.
Cytogenetic and Molecular Genetic Characteristics of Double-Hit Lymphomas
| Case | Karyotype | Fluorescence in situ hybridization |
||
|---|---|---|---|---|
| IGH-BCL2 | MYC | BCL6 | ||
| 1 | ND | + | + | − |
| 2 | 51,XY,t(2;9)(q21;p24),+3,add(5)(p15.3),add(7)(q32),add(8)(q24),+11,+12, t(14;18)(q32;q21),+15,+18,add(19)(q13.1),−22,+2mar[2] |
ND | + | Trisomy |
| 3 | 46,XX,add(3)(q2?5),add(6)(q1?2),del(6)(q1?5q2?3),t(8;22)(q24;q11), t(14;18)(q32;q21)[7] |
ND | + | − |
| 4 | 48,XX,add(3)(q2?7),t(8;22)(q24;q11),del(9)(p11),+12,add(12)(q22), t(14;18)(q32;q21),+20[10]/47,XX,add(3),t(8;22),del(9),+12, der(12)t(1;12)(q12;q24),t(14;18)[3]/47,XX,add(3),t(8;22),del(9),+12, add(12),der(12),t(14;18)[2] |
ND | ND | + |
| 5 | ND | + | + | ND |
| 6 | ND | + | + | − |
| 7 | 48,XX,add(7)(q32),add(8)(q24),i(11)(q10),dup(12)(q13q24), t(14;18)(q32;q21),+21,+1−2mar[cp3] |
+ | + | − |
| 8 | 45,X,−X,add(1)(p36),del(6)(q15),del(7)(q22),t(8;22)(q24;q11.2), t(9;15)(p13;q24),t(14;18)(q32;q21)[cp14] |
ND | ND | ND |
| 9 | 47,XY,add(2)(p13),+7,t(8;22)(q24;q11),der(13)t(3;13)(q2?6;q21), t(14;18)(q32;q21),add(17)(p13)[10] |
+ | + | Trisomy |
| 10 | ND | BCL2x8 | + | − |
| 11 | ND | + | + | ND |
| 12 | 96<4n>XXXX,+X,add(1)(p36),−3,−4,i(6)(p10),t(8;22)(q24;q11)x2, der(8)t(8;22)(q24;q11),+10,−11,del(11)(q2?2)x2,+12, der(12)ins(12;?)(q1?5;?)x3,ins(14;18)(q32;q21q21)x2,i(17)(q10),+20,−21, −21,+r,+mar1x2[10] |
+ | + | Trisomy |
| 13 | ND | + | + | − |
| 14 | ND | + | + | − |
| 15 | 52,add(X)(p22.1),del(X)(q22),+X,add(1)(p3?4),add(1)(p3?6),add(4)(q21),+5, del(6)(q13q23),+7,der(8)t(1;8)(p3?4;p2?3),t(8;22)(q24;q11),add(11)(q2?3), +12,der(14)t(14;18)(q32;q21),add(15)(q22),add(18)(q21),add(19)(q13),+21, [cp6]/53,idem,+add(4)(q21)[cp7] |
+ | + | ND |
| 16 | ND | + | + | − |
| 17 | 50,X,−Y,+X,del(2)(p21),add(5)(q35),+6,+7,der(8)t(8;22)(q24;q11), del(9)(p22),del(10)(q22q24),+11,−13,t(14;18)(q32;q21),i(17)(q10), der(22)t(8;22)(q24;q11)t(1;22)(q11;p11)x2,+mar[19] |
ND | + | ND |
| 18 | ND | + | + | − |
| 19 | 52,XY,+X,+7,t(8;22)(q24;q11),+11,+12,+13,t(14;18)(q32;q21), +der(18)t(14;18)(q32;q21)[9]/53,idem,+5[8] |
ND | ND | ND |
| 20 | 50,XY,der(2)t(1;2)(q21;q3?7),+7,t(8;22)(q24;q11),+9,der(9;17)(q10;q10), +12,t(14;18)(q32;q21),+18[12] |
ND | ND | ND |
ND: not done, +: rearranged, −: no abnormalities detected by FISH.
Among the 9 cases lacking karyotypes, 8 had MYC and IGH-BCL2 rearrangements detected by interphase FISH (Fig. 4H-I). In case 10, FISH identified a MYC rearrangement and 8 copies of an intact BCL2; further analysis with a chromosome 18 centromeric probe revealed multiple signals, confirming chromosome 18 polysomy. BCL6 FISH analysis identified a rearrangement in a single case (case 4), confirming a 3q27 rearrangement suspected by karyotype (i.e. “triple-hit”) (Fig. 4G).
Discussion
The purpose of this retrospective analysis was to define further the clinicopathologic spectrum of DHL in a well-characterized group of patients, to classify them according to the 2008 WHO Classification, and to compare the clinical and pathologic characteristics of DHL with 2 other high-grade B-cell lymphomas with which DHL may show morphologic and immunophenotypic overlap, DLBCL and BL. Our findings confirm that DHL has an aggressive clinical presentation and a poor prognosis with currently available therapies (6, 18, 19, 26, 30, 31, 36, 42, 47, 49). Our findings also suggest that DHL has clinical features distinct from both BL and DLBCL. DHL patients had a higher incidence of marrow and CNS involvement than either BL or DLBCL, and DHL patients had higher LDH levels at presentation compared with BL and IPI-matched DLBCL controls, with a median 3-4 times the upper limit of normal (Table 2). Importantly, DHL patients had a median OS of only 4.5 months, vastly inferior to that of either BL or DLBCL, and 70% of patients died within 8 months of diagnosis (Fig. 1). No clinical parameter emerged as predictive of superior OS within the DHL group, although PS <2 and age ≤60 years may suggest a slightly better prognosis. Our DHL patients were treated with either moderate intensity regimens used in DLBCL or high intensity regimens used in BL (Table 1). Neither regimen was associated with a superior outcome or OS, suggesting that highly intensive chemotherapy successfully used in BL may not offer clear benefit in DHL. Despite the retrospective nature of our data, our findings are in line with those of other studies and suggest the need for innovative therapeutic approaches to treat this disease (30, 37). Due to the high risk of CNS involvement, inclusion of CNS-directed therapy should be considered. Treatment of the majority of patients in our series with rituximab may help explain the somewhat lower mortality rate seen in our study as compared to others with similar follow-up in which fewer patients received rituximab (18, 26, 31, 42).
Because of its morphologic and immunophenotypic spectrum, recognition and classification of DHL has been difficult (30). All of the BCLU cases in our series bore some morphologic resemblance to BL, but had an immunophenotype unusual for BL such as positivity for Bcl2 or Mum1/IRF4 or a relatively low PI. All DHL cases diagnosed as DLBCL-NOS had a Ki-67 PI ≥80% and were morphologically distinct from BL, with conspicuous immunoblasts in most cases. In our study, classification as DLBCL-NOS was associated with a marginally superior OS compared with non-DLBCL morphology (Fig. 5), in contrast to the findings of Johnson et al (18) who also applied the 2008 WHO Classification to their cases and found that DLBCL morphology conferred a statistically significant OS benefit. In addition, our data showing that DHL overall has a worse prognosis that either BL or IPI-matched DLBCL underscores the importance of recognizing the presence of both MYC and IGH-BCL2 rearrangements for appropriate prognostic stratification. In particular, the fact that most DHL patients had a GCB phenotype but did so poorly suggests that pathologic prognosticators commonly used in DLBCL do not apply in DHL (14, 30).
In our series as with other published series of DHL, most patients presented with de novo disease, while a subset had a prior history of grade 1-2 FL from which the DHL was presumed to have transformed (18, 26, 30, 31, 49). Interestingly, review of the prior tumors in 2 cases revealed morphologic findings unusual for low-grade FL and associated with the blastoid variant of FL (38, 41, 55). Detection of a MYC rearrangement in the initial tumor from case 10 and the short time course from diagnosis of grade 1-2 FL to the development of DHL in case 11 suggest that both original tumors may have contained a DHL component. This is further supported by chromosomal studies of FL showing that secondary 8q24 abnormalities were found exclusively in patients with the blastoid variant of FL (38).
In our series, MYC was most frequently translocated with IGL. This is in contrast to some reports of DHL that show MYC to be more frequently translocated with IGH or a non-IG partner (18, 42) and to the majority of BL in which IGH is the most common MYC translocation partner (23). The pathogenetic significance of t(8;22) in DHL is not entirely known, but its presence implies that t(14;18) occurs primarily followed by t(8;22), since neither the expressed nor rearranged IGH is available to participate in a balanced translocation with MYC. The t(8;22) may play a diagnostic role by raising the possibility of DHL if early karyotypic analysis reveals a t(8;22) rather than a t(8;14). Careful review of the karyotype for the presence of t(14;18) or metaphase FISH to identify a cryptic IGH-BCL2 rearrangement may be warranted.
Despite the importance of conventional cytogenetic analysis in confirming a diagnosis of DHL, nearly half of the cases in our series lacked a karyotype and we relied on FISH to make the diagnosis. In many pathology laboratories, including our own, not all lymphoma cases are routinely sent for cytogenetic analysis, either due to insufficient tissue or lack of recognition of the importance of such analysis to a particular case at the time of surgery. In addition, some laboratories may not have access to routine cytogenetic analysis. In contrast, FISH is readily available at most large and reference laboratories and can confirm the diagnosis of DHL without the need for cell culture. Since DHL is relatively rare, with estimates ranging from 3-5% of high-grade B-cell lymphoma (18, 42), sending all cases of high-grade B-cell lymphoma for FISH analysis may be unnecessary. We suggest that when a karyotype is not available, FISH for both MYC and IGH-BCL2 should be limited to the following circumstances, in order to increase cost-effectiveness and minimize unnecessary testing:
high-grade B-cell lymphoma patients who present with advanced stage disease with extranodal or CNS involvement or with an LDH exceeding 3 times the upper limit of normal
all adult patients whose tumors have some morphologic resemblance to BL, particularly patients in whom adult BL is rare, such as HIV-negative or non-immunosuppressed patients
tumors resembling BL morphologically with an atypical immunophenotype for BL, including positivity for Bcl2, Mum1/IRF4, and/or a Ki-67 PI <95%
adult patients diagnosed with DLBCL-NOS with a Ki-67 ≥80%
patients with a history of low-grade FL who relapse with a high-grade B-cell neoplasm
tumors resembling low-grade FL with unusual features, such as diffuse growth, absence of centrocyte-like cells, blastoid cytologic features, very high PI, a starry-sky pattern or focal necrosis
Finally, our findings suggest that DHL warrants separate categorization in future classifications due to the clinical and molecular genetic features that distinguish it from other high-grade B-cell neoplasms, perhaps reflecting a different underlying pathogenesis (17, 30, 45). The basis for the extremely aggressive clinical behavior of DHL is likely related to MYC-induced growth promotion combined with the anti-apoptotic effect conferred by BCL2 overexpression (30). As in our series, the vast majority of reported cases exhibit a complex karyotype, pointing to a role for clonal evolution and further gene dysregulation. Most DHL cases studied using gene expression profiling have shown a molecular signature intermediate between DLBCL and BL (17), while fewer cases more closely resemble BL (7). Comparative genomic hybridization studies demonstrated that even such cases of gene expression-defined BL that do not fulfill WHO criteria for BL (so-called “discrepant BL” cases) show significant alterations from pediatric and adult BL and DLBCL (45). It is possible that DHL represents a neoplasm in which a primary genetic aberration and induction of a specific gene-expression profile was subsequently shifted in a different direction due to the acquisition of additional genetic changes (17). This may explain the morphologic and immunophenotypic heterogeneity, but shared molecular genetic and clinical features, seen in these cases.
In summary, we describe 20 patients with B-cell neoplasms with concurrent IGH-BCL2 and MYC rearrangements. Our findings confirm these neoplasms to be clinically aggressive with a poor prognosis. High intensity regimens used to treat BL have yet to demonstrate a clear benefit in DHL patients. DHL may show morphologic and immunophenotypic overlap with BL or DLBCL, or less frequently with LBL or FL with blastoid morphology. Awareness of this pathologic spectrum is important in directing ancillary testing to detect IGH-BCL2 and MYC rearrangements, particularly in laboratories where conventional cytogenetics is not routinely performed or available. Even with the use of the current WHO Classification containing intermediate categories, classification of DHL remains problematic. Despite its morphologic and immunophenotypic heterogeneity, the distinct clinical and molecular genetic features of DHL may warrant categorization as a separate entity in future classifications, as well as the development of novel treatment strategies.
Acknowledgments
The authors are grateful to Dr. A. John Iafrate of the MGH Diagnostic Molecular Pathology Laboratory for his assistance with interpretation of FISH results.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 2.Boerma EG, Siebert R, Kluin PM, et al. Translocations involving 8q24 in Burkitt lymphoma and other malignant lymphomas: a historical review of cytogenetics in the light of today’s knowledge. Leukemia. 2009;23:225–234. doi: 10.1038/leu.2008.281. [DOI] [PubMed] [Google Scholar]
- 3.Brito-Babapulle V, Crawford A, Khokhar T, et al. Translocations t(14;18) and t(8;14) with rearranged bcl-2 and c-myc in a case presenting as B-ALL (L3) Leukemia. 1991;5:83–87. [PubMed] [Google Scholar]
- 4.Cogliatti SB, Novak U, Henz S, et al. Diagnosis of Burkitt lymphoma in due time: a practical approach. Br J Haematol. 2006;134:294–301. doi: 10.1111/j.1365-2141.2006.06194.x. [DOI] [PubMed] [Google Scholar]
- 5.Cox DR. Partial likelihood. Biometrika. 1975;62:277–288. [Google Scholar]
- 6.D’Achille P, Seymour JF, Campbell LJ. Translocation (14;18)(q32;q21) in acute lymphoblastic leukemia: a study of 12 cases and review of the literature. Cancer Genet Cytogenet. 2006;171:52–56. doi: 10.1016/j.cancergencyto.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 8.De Jong D, Voetdijk BM, Beverstock GC, et al. Activation of the c-myc oncogene in a precursor-B-cell blast crisis of follicular lymphoma, presenting as composite lymphoma. N Engl J Med. 1988;318:1373–1378. doi: 10.1056/NEJM198805263182106. [DOI] [PubMed] [Google Scholar]
- 9.Dunphy CH, van Deventer HW, Carder KJ, et al. Mature B-cell acute lymphoblastic leukemia with associated translocations (14;18)(q32;q21) and (8;9)(q24;p13). A Burkitt variant? Arch Pathol Lab Med. 2003;127:610–613. doi: 10.5858/2003-127-0610-MBALLW. [DOI] [PubMed] [Google Scholar]
- 10.Fiedler W, Weh HJ, Zeller W, et al. Translocation (14;18) and (8;22) in three patients with acute leukemia/lymphoma following centrocytic/centroblastic non-Hodgkin’s lymphoma. Ann Hematol. 1991;63:282–287. doi: 10.1007/BF01698379. [DOI] [PubMed] [Google Scholar]
- 11.Galteland E, Sivertsen EA, Svendsrud DH, et al. Translocation t(14;18) and gain of chromosome 18/BCL2: effects on BCL2 expression and apoptosis in B-cell non-Hodgkin’s lymphomas. Leukemia. 2005;19:2313–2323. doi: 10.1038/sj.leu.2403954. [DOI] [PubMed] [Google Scholar]
- 12.Gauwerky CE, Hoxie J, Nowell PC, et al. Pre-B-cell leukemia with a t(8; 14) and a t(14; 18) translocation is preceded by follicular lymphoma. Oncogene. 1988;2:431–435. [PubMed] [Google Scholar]
- 13.Gluck WL, Bigner SH, Borowitz MJ, et al. Acute lymphoblastic leukemia of Burkitt’s type (L3 ALL) with 8;22 and 14;18 translocations and absent surface immunoglobulins. Am J Clin Pathol. 1986;85:636–640. doi: 10.1093/ajcp/85.5.636. [DOI] [PubMed] [Google Scholar]
- 14.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 15.Haralambieva E, Boerma EJ, van Imhoff GW, et al. Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol. 2005;29:1086–1094. [PubMed] [Google Scholar]
- 16.Horsman DE, Gascoyne RD, Coupland RW, et al. Comparison of cytogenetic analysis, southern analysis, and polymerase chain reaction for the detection of t(14;18) in follicular lymphoma. Am J Clin Pathol. 1995;103:472–478. doi: 10.1093/ajcp/103.4.472. [DOI] [PubMed] [Google Scholar]
- 17.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 18.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanungo A, Medeiros LJ, Abruzzo LV, et al. Lymphoid neoplasms associated with concurrent t(14;18) and 8q24/c-MYC translocation generally have a poor prognosis. Mod Pathol. 2006;19:25–33. doi: 10.1038/modpathol.3800500. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami K, Miyanishi S, Sonoki T, et al. Case of B-cell lymphoma with rearrangement of the BCL1, BCL2, BCL6, and c-MYC genes. Int J Hematol. 2004;79:474–479. doi: 10.1532/ijh97.03105. [DOI] [PubMed] [Google Scholar]
- 21.Kluin PM, Harris NL, Stein H, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 265–266. [Google Scholar]
- 22.Koduru PR, Offit K. Molecular structure of double reciprocal translocations: significance in B-cell lymphomagenesis. Oncogene. 1991;6:145–148. [PubMed] [Google Scholar]
- 23.Kornblau SM, Goodacre A, Cabanillas F. Chromosomal abnormalities in adult non-endemic Burkitt’s lymphoma and leukemia: 22 new reports and a review of 148 cases from the literature. Hematol Oncol. 1991;9:63–78. doi: 10.1002/hon.2900090202. [DOI] [PubMed] [Google Scholar]
- 24.Kramer MH, Hermans J, Wijburg E, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998;92:3152–3162. [PubMed] [Google Scholar]
- 25.Kramer MH, Raghoebier S, Beverstock GC, et al. De novo acute B-cell leukemia with translocation t(14;18): an entity with a poor prognosis. Leukemia. 1991;5:473–478. [PubMed] [Google Scholar]
- 26.Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92:1335–1342. doi: 10.3324/haematol.11305. [DOI] [PubMed] [Google Scholar]
- 27.Lee JT, Innes DJ, Jr., Williams ME. Sequential bcl-2 and c-myc oncogene rearrangements associated with the clinical transformation of non-Hodgkin’s lymphoma. J Clin Invest. 1989;84:1454–1459. doi: 10.1172/JCI114320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leoncini L, Raphael M, Stein H, et al. Burkitt lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 262–264. [Google Scholar]
- 29.Lillington DM, Monard S, Johnson PW, et al. The t(14;18) in a patient with de novo acute lymphoblastic leukemia is associated with t(8;9) Leukemia. 1994;8:560–563. [PubMed] [Google Scholar]
- 30.Lin P, Medeiros LJ. High-grade B-cell lymphoma/leukemia associated with t(14;18) and 8q24/MYC rearrangement: a neoplasm of germinal center immunophenotype with poor prognosis. Haematologica. 2007;92:1297–1301. doi: 10.3324/haematol.11263. [DOI] [PubMed] [Google Scholar]
- 31.Macpherson N, Lesack D, Klasa R, et al. Small noncleaved, non-Burkitt’s (Burkitt-like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol. 1999;17:1558–1567. doi: 10.1200/JCO.1999.17.5.1558. [DOI] [PubMed] [Google Scholar]
- 32.Marosi C, Bettelheim P, Chott A, et al. Simultaneous occurrence of t(14;18) and t(8;22) common acute lymphoblastic leukemia. Ann Hematol. 1992;64:101–104. doi: 10.1007/BF01715354. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Subero JI, Odero MD, Hernandez R, et al. Amplification of IGH/MYC fusion in clinically aggressive IGH/BCL2-positive germinal center B-cell lymphomas. Genes Chromosomes Cancer. 2005;43:414–423. doi: 10.1002/gcc.20187. [DOI] [PubMed] [Google Scholar]
- 34.Masir N, Campbell LJ, Goff LK, et al. BCL2 protein expression in follicular lymphomas with t(14;18) chromosomal translocations. Br J Haematol. 2009;144:716–725. doi: 10.1111/j.1365-2141.2008.07528.x. [DOI] [PubMed] [Google Scholar]
- 35.Masir N, Ventura R, Jones M, et al. Follicular lymphoma with trisomy 18 exhibiting loss of BCL-2 expression on transformation to a large cell lymphoma. J Clin Pathol. 2007;60:1061–1064. doi: 10.1136/jcp.2006.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClure RF, Remstein ED, Macon WR, et al. Adult B-cell lymphomas with Burkitt-like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol. 2005;29:1652–1660. doi: 10.1097/01.pas.0000180442.87022.08. [DOI] [PubMed] [Google Scholar]
- 37.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial) Blood. 2008;112:2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed AN, Palutke M, Eisenberg L, et al. Chromosomal analyses of 52 cases of follicular lymphoma with t(14;18), including blastic/blastoid variant. Cancer Genet Cytogenet. 2001;126:45–51. doi: 10.1016/s0165-4608(00)00383-6. [DOI] [PubMed] [Google Scholar]
- 39.Mufti GJ, Hamblin TJ, Oscier DG, et al. Common ALL with pre-B-cell features showing (8;14) and (14;18) chromosome translocations. Blood. 1983;62:1142–1146. [PubMed] [Google Scholar]
- 40.Mukhopadhyay S, Readling J, Cotter PD, et al. Transformation of follicular lymphoma to Burkitt-like lymphoma within a single lymph node. Hum Pathol. 2005;36:571–575. doi: 10.1016/j.humpath.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Natkunam Y, Warnke RA, Zehnder JL, et al. Blastic/blastoid transformation of follicular lymphoma: immunohistologic and molecular analyses of five cases. Am J Surg Pathol. 2000;24:525–534. doi: 10.1097/00000478-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Niitsu N, Okamoto M, Miura I, et al. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23:777–783. doi: 10.1038/leu.2008.344. [DOI] [PubMed] [Google Scholar]
- 43.Pegoraro L, Palumbo A, Erikson J, et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A. 1984;81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahemtullah A, Longtine JA, Harris NL, et al. CD20+ T-cell lymphoma: clinicopathologic analysis of 9 cases and a review of the literature. Am J Surg Pathol. 2008;32:1593–1607. doi: 10.1097/PAS.0b013e31817d7452. [DOI] [PubMed] [Google Scholar]
- 45.Salaverria I, Zettl A, Bea S, et al. Chromosomal alterations detected by comparative genomic hybridization in subgroups of gene expression-defined Burkitt’s lymphoma. Haematologica. 2008;93:1327–1334. doi: 10.3324/haematol.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer LG, Tommerup N, editors. An International System fot Human Cytogenetic Nomenclature. Karger; Basel, Switzerland: 2005. [Google Scholar]
- 47.Stamatoullas A, Buchonnet G, Lepretre S, et al. De novo acute B cell leukemia/lymphoma with t(14;18) Leukemia. 2000;14:1960–1966. doi: 10.1038/sj.leu.2401910. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. [Google Scholar]
- 49.Thangavelu M, Olopade O, Beckman E, et al. Clinical, morphologic, and cytogenetic characteristics of patients with lymphoid malignancies characterized by both t(14;18)(q32;q21) and t(8;14)(q24;q32) or t(8;22)(q24;q11) Genes Chromosomes Cancer. 1990;2:147–158. doi: 10.1002/gcc.2870020211. [DOI] [PubMed] [Google Scholar]
- 50.Tomita N, Nakamura N, Kanamori H, et al. Atypical Burkitt lymphoma arising from follicular lymphoma: demonstration by polymerase chain reaction following laser capture microdissection and by fluorescence in situ hybridization on paraffin-embedded tissue sections. Am J Surg Pathol. 2005;29:121–124. doi: 10.1097/01.pas.0000146027.76706.50. [DOI] [PubMed] [Google Scholar]
- 51.Tomita N, Tokunaka M, Nakamura N, et al. Clinicopathological features of lymphoma/leukemia patients carrying both BCL2 and MYC translocations. Haematologica. 2009 doi: 10.3324/haematol.2008.005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Ooteghem RB, Smit EM, Beishuizen A, et al. A new B-cell line showing a complex translocation (8;14;18) and BCL2 rearrangement. Cancer Genet Cytogenet. 1994;74:87–94. doi: 10.1016/0165-4608(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 53.Vitolo U, Gaidano G, Botto B, et al. Rearrangements of bcl-6, bcl-2, c-myc and 6q deletion in B-diffuse large-cell lymphoma: clinical relevance in 71 patients. Ann Oncol. 1998;9:55–61. doi: 10.1023/a:1008201729596. [DOI] [PubMed] [Google Scholar]
- 54.Voorhees PM, Carder KA, Smith SV, et al. Follicular lymphoma with a Burkitt translocation--predictor of an aggressive clinical course: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:210–213. doi: 10.5858/2004-128-210-FLWABT. [DOI] [PubMed] [Google Scholar]
- 55.Wang SA, Wang L, Hochberg EP, et al. Low histologic grade follicular lymphoma with high proliferation index: morphologic and clinical features. Am J Surg Pathol. 2005;29:1490–1496. doi: 10.1097/01.pas.0000172191.87176.3b. [DOI] [PubMed] [Google Scholar]
- 56.Weiss LM, Warnke RA, Sklar J, et al. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987;317:1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 57.Weremowicz S, Schofield DE. Preparation of cells from formalin-fixed, paraffin-embedded tissue for use in fluorescence in situ hybridization (FISH) experiments. Curr Protoc Hum Genet. 2007 doi: 10.1002/0471142905.hg0808s52. Chapter 8:Unit 8.8. [DOI] [PubMed] [Google Scholar]
- 58.Wong KF, Chan JK. Follicular lymphoma with trisomy 18 and over-expression of BCL2 in the absence of t(14;18)(q32;q21) Cancer Genet Cytogenet. 2000;123:52–54. doi: 10.1016/s0165-4608(00)00301-0. [DOI] [PubMed] [Google Scholar]
- 59.Young KH, Xie Q, Zhou G, et al. Transformation of follicular lymphoma to precursor B-cell lymphoblastic lymphoma with c-myc gene rearrangement as a critical event. Am J Clin Pathol. 2008;129:157–166. doi: 10.1309/NKK3FEX2BE5L7EKB. [DOI] [PubMed] [Google Scholar]